Abstract
We present the nucleotide sequence of the galactokinase gene (galK) of Escherichia coli including its 5' and 3' flanking regions. This DNA sequence derives from the lambda gal8 transducing phage and is identical to the sequence present in the galK gene fusion vectors, pKO and pKG, commonly used to study transcriptional regulatory elements. We define the precise 3' junction between the bacterial and phage sequences in lambda gal8 and demonstrate that this junction probably results from a homologous recombination event between identical 9 bp sequences common to the gal operon and phage lambda. Moreover, we examine the 300 bp region located immediately beyond galK for transcription termination function and find no gal operon terminator. Lastly, we compare the galK genes of E. coli and the yeast S. cerevisiae and find several regions of strong homology among which is a potential ATP-binding site homology shared by a variety of ATP-binding proteins including protein kinases encoded by mammalian oncogenes.
Full text
PDF

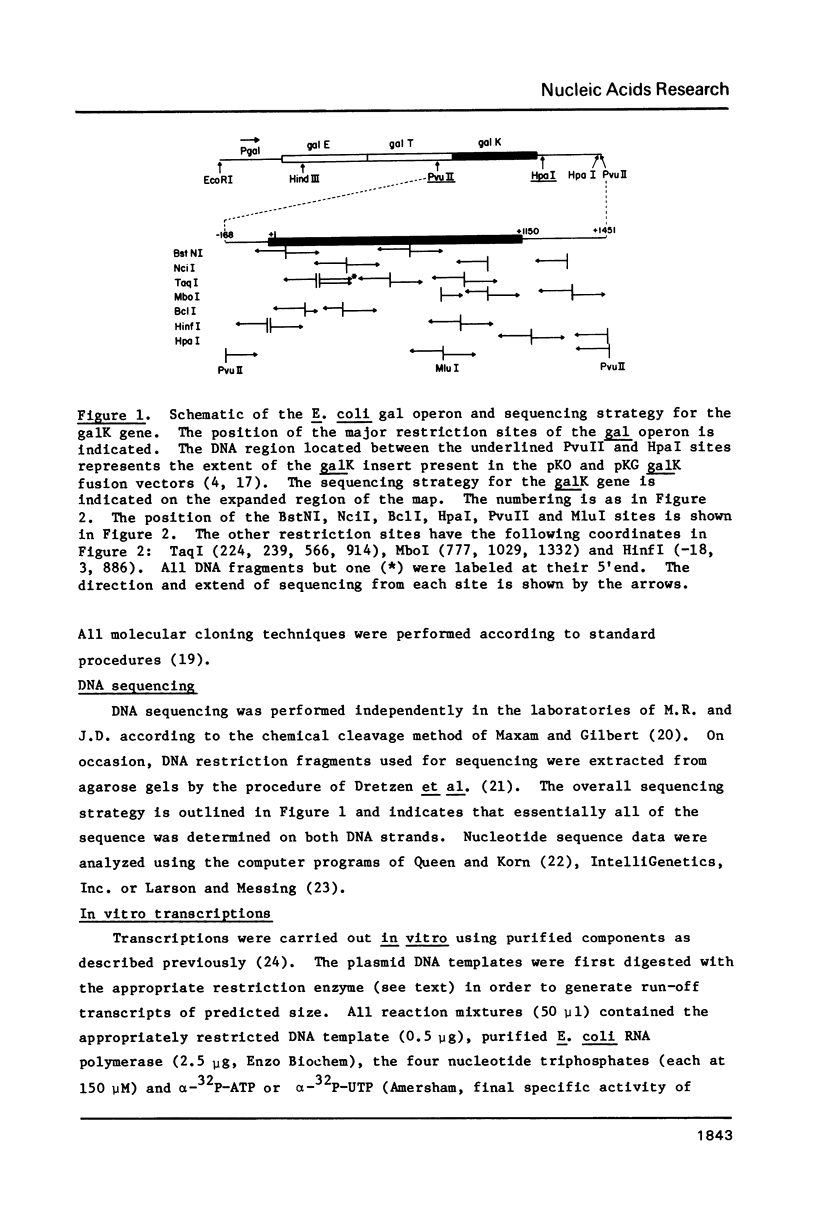










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S. L., Shapiro J. A. The galactose operon of E. coli K-12. I. Structural and pleiotropic mutations of the operon. Genetics. 1969 Jun;62(2):231–247. doi: 10.1093/genetics/62.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Berg P. E., Yu J. K., Popovic Z., Schumperli D., Johansen H., Rosenberg M., Anderson W. F. Differential activation of the mouse beta-globin promoter by enhancers. Mol Cell Biol. 1983 Jul;3(7):1246–1254. doi: 10.1128/mcb.3.7.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel F., Heusterspreute M., Merchez M., Ha Thi V., Pilaete M. F., Davison J. Gene rearrangements leading to the expression of an insertion-inactivated tetracycline resistance gene in pBR322. Plasmid. 1983 Mar;9(2):201–214. doi: 10.1016/0147-619x(83)90021-5. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Sternberg E. J., Gorman J. A., Clark P., Hamer D., Rosenberg M., Crooke S. T. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3332–3336. doi: 10.1073/pnas.81.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron B. A., Donelson J. E. Sequence of the Saccharomyces GAL region and its transcription in vivo. J Bacteriol. 1984 Apr;158(1):269–278. doi: 10.1128/jb.158.1.269-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambly-Chaudière C., Gottesman M., Debouck C., Adhya S. Regulation of the pR operon of bacteriophage lambda. J Mol Appl Genet. 1983;2(1):45–56. [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Feiss M., Adyha S., Court D. L. Isolation of plaque-forming, galactose-transducing strains of phage lambda. Genetics. 1972 Jun;71(2):189–206. doi: 10.1093/genetics/71.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Heusterspreute M., Ha Thi V., Davison J. Expression of galactokinase as a fusion protein in Escherichia coli and Saccharomyces cerevisiae. DNA. 1984 Oct;3(5):377–386. doi: 10.1089/dna.1984.3.377. [DOI] [PubMed] [Google Scholar]
