DISEASE OVERVIEW
Urinary incontinence (UI) may be defined as any involuntary or abnormal urine loss. UI is characterized by lower urinary tract symptoms (LUTS), which include both storage and voiding problems. UI can be further defined by the patient’s presentations and symptoms. Urge urinary incontinence (UUI) is defined as involuntary urine leakage associated with urgency. Stress urinary incontinence (SUI) is defined as involuntary urine leakage associated with specific activities (e.g., sneezing and coughing. Mixed urinary incontinence (MUI) includes features of both UUI and SUI.1–3
Overflow incontinence (OFI) is caused by a hypotonic bladder, bladder outlet obstruction, or other forms of urinary retention. OFI may result in LUTS and in the loss of small amounts of urine; it most often occurs in men with benign prostatic hyperplasia (BPH).4
The term overactive bladder (OAB) is often used to describe UI. OAB comprises a constellation of symptoms typically characterized by urgency, with or without UUI, accompanied by frequency and nocturia.1
Epidemiology
Approximately 10 million patients in the U.S. have UI, which is associated with significant morbidity and decreased quality of life. In 2007, it was estimated that more than 25 million people in the U.S. experienced episodes of UI. The prevalence of UI is higher in women than in men 80 years of age or younger, but both men and women are affected almost equally after age 80. UI may be associated with certain comorbidities, including hypertension and depression, although these associations are not fully understood.5,6 Among women, the incidence of UI is highest in Caucasians (7.3/100 person-years), followed by Asians (5.7/100 person-years) and African-Americans (4.8/100 person-years).7
As a result of the social stigma associated with UI or the assumption that UI is a normal part of aging, the prevalence of this disorder may be underestimated because of unreported cases.8 UI is also often undocumented upon hospital discharge; it is a neglected syndrome in nursing facilities; and it is underreported by health care professionals, who may view the condition as a symptom rather than as a medical problem.9,10
UI is primarily associated with aging, affecting up to 30% of elderly people. It occurs in 85% of long-term-care patients and is often the reason for admission to these facilities.11,12 The prevalence of UI in nursing homes remains high, and the care of nursing-home residents with UI is the subject of clinical research.13,14 In addition, UI is one of the measures used by the Centers for Medicare and Medicaid Services (CMS) to assess quality of care.15–17
Annual direct and indirect costs of managing UI in the U.S. is estimated at $25 billion for patients over 65 years of age.18,19 The direct costs of UI include diagnostic procedures and the various treatment options, including pharmacotherapy.20 Indirect costs include complications and disabilities, such as insomnia, falls, depression, caregiving, and nursing-home placement.10,21 The indirect costs of UI are associated with a significant decrease in health-related quality of life, especially in women. Other “costs” of UI are difficult to measure but are significant. These include the consequences of social withdrawal or isolation resulting from the perceived stigma of UI or from the fear of leakage or odor.22–24
Bladder Anatomy and Physiology
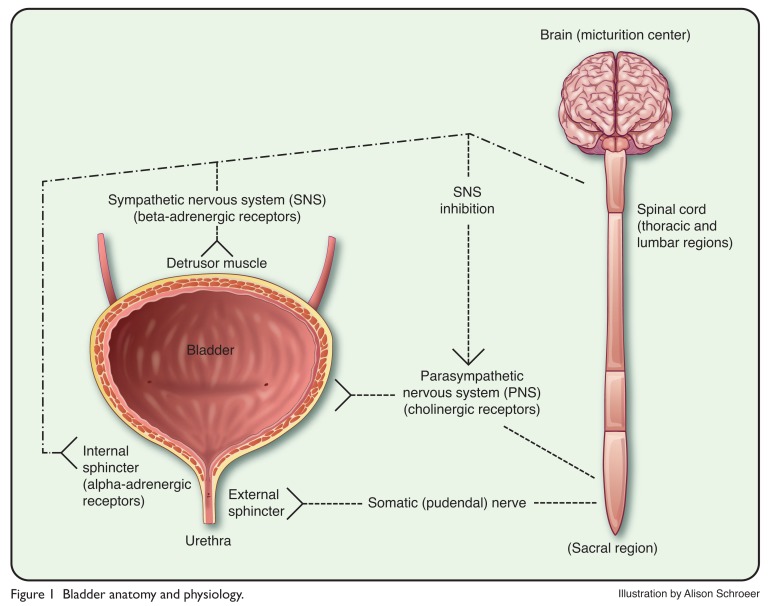
The anatomy and physiology of the bladder are complex, but a basic understanding of these topics is essential in order to appreciate the various types of UI and their management.25,26 Figure 1 illustrates the basic anatomic structures and nervous system “wiring” involved in bladder function, including the detrusor muscle, the internal and external sphincters (bladder neck and proximal urethra, respectively), and their neurological components.
Figure 1.
Bladder anatomy and physiology.
Reduced activation of the sympathetic nervous system (SNS) results in relaxation of the detrusor muscle, closure of the sphincter, and bladder filling. When the volume of urine in the bladder reaches 200 to 400 mL, the sensation of urge to void is relayed via the spinal cord to the brain centers. Voluntary voiding (micturition) involves the parasympathetic nervous system and the voluntary somatic nervous system. Influences from these systems cause contractions of the detrusor muscle and corresponding somatic nervous activity, leading to sphincter relaxation.26–31
Etiology and Risk Factors
Multiple factors, including age-related physiological changes, may result in or contribute to the various syndromes of UI. Both genitourinary and non-genitourinary factors may contribute to incontinence in aging patients. Age-related functional changes in the urinary tract (detrusor overactivity, impaired bladder contractility, decreased pressure in urethra closure, atrophy of urethral areas, and prostatic hypertrophy) may contribute to UI.32 In women, risk factors for these genitourinary changes include multiple or complex vaginal deliveries, high infant birth weight, a history of hysterectomy, and physiological changes related to the transition to postmenopause. Smoking, a high body mass index, and constipation are also associated with an increased risk of UI.33–37
Pathophysiological causes of UI include lesions in higher micturition centers, in the sacral spinal cord, and in other neurological areas as well. UI may also be associated with numerous comorbidities, such as Parkinson’s disease, Alzheimer’s disease, cerebrovascular disease, diabetes, hypertension, obstructive sleep apnea, and normal-pressure hydrocephalus. Functional factors, including mobility and dexterity, along with reaction time and lack of access to a bathroom facility, may also contribute to UI.33–37
Reversible causes of UI, often described by the mnemonic DIAPPERS, include urinary-tract infections (UTIs), stool impaction, and drugs (Table 1).35–44 Incontinence in older adults may or may not be associated with the genitourinary system. Pharmacological causes and contributors should be considered in patients with UI, especially if they are taking multiple medications (Table 2).32,38–44 Primary care providers and specialists should work as a team to manage patients with UI and to evaluate the broad spectrum of factors that may contribute to incontinence in older adults.32,38,40
Table 1.
Reversible Causes of Urinary Incontinence (DIAPPERS)
| D | Delirium |
| I | Infection (urinary tract) |
| A | Atrophic |
| P | Pharmacological |
| P | Psychological |
| E | Endocrine/excess urine output |
| R | Restricted mobility |
| S | Stool impaction |
Table 2.
Medications That Can Cause or Exacerbate Urinary Incontinence
| Classification | Medication | Activity |
|---|---|---|
| Alpha-adrenergic agonists | Nasal decongestants | Urinary retention in men with overflow incontinence related to BPH |
| Alpha-adrenergic antagonists | Prazosin, terazosin, doxazosin, silodosin, alfuzosin | Urethral relaxation; may cause or exacerbate stress incontinence in women |
| Anticholinergic drugs | Antihistamines, tricyclic antidepressants, some antipsychotics | Anticholinergic actions; urinary retention in overflow incontinence or impaction |
| Antineoplastic drugs | Vincristine | Urinary retention |
| Calcium-channel blockers | Dihydropyridines (e.g., nifedipine) | Urinary retention; nocturnal diuresis resulting from fluid retention |
| Diuretics | Furosemide, bumetanide | Polyuria; frequency; urgency |
| Narcotic analgesics | Opiates | Urinary retention; sedation |
| Sedatives/hypnotics | Long-acting benzodiazepines (e.g., diazepam, flurazepam) | Sedation; delirium; immobility |
Diagnosis and Evaluation
Patients with signs and symptoms of UI should undergo a complete medical evaluation to rule out reversible causes of the disorder. Formulating an accurate diagnosis may require the participation of clinicians with specialized training in urology. Clinically, patients with UI present with a variety of symptoms, depending on the type and severity of the condition. Patients with UUI usually experience urgency episodes that result in loss of urine. Women with SUI usually experience small amounts of leakage related to external stimuli, such as coughing or sneezing. Men with OFI secondary to BPH usually experience LUTS, including difficulty initiating a urine stream, the presence of a weak stream, a sense of incomplete emptying, nocturia, and dribbling.1,4,25,38 The importance of a correct diagnosis cannot be overemphasized. A complete review of the patient’s history, including comorbidities, is necessary for the development of an appropriate treatment plan.47,48
Urodynamic studies assist clinicians in determining the precise cause of UI and are an important part of the diagnostic process. Urodynamic assessments include a variety of measures that evaluate urine flow, including flow rate, post-void residual urine, filling cystometry, bladder pressure, and urethral pressure. These assessments provide an extensive description of lower urinary tract function and are helpful in determining the appropriate management strategy or in evaluating treatment failures.1,49–51
Because UI in older adults is associated with a high risk of institutionalization and comorbidities, including depression and UTIs, appropriate assessment of transient UI is essential. Transient UI may have an abrupt onset and may last less than 6 months. Because caregivers and health care professionals may erroneously consider UI an inevitable consequence of aging, failure to identify transient forms of the disorder may result in a permanent diagnosis and poor patient outcomes. Various tools, including bladder diaries and the mnemonic described in Table 1, should be helpful in identifying and treating underlying causes of transient UI.
