Abstract
Any tumor could be controlled by radiation therapy if sufficient dose were delivered to all tumor cells. Although technological advances in physical treatment delivery have been developed to allow more radiation dose conformity, normal tissues are invariably included in any radiation field within the tumor volume and also as part of the exit and entrance doses relevant for particle therapy. Mechanisms of normal tissue injury and related biomarkers are now being investigated, facilitating the discovery and development of a next generation of radiation protectors and mitigators. Bringing recent research advances stimulated by development of radiation countermeasures for mass casualties, to clinical cancer care requires understanding the impact of protectors and mitigators on tumor response. These may include treatments that modify cellular damage and death processes, inflammation, alteration of normal flora, wound healing, tissue regeneration and others, specifically to counter cancer site-specific adverse effects to improve outcome of radiation therapy. Such advances in knowledge of tissue and organ biology, mechanisms of injury, development of predictive biomarkers and mechanisms of radioprotection have re-energized the field of normal tissue protection and mitigation. Since various factors, including organ sensitivity to radiation, cellular turnover rate, and differences in mechanisms of injury manifestation and damage response vary among tissues, successful development of radioprotectors/mitigators/treatments may require multiple approaches to address cancer site specific needs. In this review, we discuss examples of important adverse effects of radiotherapy (acute and intermediate to late occurring, when it is delivered either alone or in conjunction with chemotherapy, and important limitations in the current approaches of using radioprotectors and/or mitigators for improving radiation therapy. Also, we are providing general concepts for drug development for improving radiation therapy.
Keywords: Acute radiation effects, radiotherapy, radiation mitigator, radioprotector, oral mucositis, lung fibrosis, radiation-induced brain damage
Introduction
Radiotherapy is an important treatment modality for many malignancies and is also used as a part of combined modality therapy with (I) conventional chemotherapeutic agents often used in modified schedules accommodating radiation, (II) molecular targeted therapy, (III) immunotherapy, and (IV) as a part of immune suppression for stem cell and organ transplantation. New technologies in radiation therapy in the past decade have led to significant improvements in tailoring the radiation dose distribution more precisely to the shape of the tumor and minimizing the dose to sensitive normal tissues. These advances also allow higher dose delivery to a defined tumor sub-volume called “dose-painting” to areas deemed having greater tumor burden and/or increased radio-resistance due to hypoxia. Molecular and functional imaging linked to physical CT scanned images are used to guide radiation targeting and adapt treatment to tumor and normal tissue changes during a course of therapy. These novel approaches reduce collateral normal tissue damage and improve the therapeutic ratio. However, the location of the tumor within the organ, errors in treatment delivery such as incorrect patient positioning, and patient movement during treatment can result in excessive doses to normal tissues. Changes in treatment plans may be required during the course of treatment to accommodate changes in location, size and shape of the tumor and the organs at risk. A key factor to the risk of radiation injury is the relationship between dose and volume treated.
Many patients suffer adverse effects from radiation therapy. These side effects may be acute, occurring during or within a few weeks after therapy, or intermediate to late, occurring months to years after therapy. Acute radiation toxicity is primarily due to cell killing, but inflammation or infection may also be contributing factors. Intermediate and late effects result from complex responses as tissues attempt to heal or fail to heal, and may be exacerbated by trauma or infection. There is a need to reduce radiation toxicity and thus provide a therapeutic benefit and improve overall quality of life. Understanding the mechanisms through which radiation toxicity develops would provide clues for developing effective radioprotectors, mitigators or treatments (1). In this review, we discuss examples of important adverse effects of radiotherapy (acute and intermediate to late-occurring, including consequential effects (2), delivered either alone or in conjunction with chemotherapy, and important limitations in the current approaches of using radioprotectors and/or mitigators for improving radiation therapy. Table 1, modified from Vikram et al. (1) illustrates important cancer types, current treatment approaches, mortality, median survival, and important adverse effects of radiation therapy either alone or as an adjuvant to chemotherapy to emphasize how development of radioprotectors can help improve radiation therapy.
Table 1.
Important adverse effects after conventional radiotherapy (1) (modified to emphasize development of radioprotectors to improve radiation therapy)
| Cancer Type | Treatment | Mortality | Median Survival (mo) | Adverse Effects |
|
|---|---|---|---|---|---|
| Acute Effects | Likely intermediate to late effects | ||||
| Glioblastoma | Temozolomide | 73.5% by 2 yr | 14.6 | Gr. 3-4 non hematologic, Fatigue, rashes and vision, nausea, vomiting | Cognitive defects (3) |
| Head and Neck (locally advanced, unresectable) | Cetuximab | 45% by 3 yr | 49 | Gr 3-5 mucosal toxicity (56%), Gr 3-5 dyspahgia (26%), Gr 3-5 dermatitis (23%) | Cognitive defects (3) |
| Head and Neck (locally advanced, resected) | Cisplatin | Not available | 48 | Gr. 4-5 non hematologic in 27% including mucositis, pharyngitis, nausea, vomiting, skin toxicity | Cognitive defects (3) |
| Larynx (locally advanced) | Cisplatin | 24% by 2 yr | Not available | Gr 3/4 non hematologic toxicity in 77% including mucositis, pharyngitis, esophagitis, laryngitis | Persistant dysphagia in 15% at 2 years |
| Lung, non small-cell locally advanced | Continuous hyperfractionated accelerated radiation therapy | 71% by 2 yr | 16.5 | Symptomatic acute pneumonitis (10%) | Persistent severe dysphagia in 7% at 2 years |
| Lung, non small-cell locally advanced | Chemotherapy before irradiation | 68% by 2 yr | 13.2 | Acute 3-5 toxicity (52%) | Late Gr 3-5 toxicity (3%) |
| Lung small-cell limited disease | Chemotherapy | 74% by 5 yr | 23 | Acute Gr 3-5 esophagitis (32%), Infection, fever, vomiting, pulmonary effects | Fibrosis |
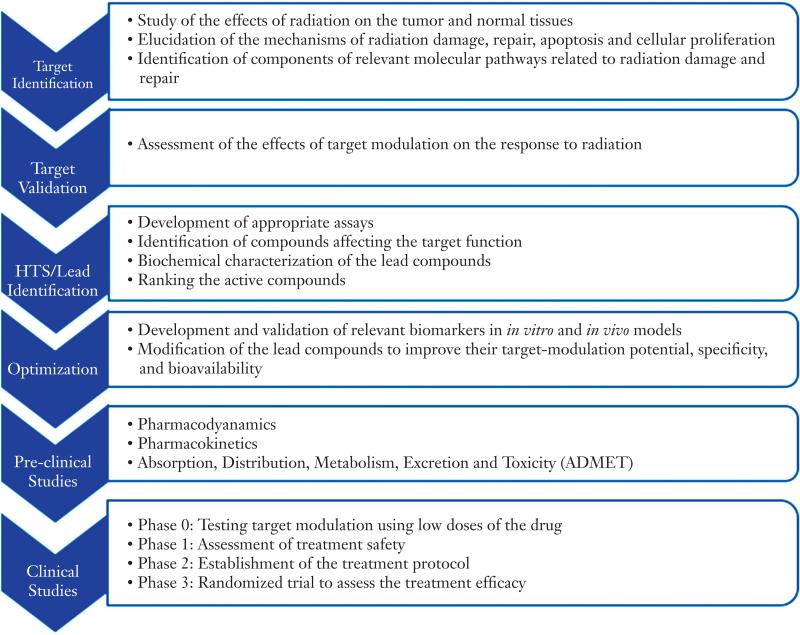
There are three categories of intervention for radiation damage: Protectors are agents given before radiation to prevent damage; mitigators are given during or shortly after a course of radiation therapy, before symptoms of toxicity appear; and treatments are given after symptoms of toxicity appear (4). Since various factors, including organ sensitivity to radiation, cellular turnover rate, and differences in mechanisms of injury manifestation and damage response vary among tissues successful development of radioprotectors/mitigators/treatments may require multiple approaches. In addition, patients cured of their primary malignancies may be susceptible to the development of secondary malignancies several years to decades after treatment. This risk is higher in younger patients in part because they have longer life expectancy for developing late effects. This review, however, will exclude carcinogenesis, and instead focus on the acute and intermediate to long-term toxicities from radiotherapy and potential strategies for protection, mitigation and treatment. Proposed general drug development process for radioprotectors to improve radiation therapy is illustrated in Figure 1 taking into consideration important adverse effects in current treatment approaches for major cancer types.