- Johansen H., Schümperli D., Rosenberg M. Affecting gene expression by altering the length and sequence of the 5' leader. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7698–7702. doi: 10.1073/pnas.81.24.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Larson R., Messing J. Apple II software for M13 shotgun DNA sequencing. Nucleic Acids Res. 1982 Jan 11;10(1):39–49. doi: 10.1093/nar/10.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Michaelis G., Starlinger P. Sequential appearance of the galactose enzymes in E. coli. Mol Gen Genet. 1967;100(2):210–215. doi: 10.1007/BF00333607. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Effects of dimethylsulfoxide on the E. coli gal operon and on bacteriophage lambda in vivo. Cell. 1974 Sep;3(1):39–46. doi: 10.1016/0092-8674(74)90035-x. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Ralston R., Bishop J. M. The membrane glycoprotein encoded by the retroviral oncogene v-erb-B is structurally related to tyrosine-specific protein kinases. Proc Natl Acad Sci U S A. 1984 Feb;81(3):704–707. doi: 10.1073/pnas.81.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Chepelinsky A. B., McKenney K. Studying promoters and terminators by gene fusion. Science. 1983 Nov 18;222(4625):734–739. doi: 10.1126/science.6356355. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Rymond B. C., Zitomer R. S., Schümperli D., Rosenberg M. The expression in yeast of the Escherichia coli galK gene on CYC1::galK fusion plasmids. Gene. 1983 Nov;25(2-3):249–262. doi: 10.1016/0378-1119(83)90229-9. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Schell M. A., Wilson D. B. The NH2-terminal sequences of galactokinase from Escherichia coli and Saccharomyces cerevisiae. FEBS Lett. 1977 Nov 1;83(1):45–47. doi: 10.1016/0014-5793(77)80638-8. [DOI] [PubMed] [Google Scholar]
- Schümperli D., Howard B. H., Rosenberg M. Efficient expression of Escherichia coli galactokinase gene in mammalian cells. Proc Natl Acad Sci U S A. 1982 Jan;79(2):257–261. doi: 10.1073/pnas.79.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D., McKenney K., Sobieski D. A., Rosenberg M. Translational coupling at an intercistronic boundary of the Escherichia coli galactose operon. Cell. 1982 Oct;30(3):865–871. doi: 10.1016/0092-8674(82)90291-4. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Taylor W. R. Modelling the ATP-binding site of oncogene products, the epidermal growth factor receptor and related proteins. FEBS Lett. 1984 Oct 1;175(2):387–392. doi: 10.1016/0014-5793(84)80774-7. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Hogness D. S. The enzymes of the galactose operon in Escherichia coli. 3. The size and composition of galactokinase. J Biol Chem. 1969 Apr 25;244(8):2137–2142. [PubMed] [Google Scholar]
- Wu A. M., Christie G. E., Platt T. Tandem termination sites in the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1981 May;78(5):2913–2917. doi: 10.1073/pnas.78.5.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]