Initial questions for patients suspected of having UI may include “Have you ever leaked urine?” or “Have you lost bladder control?” Bladder diaries may be used to assess patterns of voiding, frequency, and volume. Questionnaires may also be helpful, although they depend on the patient’s or the caregiver’s memory.45–49
Because only approximately 20% of women with UI seek medical attention, and because there is the misconception that urinary leakage is a normal part of aging, health care practitioners should aim discussions at identifying women who are experiencing UI and need further evaluation.7,41,51,52 Pharmacists should have a thorough understanding of UI and its pharmacotherapeutic management. A comprehensive understanding of UI is necessary to optimize pharmacotherapy and to allow the pharmacist to review the patient’s medical profile for medications that might be causing or exacerbating the disorder.33,34,25,42 Because many patients with UI are older, it is often necessary to make dosage adjustments in their medications. Because of changes in both pharmacokinetics and pharmacodynamics in elderly populations, additional monitoring to avoid drug-related adverse events is required.52
Nonpharmacological Management: Conservative Measures and Exercises
The management of UI should include an evaluation of potential reversible contributors and trials of nonpharmacological interventions, which depend on the type of UI identified. Clinical studies support proper nutrition, the avoidance of constipation, weight loss, and physical activity as beneficial in improving symptoms.53–61 A study of weight loss in overweight women reported a clinically relevant reduction in the frequency of both stress and urge incontinence episodes.58 Women who are able to engage in regular daily exercise of moderate intensity are reported to have a lower incidence of UI than sedentary women, although the ability to exercise may be limited by physical disabilities in elderly women.
Other non-drug interventions for UI include prompted or timed voiding, habit retraining, and praises for appropriate toileting. Success with these interventions requires the patient’s awareness of the need to void and the ability to delay voiding if necessary. These interventions, along with exercise, are associated with modest and short-term improvements in daytime UI. Absorbent products or pads may also be helpful to some patients; the use of these products should be based on the needs of the patient rather than on the convenience of the caregiver or facility staff. The drugs listed in Table 2 are often problematic in these patients and may contribute to or exacerbate UI; thus, evaluation may be necessary.62–68
Pelvic floor (Kegel) muscle training and bladder training have been beneficial in resolving or improving UI.69,70 Kegel exercises involve strengthening and retraining the detrusor bladder muscle to regain some control of urinary function. Evidence supports the use of this behavioral intervention in the treatment of UUI, SUI, and MUI. Choi et al. suggested that these exercises might be most effective in younger women with predominantly stress-related incontinence.71
The training process involved in learning these exercises may be complex for some patients, especially older adults with memory disorders.69–71 Comparisons of various conservative techniques, using a device that monitors compliance and the performance of exercises, showed that pelvic floor exercises, alone or in combination with biofeedback or electrical stimulation, may be beneficial for patients with SUI or MUI.53,54,56,72
The treatment of UI in older adults living in the community is often overlooked, but if the disorder is identified in these individuals, it can be successfully managed with conservative measures. The use of nonpharmacological interventions, including Kegel exercises and bladder retraining, can be effective even in frail older adults, especially with caregiver assistance. Medications may be necessary in some patients, however, and treatment outcomes may be less successful in patients with advanced age and severe UI.73
Pharmacotherapy: Estrogen Replacement
The loss of estrogen during menopause has multiple effects on postmenopausal women, including atrophic tissue changes in the urogenital tract. These physiological changes may result in dryness, burning, itching, dyspareunia, and infections along with additional LUTS, including frequency and urgency.74–77 Hormone therapy (HT) has always been considered a therapeutic option for the management of postmenopausal symptoms. HT offers significant benefits in the management non-urogenital features, such as hot flashes, and may relieve the vaginal dryness associated with menopause. In addition, HT has been used to improve LUTS because of its effect on estrogen receptors in the urogenital area.78,79
During the past decade, the use of exogenous estrogen in post-menopausal women has become controversial because of concerns about increased rates of breast cancer and the risk of vascular disease–related morbidity (e.g., clotting and stroke).80 The role of estrogen in the management of UI is also controversial because data have suggested that HT provides only minimal benefit in UI and may even exacerbate the disorder.81–85 The basis for the assumption that estrogen would be beneficial in UI is the presence of estrogen and progesterone receptors throughout the genital tract, bladder, and vaginal epithelium. The presence of these receptors led investigators to theorize that HT could be a useful treatment for UI, especially stress urinary incontinence (SUI).74–77,85–92
Some clinical trials, however, have not supported the use of oral HT for managing UI.84,93 In a meta-analysis of 28 clinical studies of approximately 3,000 women with UI and in controlled trials of estrogen in more than 700 women with features of UUI and SUI, greater improvement of symptoms was reported for estrogen-treated patients with UUI than for the control groups; however, no beneficial effects were observed among patients with SUI.94
Other controlled studies showed that the use of estrogen alone or in combination with progestin may contribute to or increase the incidence of UI, especially SUI, in postmenopausal women.95–102 The Nurse Health Study reported an increased risk of UI associated with the use of estrogen, with or without progestin therapy, in younger postmenopausal women (37–54 years of age).95 Additional retrospective data from this study suggested an association between the use of oral contraceptives and UI in premenopausal women.96
The Women’s Health Initiative (WHI), a randomized controlled trial involving more than 23,000 postmenopausal women 50 to 79 years of age, reported that HT increased the incidence of UI at 1 year; the highest incidence was in women with SUI. Estrogen alone or taken with progestin increased the risk of UI among continent women and worsened the features of UI among symptomatic women after 1 year.97–100 The Heart Estrogen/Progestin Replacement Study (HERS), a randomized, placebo-controlled, double-blinded trial, evaluated conjugated estrogen plus progestin for the secondary prevention of heart disease in 1,200 women. Estrogen plus progestin increased the risk of UUI and SUI within 4 months after initiation of treatment.101,102
These trials showed that conjugated estrogen alone and in combination with progestin increased the risk of UI and exacerbated existing UI in postmenopausal women. HT, therefore, should not be used for the prevention or treatment of UI. Additional associations between HT and cerebrovascular disease and breast cancer in postmenopausal women should further increase the reluctance to use HT in postmenopausal women with UI.103,104
The role of topical estrogens in the management of UI is unclear; more study is needed to investigate these formulations in UI.78,105 Evidence supports the use of topical or localized estrogen in treating UUI caused by postmenopausal atrophic changes, which result in the loss of urethral support and in symptoms of UI.74,106 Topical estrogen formulations may include creams or estradiol-impregnated vaginal rings. The mechanisms of topical estrogen in this setting may include an increased blood supply and increased mucosal thickness, resulting in improved function of the lower urogenital system. Although these benefits have been reported in elderly women with atrophic changes and concurrent OAB, they have not been reported in women with SUI.107–109
Classification and Treatment
Urinary incontinence is usually classified in the format described in Table 3, although many patients may experience symptoms that suggest a mixed disorder. An overview of the various types of UI is presented in Table 3.3,4,30,31,110–112 The next sections discuss urge UI, stress UI, overflow incontinence, and mixed UI.
Table 3.
Causes, Symptoms, and Treatment of Urinary Incontinence
| Type of Incontinence | Common Causes | Common Symptoms | Treatment Options |
|---|---|---|---|
| Urge urinary incontinence (UUI) | |||
| Idiopathic detrusor overactivity | Urinary tract infections | Urgency and frequency, day or night |
|
| Neurogenic detrusor overactivity |
|
||
| Stress urinary incontinence (SUI) | |||
| Stress incontinence (outlet incompetence) |
|
Small volumes of urine loss with coughing or sneezing |
|
| Mixed urinary incontinence (MUI) | |||
| Mixed UUI and SUI |
|
Symptoms may include urge and stress features | Treatment depends on predominant symptoms |
| Overflow incontinence (OFI) | |||
| Overflow incontinence |
|
Poor stream, incomplete emptying, and dribbling |
|
| Other types of incontinence | |||
| Post-prostatectomy incontinence | Disruption or denervation of pelvic floor muscle fibers | Stress incontinence and dribbling |
|
| Fistula (e.g., colovesical or vesicovaginal) |
|
Continuous, steady incontinence | Surgical repair |
| Functional incontinence |
|
Symptoms vary | Eliminate causes |
URGE URINARY INCONTINENCE
Urge (urgency) urinary incontinence (UUI) is a common cause of incontinence in elderly people. It is characterized by urgency, followed by involuntary loss of urine. UUI is sometimes referred to as OAB. However, the terms are not interchangeable, because about two-thirds of patients with OAB do not have UI.1,31
UUI occurs primarily as a result of detrusor muscle overactivity, resulting in uninhibited or involuntary muscle contractions.26–28 Patients with UUI describe a sudden desire to urinate that is difficult to defer, resulting in leakage of urine. These episodes may occur at various times during the day or night.31,114 The primary causes of UUI (see Table 3) include idiopathic detrusor overactivity (resulting from UTIs) and neurogenic detrusor overactivity (resulting from stroke, trauma, neurological diseases, or medications).25–28,43–46,112 The severity of age-related volumetric changes in the brain’s white matter may be associated with urinary urgency, and this process may have implications for future UI therapies.115,116
Nonpharmacological Management
The nonpharmacological management of UUI includes bladder training, behavioral treatments; pelvic floor exercises; the avoidance of caffeine; the use of pads for temporary bladder support; and, in some cases, surgery.117,118 Behavioral therapy in combination with drug therapy has produced variable results. Behavioral interventions, including educational brochures with verbal reinforcement, were beneficial in UUI patients who were dissatisfied with anticholinergic drug therapy.118 Behavioral training, including Kegel exercises and urge-suppression techniques, was found to be ineffective in improving outcomes in women with UUI.53,54,119
Pharmacotherapy
Anticholinergic (Antimuscarinic) Agents
The current focus of pharmacotherapy for UUI is control of detrusor muscle overactivity through the inhibition of M2 and M3 muscarinic (acetylcholine) receptors on the bladder.120–122 Numerous drugs that act as acetylcholine antagonists (anticholinergic agents) are available for the treatment of UUI and can reduce symptoms of urgency and improve bladder control. Because muscarinic receptors are located in other organ systems throughout the body, their inhibition can have a variety of physiological and adverse effects.
The five most commonly used types of muscarinic receptors, their anatomic locations, and the adverse effects that can result from their inhibition are presented in Table 4. Table 5 lists the available antimuscarinic (anticholinergic) agents used to treat UUI. Each of these agents is discussed on the following pages.123–126
Table 4.