Figure 1.
Proposed general drug development process for radioprotectors to improve radiation therapy
Skin and mucosal damage
Damage to skin and mucosa represents one of the most common acute adverse effects of radiotherapy and/or chemotherapy. Mucosal damage may occur in the mouth, pharynx, esophagus, and bowel. It is a particular problem in head and neck cancer, where a significant number of patients report oral mucositis as the most debilitating adverse effect of radiotherapy (5,6). Oral mucositis often results in poor treatment outcome, reduced quality of life, and increased medical costs (7). Treatment regimens involving altered fractionation, such as hyperfractionation, accelerated radiotherapy, and concomitant boost accelerated radiation, improve therapy outcome, but invariably produce severe mucositis. Prevalence, patient-associated variables, pathobiology, risk factors, impact and current management approaches of oral mucositis have been reviewed (8). The World Health Organization (WHO) distinguishes four grades of oral mucositis, Grade 0 to 4 (9). The risk factors for developing severe mucosal injury include patients’ age, sex, ethnicity, body mass index, individual radiation sensitivity, etc.
Extent of radiation-induced damage and recovery in the cell renewal systems of skin and mucosa is determined by radiation sensitivity and the cellular turnover rate. A biological model for treatment induced oral mucositis has been proposed by Sonis (10). Accordingly, the onset, development, and healing of oral mucositis occurs in five sequential and overlapping steps: initiation, upregulation, message generation, ulceration, and healing. Initiation is via generation of reactive oxygen species (ROS) and direct damage to cells, tissues and blood vessels, and a cascade of reactions contributing to tissue damage (11). Up-regulation involves activation of transcription factors (e.g., nuclear factor-κβ), leading to a local increase in pro-inflammatory cytokines (IL-6) and tumor necrosis factor (TNF). A positive feedback mechanism results in an amplification and acceleration of the process leading to ulceration, allowing oral bacteria to colonize denuded connective tissue. It is now believed that treatment-induced mucositis is not restricted to direct epithelial damage in regions surrounding the treatment area, but affects the entire alimentary tract and involves the connective tissue (12). Compared to chemotherapy, radiotherapy-induced mucositis follows a relatively more gradual clinical course, as the latter is administered in fractions over weeks (8). Not surprisingly, given this overlap in toxicity, chemoradiotherapy-induced mucositis can be quite severe.
The incidence, duration and severity of radiation-induced oral mucositis increases with dose (13). In general, radiation-induced oral mucositis begins at an accumulated dose of 10 Gy during treatment, and intensifies in severity around 30 Gy, lasting for weeks to months. The highest rates of severe mucositis are seen among patients who receive a total body irradiation of 12 Gy as a preparative regimen in combination with high dose chemotherapy before blood stem cell transplantation (14).
Current approaches in the treatment of oral mucositis
Microbial colonization exacerbates oral mucositis. Current therapies for oral mucositis therefore include non-pharmacological approaches such as maintenance of oral health and hygiene in addition to oral cryotherapy as well as pharmacological treatment regimens. Benzydamine, a non-steroidal, anti-inflammatory analgesic and antimicrobial compound, is used for palliation and to reduce microbial colonization (15,16).
Management of radiation-induced oral mucositis with drugs such as the radioprotector amifostine, KGF (keratinocyte growth factor, palifermin), benzydamine treatment, and other investigational therapies does not provide consistent results, as described below.
Amifostine, given 15-30 min before each fraction of radiation, was not effective in preventing oral mucositis in a randomized large clinical trial involving over 300 patients undergoing treatment for squamous head and neck cancer, but both acute and delayed xerostomia were reduced (17).
KGF acts specifically on epithelial cells, promoting proliferation and decreasing apoptosis. It also causes thickening of the mucosa. It was effective in reducing chemotherapy-induced oral mucositis (14,18). Based on this effect, it was FDA-approved for prophylaxis of mucositis in patients receiving etoposide, cyclophosphamide and total body irradiation of 12 Gy prior to hematopoietic stem cell transplantation for hematological malignancies (14). However, in a clinical study assessing the efficacy and safety of prophylactic KGF given to patients for three days before receiving concurrent chemoradiotherapy (CRT) for advanced head and neck squamous cell carcinoma and weekly treatment after completion of CRT, it appeared to reduce mucositis, dysphagia, and xerostomia during hyperfractionated radiotherapy, but not during standard radiation therapy (19). In a subsequent multinational, randomized, placebo controlled, double-blinded trial with (n=188) patients with locally advanced head and neck cancers, a higher dose of KGF (180 μg/kg), when administered in weekly doses throughout the treatment with conventional chemoradiation, reduced the incidence of severe oral mucositis from 69% to 54%. The median duration of mucositis was reduced from 26 to 5 days and time to onset delayed from 35 to 47 days. The side effects were tolerable (20).
