Several laboratories have shown that collections of adult cardiac stem cells can be grown directly from myocardial tissue.(1–7) Expanded cells are multipotent and clonogenic.(3–5) Spherical aggregates of cells grown from heart biopsies, termed cardiospheres, self-assemble in suspension culture and are enriched in stemness.(1, 6, 7) Andersen and colleagues, in work on neonatal murine heart tissue, used novel culture methods as a basis to question the cardiogenic potential of cardiosphere-cultured stem cells.(8) We contend that their sweeping conclusions are not applicable to studies using established methods (1, 2, 6, 7).
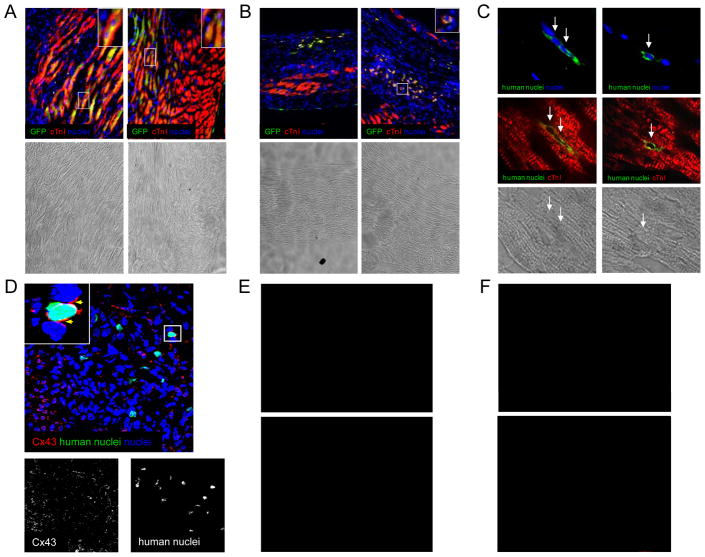
To confirm the cardiogenic nature of human cardiosphere-derived cells (CDCs) in vivo, we evaluated the ability of CDCs to engraft and form new cardiomyocytes after transplantation into infarcted SCID mice. Immunostaining for human nuclear antigen (1:50, Chemicon MAB1281) and lentivirally-mediated GFP or beta-galactosidase labelling were used to track cells after injection. While the majority of CDCs could be found throughout the infarct (57±3% of the total engrafted) and the immediate border zone (30±5%), stable engraftment also existed in the remote myocardium (13±3%). Figure 1A and 1B show GFP-labelled CDCs within the border and infarct zones. Counterstaining for cardiac troponin I (cTnI; Chemicon MAB3150) demonstrates that many CDCs have differentiated into cardiomyocytes. As shown in Figure 1, portions of the mouse heart were reconstituted by human CDCs (Figure 1A), while CDC-derived cardiomyocytes in the dense central infarct zone remained small with little cTnI expression in their cytoplasm (Figure 1B). Human nuclear antigen expression also demonstrates differentiated cardiomyocytes, derived from transplanted human CDCs, within the border zone (Figure 1C). These human-derived cells express Cx43, suggesting, but not proving, functional integration within the infarcted tissue (Figure 1D). Such functional integration has, however, been rigorously documented in vitro with myocyte-CDC co-culture.(7) Further, beta-galactosidase-labelled CDCs expressing markers of endothelial (vWF, Figure 1E) and smooth muscle (αSMA; Figure 1F) lineages highlight the in vivo multilineage potential of CDCs.
Figure 1.
Cardiogenic differentiation of transplanted cells cultured from humans biopsies using the cardiosphere method. Human CDCs were injected into SCID mice at the time of myocardial infarction. (A): Example images of the infarct border zone demonstrating engraftment and differentiation of lentiviral GFP-labelled CDCs 6 weeks after injection. (B): Example images of the central scar demonstrating rounded GFP-labelled CDCs weakly expressing markers of cardiac differentiation six weeks after injection. (C) Examples of unlabeled human CDCs within the infarct (left image) and border zone (right image) 3 weeks after injection. (D) Example of human-derived cardiomyocytes within the infarct border zone expressing Cx43 one week post-transplantation. (E) Example image of beta-galactosidase-labelled human CDCs differentiating into smooth muscle cells 3 weeks after injection. (F) Example image of beta-galactosidase-labelled human CDCs differentiating into endothelial cells 3 weeks after injection.