Muscarinic Receptor Subtypes and Adverse Effects of Receptor Inhibition
| Organ System | Receptor Subtype | Adverse Effects of Inhibition (Anticholinergic Effects) |
|---|---|---|
| Bladder (detrusor muscle) | M2, M3 | Decreased contractions; urinary retention |
| Cardiac tissue | M2 | Tachycardia; palpitations |
| Central nervous system and brain (cortex and hippocampus) | M1, M2, M3, M4, M5 | Effects on memory, cognition, and psychomotor speed; confusion; delirium; sedation; hallucinations; sleep disruption |
| Eyes (ciliary muscle and iris) | M3, M5 | Dry eyes; blurred vision; mydriasis (dilation of the pupil) |
| Gastrointestinal tract | M1, M2, M3 | Slowed transit time; constipation; effects on sphincter tone and gastric acid secretion |
| Salivary glands | M1, M3, M4 | Xerostomia (dry mouth) |
Table 5.
Anticholinergic Agents Used for Urinary Incontinence
| Drug | Recommended Adult Dose | Pharmacokinetic Properties |
|---|---|---|
| Darifenacin (Enablex ER, Novartis) | 7.5–15 mg q.d.; swallowed whole with liquid; should not be chewed, divided, or crushed |
|
| Fesoterodine (Toviaz ER, Pfizer) | 4–8 mg q.d.; swallowed whole with liquid; should not be chewed, divided, or crushed |
|
| Oxybutynin IR (Ditropan, Janssen) | 2.5–5 mg b.i.d. or t.i.d.; maximum dosage, 5 mg q.i.d. |
|
| Oxybutynin gel 10% (Gelnique, Watson) Oxybutynin gel 3% (Anturol, Antares) |
1 g (sachet) applied daily to dry, intact skin; rotate application sites (abdomen, thigh, shoulder, upper arm) Three pumps (84 mg); applied as above; may rotate site if necessary |
|
| Oxybutynin transdermal patch (Oxytrol, Watson) | 36-mg patch applied twice weekly (every 3–4 days); delivers 3.9 mg daily; rotate administration sites (abdomen, hip, buttock) |
|
| Oxybutynin XR (Ditropan XL, Janssen) | 5–10 mg q.d.; may be increased to a maximum of 30 mg/day; swallowed whole; should not be chewed, divided, or crushed |
|
| Solifenacin (vesicare,Astellas Pharma US) | 5–10 mg q.d.; swallowed whole with water |
|
| Tolterodine IR (Detrol, Pfizer) | 1–2 mg b.i.d. |
|
| Tolterodine (Detrol LA, Pfizer) | 2–4 mg q.d.; swallowed whole with liquid |
|
| Trospium IR (Sanctura, Allergan) | 20 mg b.i.d., at least 1 hour before meals or on empty stomach |
|
| Trospium (Sanctura XR, Allergan) | 60 mg q.d. in morning, at least 1 hour before breakfast, with water or on empty stomach |
|
b.i.d. = twice daily; CrCl = creatinine clearance; CYP = cytochrome P450; GI = gastrointestinal; IR = immediate release; IV = intravenous; q.d. = once daily; q.i.d. = four times daily; t.i.d. = three times daily; XR = extended release.
CYP3A4 metabolism: use lower dose if patient is taking concurrent CYP3A4 inhibitor (e.g., clarithromycin, ketoconazole).
Antimuscarinic side effects are associated with both central and peripheral adverse reactions (see Table 4). Central adverse effects include delirium, confusion, and exacerbation of existing memory loss; these effects are especially concerning in elderly patients. Peripheral adverse effects include constipation, dry eye, and urinary retention.25,120,127,128
Contraindications to the use of anticholinergic agents include uncontrolled narrow-angle glaucoma, a risk of urinary or gastric retention, the presence of underlying delirium or dementia, and a hypersensitivity to these drugs. Cautious use of anticholinergic drugs is recommended in patients with myasthenia gravis and with some gastrointestinal (GI) disorders, such as ulcerative colitis, intestinal atony, and gastroesophageal reflux disease.129–134
Gopal et al. reported high discontinuation rates for anticholinergic drugs that were used to treat LUTS in women.135 The study authors estimated overall and drug-specific discontinuation rates for nine agents in approximately 30,000 women over a 6-month period. Discontinuation rates were high for all anticholinergic drugs regardless of class. The overall discontinuation rate was 60%; oxybutynin (Ditropan, Janssen) and extended-release (ER) tolterodine (Detrol LA, Pfizer) were discontinued at rates of 71% and 54%, respectively. Some limitations of the study included diagnoses based on electronic medical data and a lack of data about why patients stopped therapy. The results suggest a need for more effective and tolerable therapies for UUI, including more vigilant use of nonpharmacological interventions, such as fluid modification, pelvic floor rehabilitation, and bladder training.135
Anticholinergics have the potential to interact with other medications that have the same side-effect profile and with other centrally acting drugs.120 The concomitant use of acetylcholinesterase inhibitors for dementia and anticholinergic drugs may exacerbate cognitive decline and should be avoided if possible.136
As shown in Table 5, all of the anticholinergic agents used to treat UI, except trospium chloride (Sanctura, Allergan/Esprit/ Indevus), are metabolized by hepatic cytochrome P450 (CYP) enzymes; inhibitors of these enzymes, therefore, may potentiate the adverse effect of anticholinergic drugs. Clinicians should monitor patients with UUI, especially older adults and those taking multiple medications, for adverse effects, drug interactions, and potential contraindications during treatment with anticholinergics.25,120,137
Older drugs, such as propantheline (Pro-Banthine, Shire), dicyclomine (Bentyl, Axcan Pharma), and flavoxate (Urispas, Ortho-McNeil), are still available, but they are rarely used because of their questionable efficacy and side-effect profiles. The tricyclic antidepressant imipramine (Tofranil, Mallinckrodt) has been used to treat patients with UUI and may have a role in MUI because of its dual anticholinergic and alpha-adrenergic properties.120, 128,138–141
Currently, the anticholinergic drugs most commonly used in clinical practice for the treatment of UUI include transdermal oxybutynin (Oxytrol, Watson Pharma), oxybutynin gel (Gelnique, Watson Pharma), tolterodine (Detrol and Detrol LA, Pfizer), trospium chloride, darifenacin (Enablex, Novartis), solifenacin (vesicare, Astellas/GlaxoSmithKline), and ER fesoterodine (Toviaz, Pfizer) (see Table 5).138–141
As mentioned, several anticholinergic agents are available in various doses, formulations, and routes of administration, providing clinicians with several treatment options for with UUI.128,132,137,138,141 These drugs are usually used to treat UUI and OAB in patients who have not achieved symptom relief and improved quality of life with conservative nonpharmacological interventions.
Clinical Efficacy
Efficacy data for anticholinergic drugs in patients with UUI have been obtained from a number of meta-analyses and head-to-head trials. Two large meta-analyses reported similar clinical efficacy among the available anticholinergic agents, as measured by reductions in episodes of urgency and incontinence, frequency, daily micturition, nocturnal awakenings, increased volume per void, patient satisfaction, and quality of life.141,142 Another meta-analysis included data from 50 randomized controlled trials and three pooled analyses that included various formulations and doses of anticholinergic agents. This study reported advantages with ER formulations in terms of efficacy and safety. Dose escalations with immediate-release (IR) formulations provided some improvement in efficacy but with an increased risk of adverse events.143
Head-to-head trials with anticholinergic agents have reported similar efficacy or insignificant differences among the various drugs. Tolerability differences were evident in some studies, especially when other drugs were compared with IR oxybutynin. One report described the available anticholinergic agents as equivalent first choices, except for oral oxybutynin administered at dosages of more than 10 mg/day, which were associated with a higher rate of adverse effects.144 The study data showed a smaller treatment effect with anticholinergics compared with placebo than what might be expected in clinical practice. This difference might have been due to the use of concurrent bladder training in some patients who were prescribed these drugs in the clinical setting, compared with the absence of this intervention in clinical trials. The literature is devoid of direct comparisons between anticholinergic drugs and bladder-training interventions.53,54,142–147
Oxybutynin (Ditropan, Oxytrol). Oxybutynin is the oldest of the agents currently used to treat UUI. It is available in IR and ER oral formulations (Ditropan and Ditropan XL, Janssen), along with a dermal patch and topical gel formulations (see Table 5). Oxybutynin is considered the gold standard with which other agents in the class are compared. Trial data indicate that the efficacy of oxybutynin is similar to that of other anticholinergic drugs. The significance of its proposed muscle-relaxant properties is unclear.148 The ER tablet, dermal patch, and topical gel may offer improved tolerability because of reduced levels of the active metabolite, N-desethyloxybutynin.149–153
In December 2011, the FDA approved oxybutynin topical gel 3% (Anturol, Watson/Antares) for the treatment of OAB in patients with symptoms of UUI, urgency, and frequency.154
Adverse events associated with oral oxybutynin include the dose-related anticholinergic effects described previously, along with erythema and pruritus resulting from the transdermal and gel formulations. The incidence of dry mouth is reported to be as high as 50% to 70% with the IR formulation, secondary to the creation of N-desethyl-oxybutynin during the drug’s extensive first-pass metabolism. In addition, oxybutynin may have a higher affinity for muscarinic receptors in the parotid (salivary) glands.148–153
The IR formulation of oxybutynin is also associated with orthostatic hypotension as a result of the drug’s alphaadrenergic–blocking properties, as well as sedation resulting from its histamine-blocking effects. The IR and ER formulations have similar efficacy, but the ER formulation allows the release of a controlled amount of drug in the GI tract over 24 hours. In addition, reduced first-pass metabolism results in greater parent-to-metabolite ratios, lower peaks, and fewer concentration-dependent side effects.