Thus, the majority of current treatment approaches for oral mucositis involve palliation or treatment after manifestation of symptoms, inducing proliferative activity of the mucosal layer to enhance repair of damage. Few attempts to prevent damage to the normal mucosa during radiation treatment have been made, largely because of the possibility of tumor protection, enhanced tumor proliferation, development of tumor resistance to other cytotoxic therapies, or inter-individual variability in response to radiation.
Standardization of dose, route, and time of administration is also essential to development and application of agents to reduce the incidence and severity of oral mucositis. These are constrained by side effects of the agents themselves, as was the case with amifostine (17). Common adverse events related to the administration of this drug included nausea/vomiting, hypotension, facial flushing and phlebitis.
Prevention of mucosal damage is preferable to mitigation, which is preferable to treatment after symptoms develop, to allow either the uninterrupted delivery of the prescribed radiation dose or dose escalation to the tumor. Phenylbutyrate, an antitumor histone deacetylase inhibitor, was recently shown in a pilot study to mitigate oral mucositis, during radiotherapy or chemoradiotherapy (21). Further development of normal-tissue-specific radioprotectors is needed. It is also important to develop and validate predictive markers useful for determining radiation sensitivity of the mucosa in individual patients in order to optimize the balance between tumor control and normal tissue toxicity.
Radiotherapy-induced lung damage
More than 60% of patients with Non-Small Cell Lung Cancer (NSCLC) are treated with radiation therapy (22). Radiation-induced lung damage is an intermediate to late-occurring side effect of radiation therapy. This damage appears as pneumonitis at the earlier times, with fibrosis occurring as a late effect.
Pneumonitis
Pneumonitis occurs at about 1-3 months after radiotherapy in some patients undergoing thoracic irradiation for cancers of lung, esophagus, breast, and lymphatic systems. The symptoms are congestion, cough, dyspnea, fever, and chest pain. Pneumonitis generally subsides after several weeks and can be treated with steroids.
Pneumonitis involves interstitial pulmonary inflammation, although the molecular mechanisms are not yet fully understood. Stone et al. (23) reviewed radiation-induced damage to lung and described the mechanisms of its onset, development, and contributing factors. At the molecular level, several cytokines such as TGF-β1 (24,25), IL-1 and IL-6 (26) seem to play important roles. Kong et al. (27) proposed a mechanism of regulation of pneumonitis and fibrosis. Accordingly, repetitive stimuli from fractionated irradiation and chemotherapy induce local damage to lung cells causing release of regulatory molecules such as cytokines that attract fibroblasts, circulating fibrocytes, and bone marrow stem cells that contribute to tissue healing and functional recovery (28). It is likely that interactions among multiple cell systems within a network of cellular and supra-cellular signaling pathways drive the processes leading to radiation-induced lung damage. Serial plasma specimens analyzed for changes in circulating cytokines before, during, and up to 12 weeks after irradiation indicated that both IL-1α and IL-6 levels were significantly higher before, during, and after radiotherapy in patients who developed pneumonitis (26).
While new conformal techniques are helpful in limiting normal tissue radiation doses, increasing lung doses will also increase the risk of developing radiation pneumonitis. This relationship is linear-quadratic from 5 to 30 Gy (27). New information on dose-volume relationships indicates that doses of radiation higher than those traditionally administered can be delivered to a majority of patients with non-small cell lung cancer (NSCLC) (29). This could allow dose escalation based on risk of toxicity in individual patients, combined with information on the lung volumes to be irradiated (29,30). Radiation toxicity to the lung can be markedly exacerbated by concurrent use of chemotherapy. For example, when gemcitabine and docetaxel were combined with radiation therapy, the combination regimen was extremely toxic with 8% deaths and 23% grade-3 lung toxicity compared to 1.6-2.1% deaths from radiotherapy alone (31). Combined modality toxicity is an ongoing concern with the advent of particle therapies, as proton therapy trials are being considered for treatment of lung and esophageal cancers using combined modality therapy. Whether the toxicities will be equivalent to standard therapies is not yet known.
Fibrosis
Recently, Hill et al. (32) concluded that radiation-induced inflammation in lung cells occurs through production of ROS contributing to DNA damage over prolonged periods. Individual patient factors including genetic predisposition, autoimmune conditions, or comorbidities can lead to aberrant wound healing, resulting in pulmonary fibrosis. Fibrosis often follows pneumonitis months to years after irradiation. It is diagnosed radiographically and in many patients does not cause clinical symptoms. It occurs after doses above about 30-40 Gy, depending on the fractionation scheme of radiation therapy and the use of chemotherapy. Fibrosis is characterized by vascular damage and collagen deposition (27).
Current approaches of treatment or mitigation of radiation pneumonitis and fibrosis
Although pneumonitis and pulmonary fibrosis are associated, the existence of pneumonitis-prone and fibrosis-prone strains of mice suggests that different mechanisms are involved in their development (33), and therefore, different approaches may be required. Since the lung is the most sensitive tissue for the delayed effects of acute radiation exposure (DEARE) following whole body exposure in terrorism and also bone-marrow transplantation, radioprotective and/or mitigation strategies could benefit all these patients. Several drugs have been evaluated, including amifostine, agents that target the renin-angiotensin system (RAS); angiotensin converting enzyme inhibitors (ACEI) and angiotensin II receptor agonists (AT2RA), genistein, pentoxyfiline, and manganese superoxide dismutase/plasmid liposomes. Some examples are reviewed below.
A Phase III randomized study by the Radiation Therapy Oncology Group (RTOG) evaluated the benefits of amifostine administration in 180 patients with stages II-III non-small-cell lung cancer receiving induction paclitaxel and carboplatin, and then concurrently with hyperfractionated radiation therapy from pretreatment to 6 weeks post-treatment. Results indicated that the use of amifostine significantly reduced pain after chemoradiation (34% vs. 21%), less difficulty in swallowing during chemoradiation, and less weight loss compared to patients not receiving amifostine. However, physician-rated assessments of dysphagia were not significantly different between the treatment arms. No other quality of life or symptom changes were found with respect to treatment arm, smoking status, alcohol use, or gender (34).
Robbins and Diz (35) reviewed the role of the RAS as a target for the modulation of radiation-induced late effects. RAS is a complex blood-borne hormonal system in which the substrate (angiotensinogen) and enzyme (renin) are released into the circulation from the liver and kidneys, respectively (35,36). Angiotensin-converting enzyme (ACE) converts angiotensin I to the active form, angiotensin II (ANG II), by binding to G protein-coupled receptors, AT1R and AT2R (37), that are widely distributed in various tissues. ACE inhibitors (ACEI) and angiotensin II receptor antagonists (AT2RA), routinely used to manage hypertension, mitigated radiation-induced lung injury in preclinical models. In irradiated Sprague Dawley rats, administration of ACEIs captopril, CL 24817, enalapril, and CGS 13945, prevented expression of markers of endothelial dysfunction. Angiotensin II appears to play an important role in the regulation of TGF-β and α-smooth muscle actin (SMA), two proteins involved in the pathogenesis of pulmonary fibrosis (38). The AT2RA 158,809 and the ACEIs, captopril and enalapril, significantly ameliorated the effects of radiation and cytoxan treatment-induced lung injury. Thus, ACEI and an AT2RA were effective in protecting lungs from radiation-induced pneumonitis and the development of lung fibrosis (39).