These results challenge the conclusions of Andersen et al. by demonstrating in vivo cardiomyogenesis of transplanted cells cultured using established cardiosphere methods. Our lab and others have shown that CDCs also decrease infarct size and improve myocardial function(1, 7), with the different sub-populations within CDCs acting synergistically to improve myocardial function.(9) Although cardiac differentiation of transplanted CDCs does occur consistently, long-term persistence of transplanted CDCs is relatively low (10), with important contributions of paracrine effects (both tissue preservation and recruitment of endogenous regeneration) to the functional benefit of CDC transplantation.(11).
In this light, Andersen and colleagues(8) may have overstated the relevance of their findings, considering that their work made use of several unprecedented experimental procedures with novel experimental findings that occurred exclusively when neonatal rat heart was used as a tissue source. These investigators stored the myocardial tissue prior to processing in a novel buffer, potentially damaging the very cells of interest; performed prolonged enzymatic digestion to collect cells from explant culture, possibly favoring the survival of contaminating cells; and finally, failed to characterize their cells in vivo.(12) If the authors seek to convincingly critique the cardiosphere approach, they would be well-advised to reproduce established methods. As such, their work is unrelated to adult cardiosphere culture and, as performed, provides no justification for the indictment of the clinical utility of cardiospheres or CDCs, which are already being tested in human subjects. (13)
Acknowledgments
This study was supported by the NIH (U54 HL081028 and R01 HL083109) and Dr Davis is funded by the Canadian Institutes of Health Research (Clinician Scientist Award).
Footnotes
Author Contribution:
Darryl Davis: Conception and design, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Rachel Smith: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Eduardo Marbán: Conception and design, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
Relationships with industry and financial disclosures: Dr Eduardo Marbán holds equity in a private company (Capricor Inc.) that licenses techniques used to manufacture cardiac stem cells. Dr Rachel Ruckdeschel Smith is employed by Capricor Inc. The remaining co-authors have nothing to disclose.
References
- 1.Smith RR, Barile L, Cho HC, et al. Regenerative Potential of Cardiosphere-Derived Cells Expanded From Percutaneous Endomyocardial Biopsy Specimens. CIRCULATION. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 2.Davis DR, Kizana E, Terrovitis J, et al. Cells cultured directly from heart biopsies express stem cell markers and improve function after myocardial infarction [abstract] CIRC RES. 2008;103(12):1493–1501. [Google Scholar]
- 3.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. PROC NATL ACAD SCI U S A. 2007;104(35):14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang YL, Shen L, Qian K, et al. A novel two-step procedure to expand cardiac Sca-1+ cells clonally. BIOCHEM BIOPHYS RES COMMUN. 2007;359(4):877–883. doi: 10.1016/j.bbrc.2007.05.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott HC, Matthiesen TS, Brechtken J, et al. The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. NAT CLIN PRACT CARDIOVASC MED. 2007;4(Suppl 1S1):S27–S39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]
- 6.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. CIRC RES. 2004;95(9):911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 7.Johnston PV, Sasano T, Mills K, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. CIRCULATION. 2009;120(12):1075–83. 7. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen DC, Andersen P, Schneider M, et al. Murine “Cardiospheres” Are Not a Source of Stem Cells with Cardiomyogenic Potential. STEM CELLS. 2009;27(7):1571–1581. doi: 10.1002/stem.72. [DOI] [PubMed] [Google Scholar]
- 9.Smith RR, Chimenti I, Marban E. Unselected human cardiosphere-derived cells are functionally superior to c-kit- or CD90-purified cardiosphere-derived cells [abstract] CIRCULATION. 2008:118. [Google Scholar]
- 10.Terrovitis J, Lautamäki R, Bonios M, et al. Noninvasive Quantification and Optimization of Acute Cell Retention by In Vivo Positron Emission Tomography After Intramyocardial Cardiac-Derived Stem Cell Delivery. JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY. 2009;54(17):1619–1626. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chimenti I, Smith RR, Li TS, et al. Relative Roles of Direct Regeneration Versus Paracrine Effects of Human Cardiosphere-Derived Cells Transplanted Into Infarcted Mice. CIRCULATION RESEARCH. 2010 doi: 10.1161/CIRCRESAHA.109.210682. CIRCRESAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLOS ONE. 2009;4(9):e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CArdiosphere-Derived aUtologous Stem CElls to Reverse ventricUlar dySfunction (CADUCEUS) 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]