The oxybutynin transdermal patch was reported to be effective in treating UUI, with a more tolerable side-effect profile than that of the other formulations, although application-site reactions, including pruritus and erythema, were more common. A large multicenter trial with the transdermal formulation reported improved quality of life and a low incidence of adverse events in 2,878 patients 65 years of age and older; 131 patients older than 85 years of age were treated with this formulation.155–158
Drug interactions include the expected additive side effects when oxybutynin is used with other anticholinergic agents. In addition, concomitant use with CYP2D6 and CYP3A4 pathway inhibitors (e.g., fluconazole or erythromycin) may potentiate oxybutynin-related adverse effects.148 The patient’s medications should be reviewed when oxybutynin is prescribed with other agents because of potential CYP450 drug interactions and additive anticholinergic effects.120,148,153
The 10% gel formulation of oxybutynin was approved in 2009. The gel’s clinical efficacy was reported to be similar to that of the other formulations, but it showed excellent patient tolerability. Significant side effects, when compared with placebo, included dry mouth and application-site reactions. Practical tips for using the oxybutynin gel include showering 1 hour after application and using sunscreen 30 minutes before or after application. The transfer of gel between individuals may occur if vigorous skin contact is made at the application site. Patients should avoid an open fire or exposure to smoking after application until the gel has dried.159–161
The use of oxybutynin in older adults should be limited to short-term treatment with the extended-release formulation, the dermal patch, or the topical gel.148
Tolterodine (Detrol). Like oxybutynin, tolterodine is available in both IR and ER oral formulations (Detrol and Detrol LA, Pfizer) (see Table 5). Tolterodine offers improved tolerability compared with that of IR oxybutynin chloride, and it provides efficacy and tolerability similar to that of the other available agents.153,162 The bioavailability, time to peak serum levels, and elimination of tolterodine depend on the CYP2D6 metabolism phenotype.
In individuals who are extensive CYP2D6 metabolizers, the active metabolite 5-hydroxymethyltolterodine is formed, resulting in a faster onset of peak concentrations. In poor metabolizers (7% of Caucasians), who are devoid of the CYP2D6 enzyme, tolterodine is metabolized to N-dealkylated tolterodine via CYP3A4, resulting in higher serum concentrations of parent tolterodine. Poor metabolizers also experience a slower onset to peak concentrations (2 and 4 hours for the IR and ER formulations, respectively).
The elimination half-life of tolterodine also depends on the metabolism phenotype and on the drug’s formulation. The IR capsules have half-lives of 3 and 9 hours in extensive and poor metabolizers, respectively. The corresponding half-lives of the ER capsules are 7 and 18 hours. Drug interactions with tolterodine are similar to those reported with oxybutynin chloride.163–166
For patients who cannot tolerate IR tolterodine, the ER product is an effective alternative and may offer improved tolerability. In one study, diary entries showed that ER tolterodine resulted in a high degree of satisfaction and improved bladder variables among patients who were previously dissatisfied with the IR formulation or other anticholinergic agents.167–170
Second-Generation Anticholinergic Agents
The search for UI drugs with improved tolerability led to the approval of three new anticholinergic agents in 2004 and one in 2009. Although these drugs appear to offer no significant advantages over oxybutynin chloride and tolterodine in terms of efficacy, they may have some individual advantages in terms of pharmacokinetic profile, delivery, and tolerability.171–180
Trospium chloride (Sanctura). One second-generation anticholinergic drug approved in 2004 was trospium chloride (see Table 5). This quaternary, amine-structured molecule has a limited ability to penetrate the blood–brain barrier because of its hydrophilic nature.181–183 Its structure suggests a reduced potential for anticholinergic CNS side effects, but the tradeoff is poor bioavailability, especially when the drug is administered with food.
The metabolism of trospium chloride is minimal. Renal elimination is via tubular secretion, for which dose adjustments are required in patients with a creatinine clearance (CrCl) below 30 mL/minute. The drug’s efficacy is similar to that of other drugs in its class, but it may be better tolerated in some patients.173,176,184–187 Potential drug interactions are limited to agents that compete for tubular secretion (metformin and digoxin) and to drugs with additive anticholinergic side-effect profiles. Contraindications are similar to those of the anticholinergics discussed earlier.173,188,189
An ER formulation of trospium chloride allows once-daily dosing (see Table 5). This ER product was reported to be convenient, effective, and well tolerated, and it may be an excellent alternative for elderly patients.190–193
Solifenacin (vesicare). Solifenacin, taken once daily, is a second-generation anticholinergic that was approved in 2004 (see Table 5). Its bioavailability is better than that of trospium chloride. Solifenacin is metabolized primarily by CYP3A4, It is renally eliminated; therefore, dosage adjustments are required in patients with a CrCl of less than 30 mL/minute.172,194,195 Solifenacin has been reported to be effective and well tolerated, with efficacy similar to that of others in the class. In clinical trials, solifenacin reduced urgency episodes, incontinence, frequency of micturition, and nocturia. The drug’s benefits were observed within 3 days after administration.196–205 In one study, fewer micturitions were reported with solifenacin than with tolterodine during a 24-hour period.174
Solifenacin may offer improved tolerability compared with IR oxybutynin, especially a lower incidence of severe dry mouth. Clinical data suggest that the overall efficacy of solifenacin is similar to that of other anticholinergics, but one trial reported improved urgency and diary-documented symptoms in patients previously treated with tolterodine.206–209
Adverse effects of solifenacin and contraindications to its use are similar to those of the other anticholinergic drugs, although prolonged corrected QT intervals have been reported with high-dose solifenacin, suggesting that this agent should be used with caution in at-risk patients. As with oxybutynin chloride and other agents in this class (see Table 5), the metabolism of solifenacin involves the hepatic CYP450 enzyme system; patients therefore require appropriate monitoring to avoid drug interactions.17,210,211
Darifenacin (Enablex). Darifenacin (Enablex, Novartis) was approved in 2004, providing another daily option for treating UUI (see Table 5). The drug’s pharmacokinetic properties include poor bioavailability and CYP2D6-dependent metabolism. Approximately 7% of Caucasian patients and 2% of African-American patients ae poor CYP2D6 metabolizers and are dependent on the CYP3A4 isoenzyme for metabolism. Dosage adjustments are recommended in patients with hepatic impairment, and caution is suggested in patients with renal disease.171,212
Darifenacin has a greater affinity for bladder M3 receptors, suggesting increased selectivity and tolerability, although clinical evidence of this advantage is lacking.212–214 The adverse effects and contraindications associated with darifenacin are similar to those of the other anticholinergic drugs.171
Clinical trials with darifenacin reported efficacy similar to that of other agents in the class, but tolerability was better than that of oxybutynin chloride. Darifenacin provided improvements in micturition variables that were similar to those of other anticholinergics, including nocturnal voids, incontinence episodes, and improved quality of life.177,214–220 A community-based survey found that patients with OAB experienced benefits with darifenacin and were generally satisfied with the drug.221
Fesoterodine (Toviaz). Pfizer’s extended-release fesoterodine entered the market in 2009 for the treatment of UUI and OAB (see Table 5). Fesoterodine is well absorbed, is not affected by food, and is metabolized by both the CYP2D6 and CYP3A4 enzyme systems. It is a prodrug, with no activity itself, but it is rapidly and completely metabolized to its active metabolite, 5-hydroxymethyl-tolterodine (5-HMT), which is responsible for all of fesoterodine’s anticholinergic effects. 5-HMT is also the active metabolite of tolterodine. With festerodine, however, the metabolite is the single active moiety; thus, fesoterodine delivers more 5-HMT via esterase metabolism compared with tolterodine.222–226
Excretion is primarily via the kidneys, with approximately 70% excreted as active and inactive metabolites.173,222 As with other agents in this class, dose-related increases in anticholinergic adverse events, including dry mouth and constipation, were reported in clinical trials of fesoterodine. However, discontinuation rates associated with these side effects were minimal.224–226
Fesoterodine has the potential to interact with inhibitors of the CYP2D6 and CYP3A4 enzyme systems. When fesoterodine is used concurrently with inhibitors of these enzymes, the dosage of fesoterodine should not exceed 4 mg daily. However, coadministration of fesoterodine with CYP3A4 inducers may result in subtherapeutic levels. Dosage adjustments may be necessary in patients with severe hepatic impairment (Child–Pugh class C) and in those with severe renal impairment (CrCl below 30 mL/minute). The recommended dosage in these patients is 4 mg daily.173,223,226
The clinical efficacy of fesoterodine in managing UUI is similar to that of other agents in the class. Clinical trials with doses of 4 mg and 8 mg reported significant reductions in daytime frequency, nocturia, urgency, and quality of life in addition to excellent tolerability.227–234 In a comparison study with extended-release tolterodine (4 mg) and placebo, fesoterodine (8 mg) provided greater decreases in incontinent episodes, volume per void, severity of urgency, and continent days per week. The drug also had a greater effect on quality-of-life measures while offering options of dosing flexibility and titration.235,236 In one trial, patients who had been dissatisfied with previous tolterodine treatment reported excellent tolerability and satisfaction with fesoterodine.237
Role of Anticholinergic Therapy
Anticholinergic drugs have a role in the management of UUI, but nonpharmacological interventions should generally be considered first. Although none of the six currently available anticholinergic agents appears to have a clear advantage in terms of efficacy, dosing convenience and drug tolerability may influence the choice of therapy. ER formulations offer daily dosing, improved compliance, and improved tolerability profiles, especially when compared with dose escalation of IR products. Trospium chloride, with its quaternary amine structure and reduced penetration of the blood–brain barrier, may be an option for patients who experience excessive CNS side effects from other drugs in the class.
Topical formulations, such as the oxybutynin transdermal patch and topical gels, may offer more tolerable and convenient dosing for some patients with UUI. Pharmacokinetic differences among the various agents may be relevant in patients with renal or hepatic impairment or when drug interactions are a concern. Pharmacogenomic metabolic profiles may also play a role in drug selection in some patients, based on their CYP450 metabolism genotype.
Finally, financial considerations may influence the selection of an anticholinergic agent because of the significant cost differences among these drugs (i.e., generic vs. brand). A cost analysis reported greater clinical benefits and improved quality of life with the newer agents, such as solifenacin, but these drugs were not cost-effective compared with IR oxybutynin for measures of frequency and incontinence.238
The initial dosage of anticholinergics should be low, especially in older adults. The dose may be titrated, if necessary, with careful monitoring for adverse effects and drug interactions. An adequate trial of 1 to 2 months is recommended before clinicians consider alternative agents or therapies.