However, administration of ACEI during radiotherapy did not reduce the risk of radiation-induced pneumonitis in a retrospective analysis of 213 eligible patients receiving 3D-CRT for lung cancer with curative intent (40). Because a relatively small fraction of patients develop pneumonitis following thoracic radiation therapy it is important to develop predictive biomarkers that will help to identify those at risk prior to initiating trials evaluating treatments with ACEIs. On the positive side, the incidence of Grade 2 or higher pneumonitis was significantly lower in 62 patients with stage I through III who were taking ACEIs during thoracic irradiation treatment compared to 100 non-users (2% vs. 11%) (39). This is consistent with preclinical evidence, but warrants further investigation in a prospective study.
Hill et al. (32) demonstrated that post-irradiation administration of EUK-207, a SOD catalase mimetic and genistein, an isoflavone with anti-inflammatory properties, decreased the frequency of radiation-induced micronuclei, a marker of radiation damage, in lung cells in mice. Similarly, genistein reduced the incidence of micronuclei in primary fibroblast cultures from female mice, indicating protection against radiation-induced genotoxicity (41). It also prevented radiation-induced reduction of COX-2 expression, TGF-β receptor (TGF-βR) I and II, and other potential biomarkers of pulmonary injury at 90 days after irradiation (41). It is hypothesized that genistein would reduce the levels of inflammatory cytokines and ROS after irradiation, resulting in reduced DNA damage and functional deficits (42).
TNF-α knockout mice had a smaller radiation-induced increase in breathing rate than wild-type mice and less severe radiation pneumonitis, indicating that TNF-α plays an important role in the development of inflammation in lung following irradiation.
Manganese superoxide dismutase-plasmid liposomes (MnSOD-PL) also protects lung from local radiation injury (43,44). It appears to stabilize antioxidant pools, including glutathione and total thiols, within cells and in normal tissues (43). Tumor radiosensitization, not protection, was observed in mice with orthotopic Lewis lung carcinomas following intratracheal administration of MnSOD-PL (45). The onset of alveolitis/pulmonary fibrosis was delayed and its extent was reduced (43). Mice treated with inhalation delivery of MnSOD-PL showed a plasmid dose-dependent increase in expression of MnSOD transgene product over the range of 250 μg to 2.5 mg. Treatment with MnSOD-PL 24 hr before 20 Gy to the lungs had slightly longer survival than irradiated controls (44).
The initial interim analysis of RTOG 0617 comparing standard 60 Gy plus chemotherapy to the higher 74 Gy plus chemotherapy + cetuximab for treatment of inoperable Stage III NSCLC, was reported at the 2011 ASTRO meeting showing no overall survival advantage with dose escalation to 74 Gy. It was also reported that there was no significant difference in treatment-related toxicities between the two radiation treatment arms after a median follow-up time of only 11 months (unpublished at the time this paper was written: http://journals.lww.com/oncology-times/blog/onlinefirst/pages/post.aspx?PostID=316). Any benefits from further dose escalation beyond 74 Gy remain to be determined, but tumor motion, location and normal tissue effects must also be considered. Normal tissue protection will be useful for improving cure rates and decreasing patient morbidity if dose escalation is to be pursued. Physical dose-volume relationships that are required for effective treatment but increase the likelihood of lung injury will need to be defined. Since the cohort that develops radiation pneumonitis is relatively small, the development of early predictive biomarkers of pneumonitis would aid in identifying the patient population that could benefit from the administration of radioprotectors or mitigators. Therefore, clinical trials are necessary to determine whether normal tissue protectors and mitigators will permit use of higher radiation doses and whether these can lead to a survival advantage in patients with non-small cell lung cancer (46).
Radiation-induced brain damage
The American Cancer Society predicts that there will be 22,910 new brain cancer cases and 13,700 deaths in 2012. Additionally, about 30% of cancer survivors will develop brain metastases. In fact over 200,000 patients/year in the US with malignant brain tumors, including primary and metastatic tumors, are treated with radiation therapy for cure and palliation. Over 100,000 of these long-term survivors (>6 months) will develop brain injury that affects their quality of life (47). In the brain, as in other tumor sites, radiation dose prescriptions and probability of tumor control are constrained by normal tissue tolerance, despite the use of state-of-the-art radiation delivery techniques and improved modeling of dose distributions. New stereotactic radiotherapy techniques that use high doses per fraction may provide benefit in the treatment of metastases, but their impact on treatment of glioblastoma may be mitigated by tumor extension beyond what is detectable in imaging.
Radiation injury to brain develops months to years after therapy, and is severe and irreversible. In the past, delayed radiation injury was thought to be solely due to a reduction in surviving clonogens of parenchymal or vascular target cell populations; this hypothesis now appears to be simplistic. Radiation injury is dynamic and involves not only loss of parenchymal and stromal cells, including vascular cells, but also impaired proliferation of precursor cells, reactive oxygen species (ROS) and waves of pro-inflammatory cytokines and leads to tissue damage and functional deficits (11,48). Research into the mechanisms of cognitive impairment presents opportunities for development of novel therapeutic intervention strategies (35). Studies in rodent models indicate that irradiation of the brain leads to a significant reduction in neurogenesis (49), inflammation of the neurons (50,51), and progressive cognitive impairment (52). Neural progenitors within the subgranular zone of the dentate gyrus are among the most radiosensitive cell types in the adult brain. Damage to these cells reduces neurogenesis and correlates with cognition deficits (50). Neural precursor cells in culture exhibit an acute dose-dependent apoptosis accompanied by an increase in ROS persisting over a 3-4-week period. Radiation also activates cell cycle checkpoints that delay or prevent cell division (42). Proliferating precursor cells and their progeny (i.e. immature neurons) exhibit a dose-dependent reduction in cell number, which is less severe in Trp53-null mice, suggesting that the apoptotic and ROS responses may be tied to Trp53-dependent regulation of cell cycle control and stress-activated pathways (51).