Patient education should focus on adequate water and fiber consumption and regular exercise to minimize constipation. Dry mouth can be reduced with the use of sugar-free candies or saliva substitutes. Excessive alcohol consumption should be avoided because of the potential for additive sedative effects.141–143,146,147
Other Therapies for Urge Urinary Incontinence
Botulinum toxin. Botulinum toxin A (BTX-A), a powerful neurotoxin produced by the bacterium Clostridium botulinum, has been studied as therapy for idiopathic detrusor overactivity in a variety of patients, including those who did not respond to anti-cholinergic drugs. BTX-A prevents the release of acetylcholine at the neuromuscular junction. This effect, in turn, inhibits depolarization of the detrusor muscle, resulting in chemical denervation of the bladder. BTX-A is administered via a cystoscopic technique that is reported to be safe and well tolerated. The toxin is injected directly into the detrusor muscle. In clinical trials, the duration of response was typically 3 to 6 months. Intravesical injections of BTX-A in patients with OAB resulted in increased bladder capacity, increased bladder compliance, and improved quality of life.239,240
A study of onabotulinumtoxinA (Botox, Allergan, Inc.) in patients with idiopathic UUI or OAB indicated that doses ranging from 100 U to 150 U were effective in managing the disorder. Adverse effects included UTIs and urinary retention.240
Although clinical trials with BTX-A have not been robust, they suggest that this agent may offer potential benefits for patients with UUI. Further research is necessary to substantiate the usefulness of BTX-A in this population.
Sacral nerve stimulation. Sacral nerve stimulation has been used as a second-line therapy for incontinence secondary to OAB since the 1980s. Several reports have demonstrated the efficacy of this treatment in UUI. The underlying principle of neuromodulation for detrusor overactivity is the induction of somatic afferent inhibition of sensory processing in the spinal cord.241
Investigational agents. Agents being evaluated for the management of UUI include imidafenacin (Ono/Kyorin), an antimuscarinic agent from Japan, and neurokinin-1 receptor antagonists.242,243 The latter agents have been useful in treating OAB but offer no advantages in efficacy compared with tolterodine.
Mirabegron (Astellas), a once-daily, oral selective beta3-adrenoceptor agonist, has been shown to reduce episodes of UI and micturition frequency in patients with OAB.244 Astellas submitted a New Drug Application for mirabegron to the FDA in August 2011. In July 2011, mirabegron was granted marketing approval in Japan. Researchers continue to develop agents that are effective and better tolerated for the treatment of UUI, especially in more sensitive older adults.242–244
STRESS URINARY INCONTINENCE
Stress urinary incontinence (SUI), the most common type of UI in elderly women, is most prevalent in Caucasians. SUI is primarily a disorder of urethral hypermobility or intrinsic sphincter deficiency. Risk factors for SUI include anatomic changes related to aging, pelvic and gynecological surgery, multiple births, medications, obesity, and neurological disorders.43,59,93,245–247 In addition to the high economic costs of treatment, the social costs and the detrimental effects of SUI on elderly women are also significant.20,248,249 Patients with SUI describe involuntary loss of urine triggered by coughing, sneezing, or rising quickly. Patients with “pure” SUI may lack urgency and nocturia, although many of them may have mixed forms, with features of UUI.246,247,250–252
Although SUI is usually considered a female disorder, it can occur in men after prostate surgery. Post-prostatectomy SUI (PPSUI) occurs in over 90% of men during the postoperative period. PPSUI is usually self-limiting and improves within 12 months with proper education and a consistent Kegel exercise regimen.253,254 Reassurance and encouragement are important parts of management. If symptoms of PPSUI last beyond 1 year postoperatively, surgical options should be considered. Surgical approaches include placing an implantable, artificial urinary sphincter (the gold standard) and urethral sling procedures. Only 5% to 10% of PPSUI patients require a continence procedure.255
Nonpharmacological Management
The treatment of SUI is primarily nonpharmacological in nature and includes prevention strategies, the use of temporary absorbent bladder-protection pads for social situations, behavioral interventions, and Kegel (pelvic floor) exercises.108,256–263 A retrospective analysis of women discharged from a large academic medical center reported that SUI inpatient procedures in this population have increased significantly over the last 25 years, with the number of inpatient procedures rising from approximately 50,000 in 1979 to 100,000 in 2004. Most of the women were older than 52 years of age. There was also a decrease in the use of some types of procedures, such as retropubic urethral suspensions, and an increase in the use of others (pubovaginal and transobturator sling procedures).264
Numerous procedures are available for men and women with SUI and are necessary in a high percentage of patients. Surgery is typically used to improve urethral resistance, thereby reducing urine leakage and preserving normal bladder function.108 Surgery is usually recommended when conservative measures have failed in postmenopausal women; however, an operation may increase the risk of postoperative voiding complications. A comprehensive evaluation is necessary in patients with apparent SUI to clarify the specific type of UI being treated and to consider comorbidities, age, childbearing preferences, and urodynamics.265–267
Women with SUI are often treated with sling procedures, which are designed to correct sphincter deficiencies and urethral hyper-mobility. Tissue or various materials are placed below the urethra to elevate it and to increase urethral compression. A commonly used, minimally invasive procedure, the mid-urethral sling, uses tension-free vaginal tape to provide urethral support and to decrease urethral hypermobility by compressing the urethra when intra-abdominal pressure increases.268
Other urethral suspension techniques include suprapubic arc, transobturator, and colposuspension (Burch) procedures. These approaches are reported to be effective for the treatment of SUI, with similar complication rates.266,269,270
Surgery is also associated with improved quality of life and is an excellent option for some patients.108,271,272
Conservative (Nonsurgical) Management
Conservative management should be considered as a first-line option in patients with SUI, especially younger women of childbearing age. Clinical evidence suggests that postpartum SUI may be self-limiting and may resolve on its own. Treatment should be reserved for women whose symptoms continue for 6 months or longer.
The risk of SUI increases with multiple pregnancies, and the benefits of surgical continence procedures may be negated by future pregnancies and childbirth. Although tampons and absorbent bladder-protection pads are usually inadequate or inappropriate for most situations, many women with SUI use these items as a temporary first-line treatment to decrease leakage in situations when abdominal pressure may increase (during exercise or physical activity). Nonsurgical methods include the use of post-partum pelvic floor exercises, weight loss, biofeedback, weighted vaginal cones, electrical stimulation units, and pessaries.273–277
Kegel exercises help to rehabilitate the muscles of the pelvic floor. These muscles consist of slow-twitch (70%) and fast-twitch (30%) fibers. During urination, these muscles, especially the fast-twitch type, are used to close the urethra.108 The exercises involve the conscious contraction and relaxation of the pubococcygeus muscle, with the goal of increasing the resting tension of the sphincter components in this region.277–279 Motivated patients who follow a rigid exercise regimen for up to 3 months typically experience beneficial effects.278 The best results are achieved with the use of verbal instructions, along with supervision by trained clinical professionals.279,280
Lifestyle changes include smoking cessation, fluid restriction (1.5 to 2.0 L daily), and reduced daily caffeine and alcohol intake. Patients’ awareness of their continence status should always be taken into consideration in the evaluation process.273,274,276
To achieve the maximum benefits from conservative management of SUI, proper education is necessary along with an emphasis on the importance of the patient’s role in therapy. Support and encouragement are vital because some interventions may take time to produce results. Patients need to understand the importance of working with their practitioners and of having an active role to achieve positive outcomes.108,274
Biofeedback
Biofeedback, in combination with pelvic floor exercises, offers a cost-effective method of reducing SUI. Vaginal or rectal sensors are used to obtain a visual indication of contraction activity and muscle strength. The purpose of biofeedback is to guide women regarding which muscles to contract to maximize the benefits of pelvic floor exercises.281–284
Intravaginal Devices
Weighted cone devices attached to vaginal muscles may also help women with SUI. These devices are designed to help patients improve pelvic-floor tone through active, continuous muscle contractions. The weight of the cone retained by the patient is in direct proportion to the improvement in muscle tone and to subsequent improvement in SUI.273
A similar device, the Colpexin Sphere, is placed in the vaginal canal to provide support for pelvic floor muscles. This device improves prolapse defects and the utility of pelvic floor exercises. Proper counseling and training are necessary, and small trials have reported success in motivated patients.275
Pessaries
Vaginal continence pessaries are used for the treatment of various pelvic floor disorders, including UI and prolapse.285,286 Although these devices may be considered first-line options for the treatment of SUI, they are not used extensively in this setting because of their perceived inconvenience. Specific types of pessaries have effectively treated SUI by providing support for the bladder neck at the urogenital angle. One short-term trial reported greater patient satisfaction and less bothersome incontinence symptoms with behavioral therapy compared with the use of pessaries at 3 months, but these differences were not sustained at 12 months; further, combination therapy was not superior to behavioral therapy alone or the use of pessaries alone.287 Pessaries may have a role as a temporary management strategy before surgery or when surgery is contraindicated, or they may be useful in women with SUI who are planning to become pregnant.286
To obtain the maximum benefit from pessaries, patients must be instructed in their appropriate use by trained practitioners. Complications associated with the use of pessaries include vaginal discharge, odor, pelvic pain, and bleeding. Other problems may include the failure to retain the pessary or vaginal prolapse, which may be more common in women who have undergone a hysterectomy. Pessaries are a conservative, safe, and effective method for managing SUI in women and may be considered an alternative to surgery in some patients.286
Electrical Stimulation Units
A rectal or vaginal probe is used to apply electrical stimulation to the pelvic floor, with the aim of inhibiting the micturition reflex and improving contraction of the pelvic floor musculature.108,273,278 These units may provide a less invasive alternative to surgery in patients with SUI. However, the devices are time-consuming to use. Kegel exercises may be equally effective and less expensive.273,284
Other Conservative Approaches
Other minimally invasive options for managing women with less severe symptoms of SUI include the injection of transurethral bulking agents, such as collagen, and transurethral collagen denaturation (the Renessa procedure). Transurethral collagen denaturation uses nonablative radiofrequency to reduce tissue compliance. Both transurethral bulking and transurethral collagen denaturization can be performed in the office, and both provide an option for high-risk surgical candidates and for patients with less severe symptoms of SUI.288
In a randomized controlled study, acupuncture of the hand had positive effects on vaginal contraction pressure, sexual life, and social activity. Kim et al. established 11 acupuncture points on the hand as a basic treatment formula.289
Pharmacotherapy
No medications have been approved for the treatment of SUI in the U.S. Alpha-adrenergic agonists, such as pseudoephedrine and phenylephrine, are used off-label for this indication based on the urethral smooth-muscle response to alpha stimulation and on improvements in intrinsic sphincter deficiency. However, the clinical utility of these drugs in SUI is limited by the lack of proven efficacy and by concerns regarding adverse side effects, including insomnia, anxiety, hypertension, arrhythmias, and stroke.43,164,290–292
Imipramine. The tricyclic antidepressant imipramine (Tofranil) has been used off-label to treat patients with SUI. The alpha-adrenergic and anticholinergic properties of this agent may provide the dual benefit needed in these patients. However, the use of imipramine in patients with SUI, especially elderly patients, is limited by its anticholinergic side-effect profile.25,120,293
Duloxetine. The lack of approved drugs for SUI has led to studies of alternative agents, including duloxetine (Cymbalta, Eli Lilly). Although duloxetine, a dual serotonin–norepinephrine reuptake inhibitor (SNRI), is approved for the treatment of SUI in Europe, it is indicated only for the treatment of depression and neuropathic pain in the U.S. Duloxetine is believed to influence neurotransmitters on the pudendal nerve. As a result, urethral sphincter contractions are strengthened, and the increased urethral closure forces prevent urine leakage.274,293,294
In clinical trials, duloxetine has reduced incontinence episodes and has increased the quality of life in women with SUI. Side effects leading to discontinuation included dry mouth, fatigue, nausea, constipation, and hyperhidrosis. In one study, treatment-related nausea was noted in 40% of patients; in most of these patients, the nausea occurred early in treatment, was transient, and was mild to moderate in severity.295–298
Duloxetine has also been evaluated as a potential treatment option for men with SUI after radical prostatectomy. The drug reduced incontinence episodes and improved quality of life.299
Venlafaxine. Venlafaxine (Effexor, Pfizer), another dual SNRI, has been evaluated for the management of SUI. It was reported to be effective in a double-blind, randomized, placebo-controlled study of women with SUI. Nausea occurred in 40% of the venlafaxine group compared with 15% of the placebo group (P < 0.05).300
The use of antidepressants with dual neurotransmitter mechanisms for the treatment of SUI requires further study, but these drugs may have future utility in some patients.