Histological characteristics of brain injury appear to be non-specific to radiation, but after high doses, white matter necrosis with demyelination is a prominent histopathological feature. Endothelial cell loss appears to contribute to the demyelination, because significant demyelination was observed and neural precursor cell populations were reduced when endothelial cells were selectively irradiated using boron neutron capture therapy employing a boron compound that remained within the vasculature (53,54). Also, excessive generation of ROS, including oxygen radicals, free radicals, and inorganic and organic peroxides, causes an “oxidative stress” and overwhelms the “antioxidant defense system”, resulting in the development of delayed effects in the brain (55). Gradual upregulation of vascular endothelial growth factor (VEGF) occurs several weeks prior to manifestation of tissue pathology (56), which seems to gradually diminish the integrity of blood brain barrier (BBB) (57). This leads to a vicious cycle of reduction in endothelial cell density and disruption of BBB, ultimately causing functional deficits.
The RAS described previously is also found in the brain (35), where it is involved in brain-specific functions, including modulation of the BBB, pain perception, stress, memory, and cognition (58,59).
Therapeutic strategies for radiation-induced brain damage
Drugs currently used in animal models to counter radiation-induced brain damage block pro-inflammatory cytokines and prevent formation of ROS. These include ACEIs, statins, superoxide mimetics, and VEGF inhibitors.
An ACEI, ramipril, ameliorated demyelination of optic nerves in a rat model of optic neuropathy after a single stereotactic dose of 30 Gy (60) and preserved the functional integrity of the nerve (61). Putative mechanisms of amelioration of radiation-induced brain injury, including cognitive impairment, by RAS inhibitors include a blockade of Ang II/NADPH oxidase-mediated oxidative stress and neuro-inflammation and a change in the balance of angiotensin (Ang) peptides from the pro-inflammatory and pro-oxidative Ang II to the anti-inflammatory and anti-oxidative Ang-1-7 (62). Treatment with the AT1RA L-158,809 before, during, and after, fractionated whole-brain irradiation prevents or ameliorates radiation-induced cognitive deficits in adult rats, although it does not appear to modulate chronic inflammatory mechanisms (63,64). Both ACEIs and AT1RAs are routinely prescribed for hypertension and are well-tolerated drugs that also exhibit some antitumor properties and can prevent/ameliorate radiation-induced brain injury (62).
Statins, a class of drugs routinely used to treat hypercholesterolemia and atherosclerosis, have pleiotropic effects, which may include neuroprotection and promotion of tissue repair via modulation of endothelial nitric oxide synthetase (eNOS) antioxidant and anti-inflammatory pathways (65-68). Jenrow et al. (69) investigated whether atorvastatin, administered alone or in combination with the ACEI, ramipril, following radiation injury, protects progenitors and/or preserves neurogenic potential within the subgranular zone of the dentate gyrus. Although chronic administration of atorvastatin alone was relatively ineffective as a mitigator, its combination with ramipril appeared to interact synergistically to mitigate radiation-induced disruption of neurogenic signaling. Cognitive functions were not evaluated in this study in adult male rats.
Since oxidative stress via excessive generation of ROS appears to play a role in the development of delayed effects in brain (55), it was speculated that superoxide dismutase (SOD), may help mitigate late effects of irradiation on brain. VEGF family of signal proteins stimulates vasculogenesis and angiogenesis, which promote tumor growth. Anti-VEGF therapies have been found useful in the treatment of certain cancer types, but their benefit in protecting against radiation-induced normal tissue damage and/or mitigation is not clear. Winkler et al. (70) showed that VEGF receptor 2 (VEGFR2) blockade creates a “normalization window”, because of a transient stabilization of blood vessels and improved oxygen delivery to hypoxic regions within a rat orthotopic glioma tumor in which radiation therapy may be more effective. The benefit to counter radiation-induced normal tissue damage in brain is not clear. Bevacizumab, alone and in combination with other agents, was found to reduce radiation necrosis by decreasing capillary leakage and the associated brain edema in a clinical trial involving a very small number of patients (n=15) with malignant brain tumors, but these findings need to be confirmed in a randomized trial (71).
Development of radioprotectors/mitigators -translational path to clinic
Decades of preclinical and clinical research efforts have been spent with the aim of protecting normal tissue from acute radiation-induced damage and mitigating intermediate to late effects with some limited success such as with amifostine. The importance of developing agents that protect or mitigate radiation-induced damage in normal tissue, improve survival and quality of life, as well as improve palliative care in cancer patients was emphasized in an NCI workshop, “Advanced Radiation Therapeutics - Radiation Injury Mitigation”. The proceedings of this workshop include guidelines for preclinical (72) and clinical development (73) of promising agents for reducing the adverse effects of radiation therapy.
A three-stage approach is recommended for preclinical radioprotector/mitigator development (74). In Stage I, maximum tolerated dose (MTD) and toxicity of the agent is determined using Good Laboratory Practices (GLP). In stage II, protective/mitigative effects are determined using both in vitro and in vivo testing in both normal tissues and tumors. If both absence of tumor protection and sufficient normal tissue protection/mitigation is found, then the mechanism of action should be identified, if not already available. In Stage III, comprehensive toxicological and pharmacological testing is performed to address the regulatory requirement for data on Absorption, Distribution, Metabolism, Excretion and Toxicity profiles (ADMET) before proceeding to the clinical investigation (73). A consensus was reached among the workshop participants on (I) best practices for agent evaluation for normal tissue protection and radiation injury mitigation in cancer patients, (II) clinical trial designs that could efficiently and empirically move the most promising agents into appropriate clinical trials, and (III) scientific rationale that might be applied by regulatory agencies to evaluate agents for investigational new drug (IND) applications and approval. An algorithm to guide clinical trials for such agents in patients receiving radiotherapy or chemotherapy has already been published (73).
The search for a universal radioprotector that works across all tissue types and anatomical sites is likely to yield limited success, because various organs and tissues differ in such factors as radiation sensitivity, DNA damage response, proliferative and oxygenation status of tissue, vasculature, drug uptake, and activation, release, and response to inflammatory cytokines. For example, the radioprotection afforded to normal tissues by amifostine varies widely, with some of the most responsive tissues showing low levels of absorbed drug and vice versa, possibly due to differences in oxygen tension (74). In addition, tumors can also affect the biology and radiation response of normal tissues, before, during, and after irradiation. Therefore, although there may be some commonality among tissues, efforts must be focused on discovering and developing radioprotectors/mitigators that are specific to each anatomical site.
The extent of initial DNA damage induced by a given radiation dose to different tissues will be similar in the absence of differences in tissue oxygenation, although differences in DNA conformation resulting from cell cycle differences might occur. The outcome of this damage will largely be determined by DNA damage responses in the different tissues. There is significant inter-individual variation in responses and susceptibility to radiation effects on normal tissues, doubtless influenced by genetic factors. These are at present not well defined, but are the subject of active investigation. In the absence of mutation in DNA damage response genes, the response to DNA damage will be influenced by the proliferative status, cell cycle distribution and propensity for apoptosis of the cells in the irradiated tissue. More rapidly dividing tissues with a higher rate of cell turnover, such as those of the oral mucosa and lung epithelial lining will demonstrate greater acute reactions and consequential late effects, while the more slowly dividing CNS tissues (brain and spinal cord) are susceptible to late effects including leukoencephalopathies and radiation necrosis (75). The protection and mitigation strategies for these two types of responses could, in the future, differ as a result. For example, while anti-apoptotic approaches might be applied to epithelium, they could be of limited benefit in neural injury.
The principal factors currently determining therapeutic approach are the location and accessibility of these tissues. While both pneumonitis and CNS inflammation can be treated with steroids, epithelial surfaces in the lung, oral and upper aerodigestive mucosa are candidates for topical approaches including the application of radical scavengers. This could not be used in the CNS. Soy isoflavones and SOD mimetics have been proposed for prevention of pneumonitis (76). Bevacizumab has recently been proposed as a treatment to prevent the vascular endothelial dysfunction that contributes to radiation necrosis in the CNS (77).
It is well known that hypoxic cells, which are present in many tumors, are radioresistant. Because these may give the tumor a survival advantage, any additional potential for protection of tumors in relation to normal tissue, whose oxygen levels also vary, is a concern in the radioprotector field. Assays for tumor protection using cultured cell lines do not translate well into in vivo studies and hence to the clinic, because they do not mimic oxygen levels and other microenvironmental factors that affect the responses of tumors and normal tissues in situ. Functional radiobiological endpoints for cell killing such as the clonogenic assay are necessary to fully assess the impact of any protector/mitigator for normal and cancer cell lines. Since irradiated cells may remain metabolically viable and undergo several cell divisions before they die (78), assays based on uptake or exclusion of dyes are inappropriate (79).
Differential radioprotection may be achieved if the normal tissue selectively takes up the radioprotector or if it has mechanisms of tissue protection not utilized by the tumor. Therefore, data demonstrating a higher concentration of the drug in the target normal tissue than the tumor in in vivo models are essential. Studies of structure-activity relationships using analogs of lead compounds can aid in understanding mechanisms of action and finding the most effective radioprotectors. Preclinical and/or early phase clinical studies demonstrating safety, efficacy, dose, schedule, pharmacokinetics (PK), pharmacodynamics (PD), and metabolism is necessary. It is important to demonstrate that an effective concentration of an agent in the target tissue can be achieved. This may differ among various organs. For example, the normal tissues in which the highest concentrations of amifostine are achieved include kidney, salivary gland, bone marrow, liver, heart, lung and small intestine (80). Not surprisingly, the most impressive clinical benefits were reported for amifostine for protecting kidneys and preventing xerostomia (81). Exploitable differences between tumors and normal tissues may include differences in vasculature and membrane properties related to drug or prodrug uptake and conversion to an active metabolite.
Protectors and mitigators, especially those intended for use in patients treated with radiotherapy, must be evaluated in relevant in vitro and in vivo systems to determine whether they also protect tumors or increase metastasis while protecting normal tissue or aiding normal tissue recovery. In addition, they should have limited normal tissue toxicity. The protective/mitigative effect of the candidate agents ideally should be determined using in vivo human orthotopic xenograft mouse models (82), where possible to demonstrate protection/mitigation in the target tissue, but not the tumor.
Finally, a clear understanding of regulatory requirements including a regulatory plan with key steps such as a pre-IND meeting with FDA, submitting an investigational new drug (IND) application, approval of clinical trial design, and ultimately drug registration also are critical.
Phase zero trials
Traditionally new drugs in oncology undergo Phase I trials for evaluating their toxicity profile, then Phase II trials for demonstrating efficacy proof-of-principle, followed by Phase III trials for the evaluation of efficacy. The most common reason that drugs fail is lack of efficacy in Phase II or III trials. That may be due to inadequate biological understanding of the underlying mechanisms, inadequate animal models, inadequate understanding of the optimal scheduling of the drug and/or suboptimal design of the clinical trial itself.
Toxicity is a major concern for many cancer drugs. The Phase 0 trial is a new approach for evaluating the PK and PD properties of a new investigational agent in a small number of patients before initiating larger, traditional Phase I trials (83). It involves administration of very low doses of the new drug over a short time period and measuring the effect of the drug on its molecular target and/or pathways in humans employing procedures validated in preclinical models.
FDA Exploratory IND Guidance may be found at: (http://www.fda.gov/downloads/Drugs/Guidance.%20Compliance%20Regulatory%20Information/Guidances/ucm078933.pdf). Because of the low doses involved, Phase 0 trials require less preclinical toxicity data than for traditional first-in-human phase I studies. Issues to be addressed in the design of such trials for radioprotectors and mitigators will include obtaining relative distribution of candidate drugs in tumor vs. normal tissue and the identification of appropriate biomarkers.
Acknowledgements
National Cancer Institute's Radiation Research Program supported the manuscript preparation.
Footnotes
Disclosure: This manuscript is not being submitted for consideration of publication elsewhere. All authors do not have any financial interests in any or all of the companies that are mentioned in this manuscript.