OVERFLOW INCONTINENCE
Etiology and Diagnosis
Overflow incontinence (OFI) is described with variable nomenclature in the literature. It is a condition of paradoxical incontinence caused by chronic urinary retention. In this situation, the intra-vesical pressure eventually equals the urethral resistance, resulting in periodic leakage or dribbling. OFI may be caused by obstructive processes anywhere in the lower urinary tract or by impaired disorders of bladder emptying.4
The most common cause of this type of UI is bladder outlet obstruction secondary to BPH in men. Other bladder-outlet obstructive disorders include urethral stricture disease, post-prostatectomy bladder neck contracture, and pelvic organ prolapse. Another common cause of OFI is impaired emptying of the bladder owing to decreased bladder contractility. Common causes of impaired contractility include hypotonic or neurogenic bladder states, often resulting from diabetes, spinal cord injuries, prolonged urinary obstruction, and adverse drug effects.4
Although OFI is less common in women than in men, bladder prolapse or alignment problems can contribute to OFI in women. Extrinsic factors include multiple medications with anticholinergic side effects that can lead to UI and OFI symptoms (see Table 2).4
OFI most commonly occurs in men with benign prostatic hypertrophy (BPH). BPH is defined as the proliferation of epithelial and stromal cells in the prostate gland, characterized by discrete nodules in the periurethral area, which can cause various degrees of bladder outlet obstruction secondary to compression of the prostate urethra. Studies suggest that BPH affects more than 30% of men 60 to 70 years of age and 90% of men in their 70s and 80s.25,301
In men with BPH, an enlarged prostate is noted on physical examination. In some cases, further evaluation may be necessary to rule out prostate cancer or other obstructive disorders.301 The clinical presentation of OFI is characterized by lower urinary tract symptoms (LUTS). This spectrum of symptoms results from compression of the urethra, which in turn leads to an unstable detrusor muscle or to bladder distention and hypertrophy caused by the patient’s chronic inability to completely empty the bladder. Symptoms of OFI include difficulty initiating a urine stream, a weak stream, a sense of incomplete emptying, nocturia, and dribbling. The severity of the symptoms might not be correlated with the degree of BPH, and a presentation of LUTS can be due to other causes.
Findings that suggest a further differential diagnosis in men with suspected OFI include fever or prostate tenderness, abnormal sphincter tone, hematuria, and prostate nodules or induration. These signs and symptoms may indicate prostatitis, neurogenic bladder, or malignancy of the bladder or prostate. A complicating clinical feature of LUTS associated with BPH is urgency symptoms, which may occur in up to two-thirds of patients.301–305
The prevalence of OFI in men with BPH is unclear. One study indicated that incontinence occurs in approximately 2,700 of every 100,000 men, although the specific type of incontinence was not specified. In men who underwent BPH surgery or received alpha-blocker therapy, the risk of incontinence was increased, especially in the postsurgical group. The BPH-related incontinence in this study might have been a result of postsurgical complications, such as new-onset urgency or frequency, and other irritative symptoms. Additional diagnostic procedures, such as cystourethroscopy and urodynamic testing, might be necessary in men with BPH, especially post-BPH surgery patients, before therapy is initiated.306
Nonpharmacological Management
General Considerations
The nonpharmacological treatment of OFI associated with BPH includes the elimination of potential triggers, such as alcohol, caffeine, and medications, along with various invasive and non-invasive procedures, including transurethral resection of the prostate (TURP).301,307–309 The American Urological Association Symptom Index provides an objective, validated tool to determine symptom severity and to provide guidance for management. An initial digital rectal examination and, in some cases, a urinalysis are recommended to rule out other urological disorders or problems.
Watchful waiting with yearly follow-up is usually recommended for men with mild BPH symptoms when other conditions have been excluded. Failure to respond to nonpharmacological interventions or the presence of hematuria, renal insufficiency, bladder stones, hydronephrosis, or recurrent infections requires further evaluation.301,304
Surgical Options
The surgical management of BPH continues to evolve. Although TURP has long been the standard of care, it is not without complications. In one cohort study that looked at the complications of TURP, the cumulative incidence of repeated interventions was approximately 15% among 23,000 cases.310,311
Alternative procedures have emerged over the last 15 years, but few have shown significant advantages over TURP. Transurethral incision of the prostate (TUIP) was developed for men with a smaller prostate gland (less than 40 g). Although TUIP offers a shorter procedure time, less bleeding, and fewer complications, it might not be as effective as TURP in reducing urinary symptoms.312
Resection techniques using electrical currents have included monopolar TURP, bipolar transurethral vaporization of the prostate, bipolar transurethral resection of the prostate, and bipolar enucleation of the prostate. These procedures have both advantages and disadvantages, and none has replaced TURP as the gold standard.313–318
Several laser procedures have also been evaluated, including interstitial laser coagulation of the prostate, holmium laser ablation of the prostate (HoLaP), holmium laser enucleation of the prostate (HoLEP), photoselective vaporization of the prostate (PVP), and thulium laser resection of the prostate (TmLRP).319–325
Other minimally invasive treatments include transurethral microwave thermotherapy (TUMT), water-induced thermotherapy, high-intensity focused ultrasonography (HIFU), and transurethral needle ablation (TUNA).326
Clinical studies have compared various minimally invasive procedures with TURP and other surgical interventions, such as open simple prostatectomy. Although these noninvasive techniques may have advantages over simple prostatectomy, no clear superiority over TURP has been shown. Some laser procedures (e.g., HoLaP) may be benefit certain patients, including critically ill patients or those with a high risk of bleeding.318–325 More invasive procedures include conventional open laparoscopic prostatectomy and, more recently, robotic-assisted laparoscopic prostatectomy.327–329
Open prostatectomy procedures are being replaced by less invasive surgery in the management of BPH. Some of the new technological treatment options for BPH may also challenge TURP because of their potential for fewer complications, reduced hospital admission time, and cost effectiveness. The advantages of some of these procedures over TURP are not entirely clear.330, 331
Most patients with BPH are treated based on symptom severity. These treatments may include watchful waiting rather than initial pharmacotherapy. Because many men do not undergo TURP or other procedures until they reach their 60s or 70s, patients in this age group may have a larger prostate gland and additional comorbidities. The aging status of many patients in this category has triggered research to develop less invasive therapies in patients who do not respond to initial treatment.331,332
Pharmacotherapy
Pharmacotherapeutic options for OFI secondary to BPH include peripheral alpha-adrenergic blockers (AABs), 5-alpha-reductase inhibitors (ARIs), or a combination of the two (Table 6, page 359).301,304 For initial pharmacotherapy, the choice between the two classes is usually based on symptoms and prostate size. Patients with moderate-to-severe symptoms of BPH usually begin with an AAB to provide a more rapid onset of action and symptom relief. Men with larger baseline prostate volumes (greater than 40 mL) may start with an ARI or an ARI/AAB combination. ARIs are more effective than AABs at reducing the progression of BPH.
Table 6.
Medications Used in the Treatment of Benign Prostatic Hyperplasia (BPH)
| Nonselective Alpha-Adrenergic Blockers | |
| General comments |
|
| Doxazosin (Cardura and Cardura XL, Pfizer) |
|
| Prazosin (Minipress, Pfizer) |
|
| Terazosin (Hytrin, Abbott) |
|
| Uroselective Alpha-Adrenergic Blockers (Alpha1A Receptors) | |
| General comments |
|
| Alfuzosin (Uroxatral, Sanofi) |
|
| Silodosin (Rapaflo, Watson) |
|
| Tamsulosin (Flomax, Boehringer Ingelheim) |
|
| 5-Alpha-Reductase Inhibitors | |
| General comments |
|
| Dutasteride (Avodart, Glaxo-SmithKline) |
|
| Dutasteride/tamsulosin (Jalyn, GlaxoSmithKline) |
|
| Finasteride (Proscar, Merck) |
|
Alpha-adrenergic blockers. The early, nonselective AABs were developed to treat hypertension, although they are rarely used for that indication today (see Table 6). The first available drugs in this class were phenoxybenzamine (Dibenzyline, Glaxo-SmithKline), approved for the treatment of pheochromocytoma, and prazosin (Minipress, Pfizer), approved for the treatment of hypertension. AABs have evolved over the last 30 years, and more prostate-selective agents are now used for the management of BPH. As their class designation indicates, the mechanism of action of the nonselective AABs is peripheral alpha-adrenergic blockade.