References
- 1.Vikram B, Coleman CN, Deye JA. Current status and future potential of advanced technologies in radiation oncology. Part 1. Challenges and resources. Oncology. 2009;23:279–83. Williston Park. [PubMed] [Google Scholar]
- 2.Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61:223–31. doi: 10.1016/s0167-8140(01)00429-7. [DOI] [PubMed] [Google Scholar]
- 3.Johannesen TB, Lien HH, Hole KH, et al. Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother Oncol. 2003;69:169–76. doi: 10.1016/s0167-8140(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 4.Coleman CN, Blakely WF, Fike JR, et al. Molecular and cellular biology of moderate-dose (1-10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17-18, 2001. Radiat Res. 2003;159:812–34. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 5.Rose-Ped AM, Bellm LA, Epstein JB, et al. Complications of radiation therapy for head and neck cancers. The patient's perspective. Cancer Nurs. 2002;25:461–7. doi: 10.1097/00002820-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Bellm LA, Epstein JB, Rose-Ped A, et al. Patient reports of complications of bone marrow transplantation. Support Care Cancer. 2000;8:33–9. doi: 10.1007/s005209900095. [DOI] [PubMed] [Google Scholar]
- 7.Nonzee NJ, Dandade NA, Patel U, et al. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer. 2008;113:1446–52. doi: 10.1002/cncr.23714. [DOI] [PubMed] [Google Scholar]
- 8.Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452–6. doi: 10.1016/j.oraloncology.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO handbook for reporting results of cancer treatment. World Health Organization; Geneva: Albany, N.Y.: 1979. sold by WHO Publications Centre USA. [Google Scholar]
- 10.Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34:39–43. doi: 10.1016/s1368-8375(97)00053-5. [DOI] [PubMed] [Google Scholar]
- 11.Rubin P, Johnston CJ, Williams JP, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 12.Sonis ST. A biological approach to mucositis. J Support Oncol. 2004;2:21–32. [PubMed] [Google Scholar]
- 13.Russo G, Haddad R, Posner M, et al. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13:886–98. doi: 10.1634/theoncologist.2008-0024. [DOI] [PubMed] [Google Scholar]
- 14.Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–8. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Chu FC, Lakshmi V, et al. Benzydamine HCl, a new agent for the treatment of radiation mucositis of the oropharynx. Am J Clin Oncol. 1986;9:132–4. doi: 10.1097/00000421-198604000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Sonis ST, Clairmont F, Lockhart PB, et al. Benzydamine HCL in the management of chemotherapy-induced mucositis. I. Pilot study. J Oral Med. 1985;40:67–71. [PubMed] [Google Scholar]
- 17.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–45. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 18.Rosen LS, Abdi E, Davis ID, et al. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. J Clin Oncol. 2006;24:5194–200. doi: 10.1200/JCO.2005.04.1152. [DOI] [PubMed] [Google Scholar]
- 19.Brizel DM, Murphy BA, Rosenthal DI, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. J Clin Oncol. 2008;26:2489–96. doi: 10.1200/JCO.2007.13.7349. [DOI] [PubMed] [Google Scholar]
- 20.Le QT, Kim HE, Schneider CJ, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29:2808–14. doi: 10.1200/JCO.2010.32.4095. [DOI] [PubMed] [Google Scholar]
- 21.Yen SH, Wang LW, Lin YH, et al. Phenylbutyrate mouthwash mitigates oral mucositis during radiotherapy or chemoradiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol. 2012;82:463–70. doi: 10.1016/j.ijrobp.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Tyldesley S, Boyd C, Schulze K, et al. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49:973–85. doi: 10.1016/s0360-3016(00)01401-2. [DOI] [PubMed] [Google Scholar]
- 23.Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 24.Anscher MS, Kong FM, Marks LB, et al. Changes in plasma transforming growth factor beta during radiotherapy and the risk of symptomatic radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys. 1997;37:253–8. doi: 10.1016/s0360-3016(96)00529-9. [DOI] [PubMed] [Google Scholar]
- 25.Yuan X, Liao Z, Liu Z, et al. Single nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol. 2009;27:3370–8. doi: 10.1200/JCO.2008.20.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Williams J, Ding I, et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol. 2002;12:26–33. doi: 10.1053/srao.2002.31360. [DOI] [PubMed] [Google Scholar]
- 27.Kong FM, Ten Haken R, Eisbruch A, et al. Non-small cell lung cancer therapy-related pulmonary toxicity: an update on radiation pneumonitis and fibrosis. Semin Oncol. 2005;32:S42–S54. doi: 10.1053/j.seminoncol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Greenberger JS. Gene therapy approaches for stem cell protection. Gene Ther. 2008;15:100–8. doi: 10.1038/sj.gt.3303004. [DOI] [PubMed] [Google Scholar]
- 29.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(Suppl):S70–6. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324–33. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Kouroussis C, Mavroudis D, Kakolyris S, et al. High incidence of pulmonary toxicity of weekly docetaxel and gemcitabine in patients with non-small cell lung cancer: results of a dose-finding study. Lung Cancer. 2004;44:363–8. doi: 10.1016/j.lungcan.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Hill RP, Zaidi A, Mahmood J, Jelveh S. Investigations into the role of inflammation in normal tissue response to irradiation. Radiother Oncol. 2011;101:73–9. doi: 10.1016/j.radonc.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skwarchuk MW, Travis EL. Murine strain differences in the volume effect and incidence of radiation-induced colorectal obstruction. Int J Radiat Oncol Biol Phys. 1998;41:889–95. doi: 10.1016/s0360-3016(98)00145-x. [DOI] [PubMed] [Google Scholar]
- 34.Sarna L, Swann S, Langer C, et al. Clinically meaningful differences in patient-reported outcomes with amifostine in combination with chemoradiation for locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. Int J Radiat Oncol Biol Phys. 2008;72:1378–84. doi: 10.1016/j.ijrobp.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Robbins ME, Diz DI. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2006;64:6–12. doi: 10.1016/j.ijrobp.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 36.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144:2179–83. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 37.Nakajima M, Hutchinson HG, Fujinaga M, et al. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci USA. 1995;92:10663–7. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molteni A, Wolfe LF, Ward WF, et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13:1307–16. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 39.Kharofa J, Cohen EP, Tomic R, et al. Decreased Risk of Radiation Pneumonitis with Incidental Concurrent Use of Angiotensin-Converting Enzyme Inhibitors and Thoracic Radiation Therapy. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2011.11.013. DOI: S0360-3016(11)03501-2 [pii] 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Wang LW, Fu XL, Clough R, et al. Can angiotensin-converting enzyme inhibitors protect against symptomatic radiation pneumonitis? Radiat Res. 2000;153:405–10. doi: 10.1667/0033-7587(2000)153[0405:caceip]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Day RM, Barshishat-Kupper M, Mog SR, et al. Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. J Radiat Res (Tokyo) 2008;49:361–72. doi: 10.1269/jrr.07121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calveley VL, Jelveh S, Langan A, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173:602–11. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenberger JS, Epperly MW. Review. Antioxidant gene therapeutic approaches to normal tissue radioprotection and tumor radiosensitization. In Vivo. 2007;21:141–6. [PubMed] [Google Scholar]
- 44.Carpenter M, Epperly MW, Agarwal A, et al. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes protects the murine lung from irradiation damage. Gene Ther. 2005;12:685–93. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- 45.Guo H, Epperly MW, Bernarding M, et al. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) intratracheal gene therapy reduction of irradiation-induced inflammatory cytokines does not protect orthotopic Lewis lung carcinomas. In Vivo. 2003;17:13–21. [PubMed] [Google Scholar]
- 46.Rosenman J. Can the use of amifostine improve cure rates for patients with advanced non-small cell lung cancer? Semin Oncol. 2004;31:52–8. doi: 10.1053/j.seminoncol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 47.American Cancer Society I. Learn about cancer. [05/05/2012];American Cancer Society. 2012 [updated 01/05/2012]; Available from: http://www.cancer.org/Cancer/BrainCNSTumorsinAdults/DetailedGuide/brain-and-spinal-cord-tumors-in-adults-key-statistics.
- 48.Kim JH, Brown SL, Jenrow KA, et al. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87:279–86. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 49.Ramanan S, Kooshki M, Zhao W, et al. The PPARalpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys. 2009;75:870–7. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limoli CL, Rola R, Giedzinski E, et al. Cell-density-dependent regulation of neural precursor cell function. Proc Natl Acad Sci USA. 2004;101:16052–7. doi: 10.1073/pnas.0407065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Limoli CL, Giedzinski E, Rola R, et al. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat Res. 2004;161:17–27. doi: 10.1667/rr3112. [DOI] [PubMed] [Google Scholar]
- 52.Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 53.Coderre JA, Morris GM, Micca PL, et al. Late effects of radiation on the central nervous system: role of vascular endothelial damage and glial stem cell survival. Radiat Res. 2006;166:495–503. doi: 10.1667/RR3597.1. [DOI] [PubMed] [Google Scholar]
- 54.Otsuka S, Coderre JA, Micca PL, et al. Depletion of neural precursor cells after local brain irradiation is due to radiation dose to the parenchyma, not the vasculature. Radiat Re. 2006;165:582–91. doi: 10.1667/RR3539.1. [DOI] [PubMed] [Google Scholar]
- 55.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–9. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 56.Schuller BW, Binns PJ, Riley KJ, et al. Selective irradiation of the vascular endothelium has no effect on the survival of murine intestinal crypt stem cells. Proc Natl Acad Sci USA. 2006;103:3787–92. doi: 10.1073/pnas.0600133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li YQ, Ballinger JR, Nordal RA, et al. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res. 2001;61:3348–54. [PubMed] [Google Scholar]
- 58.McKinley MJ, Albiston AL, Allen AM, et al. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–18. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 59.Gard PR. The role of angiotensin II in cognition and behaviour. Eur J Pharmacol. 2002;438:1–14. doi: 10.1016/s0014-2999(02)01283-9. [DOI] [PubMed] [Google Scholar]
- 60.Kim JH, Brown SL, Kolozsvary A, et al. Modification of radiation injury by ramipril, inhibitor of angiotensin-converting enzyme, on optic neuropathy in the rat. Radiat Res. 2004;161:137–42. doi: 10.1667/rr3124. [DOI] [PubMed] [Google Scholar]
- 61.Ryu S, Kolozsvary A, Jenrow KA, et al. Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: importance of ramipril dose and treatment time. J Neurooncol. 2007;82:119–24. doi: 10.1007/s11060-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 62.Robbins ME, Zhao W, Garcia-Espinosa MA, et al. Renin-angiotensin system blockers and modulation of radiation-induced brain injury. Curr Drug Targets. 2010;11:1413–22. doi: 10.2174/1389450111009011413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conner KR, Payne VS, Forbes ME, et al. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res. 2010;173:49–61. doi: 10.1667/RR1821.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robbins ME, Payne V, Tommasi E, et al. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2009;73:499–505. doi: 10.1016/j.ijrobp.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu D, Goussev A, Chen J, et al. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 66.Shishehbor MH, Brennan ML, Aviles RJ, et al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426–31. doi: 10.1161/01.CIR.0000080895.05158.8B. [DOI] [PubMed] [Google Scholar]
- 67.Shishehbor MH, Patel T, Bhatt DL. Using statins to treat inflammation in acute coronary syndromes: Are we there yet? Cleve Clin J Med. 2006;73:760–6. doi: 10.3949/ccjm.73.8.760. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Zhang ZG, Li Y, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–51. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 69.Jenrow KA, Liu J, Brown SL, et al. Combined atorvastatin and ramipril mitigate radiation-induced impairment of dentate gyrus neurogenesis. J Neurooncol. 2011;101:449–56. doi: 10.1007/s11060-010-0282-x. [DOI] [PubMed] [Google Scholar]
- 70.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 71.Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–6. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 72.Movsas B, Vikram B, Hauer-Jensen M, et al. Decreasing the adverse effects of cancer therapy: National Cancer Institute guidance for the clinical development of radiation injury mitigators. Clin Cancer Res. 2011;17:222–8. doi: 10.1158/1078-0432.CCR-10-1402. [DOI] [PubMed] [Google Scholar]
- 73.Ryan JL, Krishnan S, Movsas B, et al. Decreasing the adverse effects of cancer therapy: an NCI Workshop on the preclinical development of radiation injury mitigators/protectors. Radiat Res. 2011;176:688–91. doi: 10.1667/rr2704.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuhas JM, Afzal SM, Afzal V. Variation in normal tissue responsiveness to WR-2721. Int J Radiat Oncol Biol Phys. 1984;10:1537–9. doi: 10.1016/0360-3016(84)90498-x. [DOI] [PubMed] [Google Scholar]
- 75.Rane N, Quaghebeur G. CNS effects following the treatment of malignancy. Clin Radiol. 2012;67:61–8. doi: 10.1016/j.crad.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 76.Sonis ST. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009;45:1015–20. doi: 10.1016/j.oraloncology.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 77.Matuschek C, Bolke E, Nawatny J, et al. Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol. 2011;187:135–9. doi: 10.1007/s00066-010-2184-4. [DOI] [PubMed] [Google Scholar]
- 78.Thompson LH, Suit HD. Proliferation kinetics of x-irradiated mouse L cells studied with time-lapse photography. Int J Radiat Biol Relat Stud Phys Chem Med. 1969;15:347–62. doi: 10.1080/09553006914550571. [DOI] [PubMed] [Google Scholar]
- 79.Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391–9. [PubMed] [Google Scholar]
- 80.Rasey JS, Nelson NJ, Mahler P, et al. Radioprotection of normal tissues against gamma rays and cyclotron neutrons with WR-2721: LD50 studies and 35S-WR-2721 biodistribution. Radiat Res. 1984;97:598–607. [PubMed] [Google Scholar]
- 81.Cassatt DR, Fazenbaker CA, Bachy CM, et al. Preclinical modeling of improved amifostine (Ethyol) use in radiation therapy. Semin Radiat Oncol. 2002;12:97–102. doi: 10.1053/srao.2002.31382. [DOI] [PubMed] [Google Scholar]
- 82.Rubio-Viqueira B, Hidalgo M. Direct in vivo xenograft tumor model for predicting chemotherapeutic drug response in cancer patients. Clin Pharmacol Ther. 2009;85:217–21. doi: 10.1038/clpt.2008.200. [DOI] [PubMed] [Google Scholar]
- 83.Murgo AJ, Kummar S, Rubinstein L, et al. Designing phase 0 cancer clinical trials. Clin Cancer Res. 2008;14:3675–82. doi: 10.1158/1078-0432.CCR-07-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]