Alpha1A receptors, the most common receptor subtype in the prostate gland, play a key role in mediating the contraction of smooth muscle. Blocking these receptors at the prostate level results in muscle relaxation and improved urine outflow. Alpha1B receptors are found in arterial vessels, and their blockage is associated with blood pressure (BP) reduction along with certain BP-related adverse effects, such as orthostatic hypotension. Research has focused on developing agents with minimal alpha1B effects to minimize BP lowering, especially in patients at risk of orthostatic hypotension, such as the elderly.
Nonselective AABs may have a greater effect on resting BP compared with uroselective AABs and may be associated with a greater risk of orthostatic hypotension. The labeling for all of the nonselective AABs includes a warning regarding the potential for hypotension.332,333 The AABs have differing pharmacokinetic profiles, including half-life and elimination properties, and bioavailability varies among formulations (see Table 6).333–338 AABs are metabolized by the hepatic CYP450 enzyme system, predominantly the CYP2D6 and CYP3A4 pathways. Awareness of potential drug interactions should be part of the monitoring plan when AABs are used with other medications.336–339
Adverse effects of AABs include the potential for orthostatic hypotension, in addition to dizziness, peripheral edema, sedation, ejaculatory dysfunction, flu-like symptoms, headache, and GI effects. Because the nonselective AABs terazosin (Hytrin, Abbott) and doxazosin (Cardura, Pfizer) lack prostate selectivity and have increased vasodilatory properties, side effects (hypotension, dizziness, fatigue) are more common with these drugs. The uroselective AABs alfuzosin (Uroxatral, Sanofi) and tamsulosin (Flomax, Boehringer Ingelheim) are associated with a greater incidence of hypotension and ejaculatory dysfunction, respectively. The newest AAB, silodosin (Rapaflo, Watson), was reported to be effective in managing the symptoms of BPH, but the clinical and tolerability advantages of this agent have not been determined.333,338–342
One adverse effect of AABs that may be significant in some patients is intraoperative floppy-iris syndrome (IFIS). IFIS was first described in 2005 as a clinical triad observed by ophthalmologists during cataract surgery. The triad is described as a billowing and fluttering of the iris stroma, resulting in susceptibility for prolapse of the iris and constriction of the pupil. This scenario imparts a greater risk of surgical complications, including trauma to and atrophy of the iris, posterior capsule rupture with vitreous loss, and postoperative macular edema. IFIS is irreversible and does not diminish or subside after the AAB is discontinued. Numerous reports have linked IFIS to the use of tamsulosin, possibly because of that drug’s propensity to selectively block alpha-1A receptors in the iris dilator muscle, thereby preventing mydriasis during cataract surgery. Although other AABs have been associated with IFIS, their relationship to that disorder is not as clearly defined.343–345
In a study comparing men who received tamsulosin or alfuzosin, there was a significantly higher risk of IFIS and subsequent complications with tamsulosin during cataract surgery A meta-analysis showed similar associations with IFIS among the various AABs, including tamsulosin, alfuzosin, terazosin, and doxazosin, along with a history of hypertension as an additional risk factor.
An awareness of a patient’s exposure to peripheral AABs should help ophthalmologists prepare for cataract surgery and alert them to the need for corrective measures to reduce the risk of complications. Various attempts to minimize IFIS and its complications during cataract surgery have included washout periods and ophthalmological interventions, including intracameral phenylephrine, preoperative atropine, and iris expansion hooks. Pharmacists and other health care providers should be aware of the risk of IFIS, and patients should be advised about the importance of sharing information about their use of peripheral AABs. Pharmacists should ask all patients receiving AABs about their cataract history and should share this information with the patient’s ophthalmologist when necessary.343–345
Contraindications to the use of peripheral AABs include heart failure, hypotension, and the potential to exacerbate SUI in women. Because men usually present with symptomatic BPH later in life, the possibility of concurrent comorbidities exists. Sexual dysfunction, heart disease, hypertension, diabetes, and the metabolic syndrome may further complicate treatment decisions and may warrant the use of uroselective AABs.
The large Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) reported a higher risk of the combined endpoint of cardiovascular disease, stroke, and heart failure in hypertensive patients receiving the nonselective AAB doxazosin versus the diuretic chlorthalidone (e.g., Thalitone, Monarch). These data support the use of nonselective AABs as second-line or third-line options for hypertension, especially in patients with a history of cardiovascular disease.334–338,341,346
The uroselective AABs may offer a more tolerable side-effect profile than nonselective agents in patients with cardiovascular and sexual function disorders (see Table 6). Alfuzosin was well tolerated in men with multiple comorbidities, including those receiving phosphodiesterase type-5 (PDE5) inhibitors for erectile dysfunction. Tamsulosin was effective in men with BPH and LUTS without increasing the risk of cardiovascular disease.340,342,346 Peripheral AABs remain a mainstay of the pharmacological management of BPH-associated LUTS. These agents have demonstrated a class effect and provide benefits within 5 to 7 days, improving both symptoms and urinary flow rates. Although open-label and controlled trials have reported clinical benefits for up to 5 years, peripheral AABs have not reduced long-term complications or disease progression.334,335,347–349
When choosing among the five peripheral AABs that are available for the treatment of BPH, clinicians must consider several factors. Patients who can benefit from the antihypertensive properties of these drugs may be given a nonselective agent, such as terazosin. Patients who cannot tolerate the vasodilator properties of AABs, such as elderly patients with orthostatic hypotension, may benefit from one of the uroselective agents, such as alfuzosin.332 An important clinical consideration is that alfuzosin and tamsulosin can be initiated without dose titration. The cost of treatment should also be considered, especially since the older AABs are available in generic formulations.
Because comparable efficacy has been reported within the class, the focus of new drug development has been on improving convenience and tolerability. To choose the most appropriate AAB, clinicians need to identify patient-specific needs and should take into account several drug-related factors, including receptor selectivity, dosing frequency, the adverse-event profile, and concurrent comorbidities.335–338,341,342
When dispensing AABs, physicians and practitioners should review with patients the potential side effects, drug interactions, effects on BP, and the possibility of sedation and dizziness, as well as discuss future cataract surgery if relevant.333,334 When prescribing a nonselective AAB, the clinician should reiterate the importance of dose titration to reduce the risk of these complications.
5-alpha-reductase inhibitors. The ARIs finasteride (Proscar, Merck) and dutasteride (Avodart, GlaxoSmithKline) are also used to treat BPH, but usually in men with advanced disease or when AABs are contraindicated (see Table 6). ARIs are often combined with AABs because of their slow onset of action, which necessitates the use of AABs for more rapid symptom relief. The clinical use of ARI/AAB combinations has led to the development of a fixed-combination product containing the ARI dutasteride and the uroselective AAB tamsulosin (Jalyn, GlaxoSmithKline) (see Table 6).301,334 Dutasteride inhibits 5-alpha-reductase, the enzyme that converts intracellular testosterone to dihydrotestosterone (DHT). DHT is a more potent androgen than testosterone. Its production remains normal in aging men because of the increased activity of intra-prostatic 5-alpha-reductase. Inhibition of the 5-alpha-reductase enzyme leads to a reduction in androgenic prostate stimulation, which in turn decreases the size and volume of the prostate gland and improves restricted urine outflow and other symptoms of BPH.
The 5-alpha-reductase enzyme consists of two types. Type-1 is found in hair follicles, sebaceous glands, the liver, and skin, whereas type-2 occurs in prostate and genital tissues and in the scalp. Type-2 receptors appear to be involved in prostate gland enlargement and are overexpressed in prostate tissue in men with BPH and some prostate cancers.301,350
The pharmacokinetic characteristics of the ARIs include hepatic metabolism via the CYP3A4 pathway, with active metabolites. Clinicians should monitor patients for the use of concurrent inhibitors of this pathway during ARI therapy because of the potential for toxicity. Dutasteride is eliminated primarily in the feces and has limited renal clearance, with a half-life of 5 weeks. Finasteride has a substantially shorter half-life (6 to 8 hours), with elimination in the feces and urine. No dose adjustments are required for patients with renal impairment, but caution is recommended for patients with hepatic impairment.301,350,351
Although ARIs are effective in treating symptoms of BPH and are well tolerated, their side-effect profiles, especially the potential for sexual dysfunction, may be problematic in some men. ARIs may decrease ejaculate volume, affect libido and sexual function, and cause gynecomastia. Although these adverse effects may be self-limited, ARIs can create compliance issues for some patients.
ARIs are Pregnancy Category X drugs. Contact with these agents should be avoided by pregnant women or by women trying to conceive. Exposure of a pregnant woman to an ARI may result in a pseudohermaphroditic fetus with ambiguous genitalia. In addition, patients with a history of heart failure and those at risk of heart failure may require further evaluation before using ARIs because of an increased incidence of this event.301,305,334,350–356
As noted previously, ARIs are usually used as second-line drugs in the management of BPH and its associated symptoms because of their slow onset of action. Full clinical efficacy may take up to 3 to 6 months to be achieved. ARIs are approved for the treatment of moderate-to-severe symptomatic BPH to improve symptoms, to reduce the risk of acute urinary retention, and to reduce the need for BPH-related surgery. Similar efficacy was reported in clinical trials of dutasteride and finasteride. Their therapeutic benefits are due to a decrease in prostate volume, which results in the relief of LUTS, a reduced need for surgery, and a reduction in acute urinary retention, along with a delay in disease progression. Improvements in symptoms of BPH were maintained for up to 4 years in open-label extension trials.340,341,350,351,354
ARIs may be considered for first-line therapy in men with more severe symptoms of BPH, a prostate volume of 40 mL or more, and increased levels of prostate-specific antigen (PSA), such as 1.5 ng/mL or more, although an ARI/AAB combination might be warranted in these patients. Combination therapy appears to be more effective than monotherapy for prostate volumes above 60 g.
Initial combination therapy with an ARI and an AAB may be changed to ARI monotherapy after several months when the benefits of the ARI become clinically evident. Studies have shown, however, that an ARI/AAB combination can be more effective than monotherapy in improving BPH-related symptoms and urine flow. Although adverse events were more frequent with combination treatment, they did not significantly affect compliance. In addition, studies reported that an ARI/AAB combination may reduce disease progression in some patients. Limited data suggest that combination therapy might be more effective in patients with underlying prostatic inflammation and that the use of long-term combination therapy may be warranted in some patients.356–361
In one trial, patients treated with an AAB had an increased risk of BPH-related surgery compared with patients treated with an ARI. This finding suggests the need for additional research to assess long-term outcomes and to identify optimal treatments for BPH patients based on their baseline characteristics.301,358–361
Another area of research is the role of ARIs in the prevention of prostate cancer.362 Two large controlled trials of ARIs in the prevention of prostate cancer reported an overall relative reduction in low-grade tumors of approximately 24%. However, it is debatable as to whether the use of this class of drugs is associated with higher grades of prostate cancer in patients who are ultimately found to have cancer.363,364 Some experts have suggested that ARIs may reduce the size of existing low-risk lesions rather than prevent the development of new cancers. ARIs continue to be studied as a means of reducing the risk of prostate cancer or slowing disease progression.365–367 Because ARIs can suppress PSA levels, monitoring of patients receiving ARI therapy should include the evaluation and adjustment of this antigen. It has been recommended that a multiple of 2 should be used to adjust PSA values in patients receiving chronic ARI therapy.301,334,354,356,357
Antimuscarinic (anticholinergic) drugs. Men with BPH who continue to experience significant lower urinary tract symptoms (LUTS, urgency, nocturia, hesitancy, and weak stream) or overactive bladder (OAB) while taking an AAB may benefit from the addition of an antimuscarinic agent. This combination may be useful for relieving symptoms of bladder outlet obstruction and detrusor overactivity. This treatment approach has been clinically effective, but clinicians experienced in the use of AAB/antimuscarinic combinations should carefully monitor treated patients to avoid acute urinary retention.305,359,368–374
Before antimuscarinic drugs are used in BPH patients with LUTS or OAB, a risk assessment is necessary. This assessment should include an evaluation of the patient’s post-void residual volume. Clinical trials have reported average post-void residual volumes of 25 to 50 mL in patients receiving antimuscarinic agents. These studies included a variety of antimuscarinic drugs in combination with AABs. Extended-release tolterodine, ER oxybutynin, and solifenacin were all studied in combination with the AAB tamsulosin (Flomax). These trials reported improvements in urinary frequency, urgency, nocturnal micturition, patient perceptions of bladder conditions, and the International Prostate Symptom Score (IPSS) compared with AAB monotherapy.305,359,368–374
A selected cohort of men were evaluated by clinicians with appropriate training in this area. In one trial, post-void residual volumes were higher with the combination, further emphasizing the importance of monitoring for potential urinary retention. It is noteworthy that antimuscarinic agents are not approved for the treatment of BPH. If they are used to treat LUTS in these patients, monitoring is essential, especially within the first 30 days after starting therapy, because of the potential for acute urinary retention.305,359,368–374
Other Therapies for Overflow Incontinence
PDE5inhibitors. These drugs have shown promise in the treatment of BPH-associated LUTS and may have a role in men with concurrent erectile dysfunction.375 In October 2011, the FDA approved tadalafil (Cialis, Eli Lilly) for the treatment of BPH. Tadalafil had been approved for the management of erectile dysfunction in 2003, and this recent approval provides clinicians with an option for managing concurrent erectile dysfunction and BPH. Clinical trials have reported significant improvement in BPH symptoms with tadalafil 5 mg daily versus placebo, as indicated by reductions in the IPSS. Studies have also shown that tadalafil is safe and effective in reducing LUTS. Tadalafil should be avoided in men who are taking nitrates, and it should be used with caution when combined with AABs because of the potential for additive effects on BP.376,377
Although PDE5 inhibitors provide effective symptom relief in BPH, they appear to have limited effects on urinary flow rates. The precise mechanism of action of PDE5 inhibitors is unknown, but these agents may have multiple effects on pathways that contribute to LUTS, including smooth-muscle relaxation, smooth-muscle and endothelial-cell proliferation, nerve activity, and tissue perfusion.376–380
Botulinum toxin. A small trial of intraprostatic BTX-A in patients with BPH-associated LUTS reported clinical efficacy and improved quality of life. Some patients experienced sustained effects of treatment for up to 12 months. The mechanism of action of BTX-A in this setting may involve inhibitory effects on smooth-muscle tone rather than a change in prostate volume.381
Herbal remedies and investigational agents. Other therapies that have been used in the management of OFI secondary to BPH include saw palmetto (which may have some ARI activity), rye-grass pollen extract, Pygeum africanum, and the Chinese herbal medicine gosha-jinki-gan.301,304,382 Although some evidence supports the use of saw palmetto extract in BPH patients, a recent dose-escalation trial reported no beneficial effects on LUTS with this herbal preparation compared with placebo.383
Various investigational agents, including long-acting AABs with improved tolerability profiles, are being studied for the treatment of BPH-associated LUTS.384 A randomized, placebo-controlled study reported that transdermal DHT had no effect on prostate growth in healthy men.385
MIXED URINARY INCONTINENCE
The International Continence Society defines mixed urinary incontinence (MUI) as “involuntary leakage associated with exertion and urgency.” MUI can be challenging to clinicians because successful treatment must address two components––urge incontinence (UI) and stress incontinence (SUI).31,250 The failure to understand the role of both types of incontinence in patients with MUI may result in the persistence of UI after surgery for SUI, which patients may perceive as a treatment or surgical failure.386
From 15% to 90% of patients with UI have MUI, but these estimates may depend on the questions that were asked during the assessment process. One study reported that when objective evaluation methods (e.g., urodynamics) were used, as few as 8% of cases were identified as MUI.387
Patients who experience symptoms suggestive of MUI require a comprehensive urological evaluation. Treatment may involve an array of procedures and pharmacotherapies (Table 7) (see UUI and SUI, pages 348 and 354).25,43,47,110,388,389 Generally, in patients with MUI, the predominant condition should be treated first.389
Table 7.
Management Options for Mixed Urinary Incontinence
| Men | Women | |
|---|---|---|
| Initial management options (treat most bothersome symptom first) |
|
|
| Specialized management options (if initial therapy fails) |
|
|
OAB = overactive bladder; SUI = stress urinary incontinence; UUI = urge urinary incontinence.
Data from Thüroff JW, et al. Eur Urol 2011;59:387–400.389
CONCLUSION
The pharmacological management of urinary incontinence requires appropriate evaluation by qualified clinicians. Pharmacists can offer educational support to patients by questioning them about their understanding of the disorder and by monitoring the effectiveness and tolerability of the agents prescribed. Taking time to get to know the patient in ambulatory and clinical settings allows the pharmacist and health care provider the opportunity to provide valuable instruction, intervention, and recommendations to improve patient outcomes in the management of UI.
Key Abbreviations.
- BPH
benign prostatic hyperplasia
- LUTS
lower urinary tract symptoms
- MUI
mixed urinary incontinence
- OAB
overactive bladder
- OFI
overflow incontinence
- SUI
stress urinary incontinence
- UI
urinary incontinence
- UTI
urinary tract infection
- UUI
urge urinary incontinence
Footnotes
Disclosure: The authors report that they have no financial, commercial, or industrial relationships in regard to this article.
REFERENCES
- 1.Abrams P, Andersson K-E, Birder L, et al. Evaluation and treatment of urinary incontinence, pelvic organ prolapse, and fecal incontinence. Neurourol Urodyn. 2010;29(1):213–240. doi: 10.1002/nau.20870. [DOI] [PubMed] [Google Scholar]
- 2.Hunskaar S, Burgio K, Diokno AC, et al. Epidemiology and natural history of urinary incontinence. In: Abrams PC, Khoury S, Wein A, editors. Incontinence: 2nd International Consultation on Incontinence. Plymouth, U.K.: Health Publications Ltd; 2002. pp. 165–201. [Google Scholar]
- 3.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 4.Smith DR, Tanagho EA, McAninch JW. Smith’s General Urology. 17th ed. New York: Lange Medical Books/McGraw-Hill; 2008. [Google Scholar]
- 5.Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: Variation in prevalence estimates and risk factors. Obstet Gynecol. 2008;111(2 Part 1):324–331. doi: 10.1097/01.AOG.0000267220.48987.17. [DOI] [PubMed] [Google Scholar]
- 6.Markland AD, Goode PS, Redden DT, et al. Prevalence of urinary incontinence in men: Results from the national health and nutrition examination survey. J Urol. 2010;84(3):1022–1027. doi: 10.1016/j.juro.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Townsend MK, Curhan GC, Resnick NM, et al. The incidence of urinary incontinence across Asian, black, and white women in the United States. Am J Obstet Gynecol. 2010;202(4):378 e1–378e7. doi: 10.1016/j.ajog.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw C, Tansey R, Jackson C, et al. Barriers to help seeking in people with urinary symptoms. Fam Pract. 2001;18(1):48–52. doi: 10.1093/fampra/18.1.48. [DOI] [PubMed] [Google Scholar]
- 9.Lawhorne LW, Ouslander JG, Parmelee PA, et al. Urinary incontinence: A neglected geriatric syndrome in nursing facilities. J Am Med Dir Assoc. 2008;9(1):29–35. doi: 10.1016/j.jamda.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Berlowitz DR, Brand HK, Perkins C, et al. Geriatric syndromes as outcome measures of hospital care: Can administrative data be used? J Am Geriatr Soc. 1999;47(6):692–696. doi: 10.1111/j.1532-5415.1999.tb01591.x. [DOI] [PubMed] [Google Scholar]
- 11.Gaugler JE, Duval S, Anderson KA, Kane RL. Predicting nursing home admission in the U.S.: A meta-analysis. BMC Geriatr. 2007;7:13. doi: 10.1186/1471-2318-7-13. (online). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison A, Levy R. Fraction of nursing home admissions attributable to urinary incontinence. Value Health. 2006;9(4):272–274. doi: 10.1111/j.1524-4733.2006.00109.x. [DOI] [PubMed] [Google Scholar]
- 13.Tannenbaum C, DuBeau CE. Urinary incontinence in the nursing home: Practical approach to evaluation and management. Clin Geriatr Med. 2004;20:437–452. doi: 10.1016/j.cger.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Palmer MH, Johnson TM. Quality of incontinence management in U.S. nursing homes: A failing grade. J Am Geriatr Soc. 2003;51(12):1810–1812. doi: 10.1046/j.1532-5415.2003.51570.x. [DOI] [PubMed] [Google Scholar]
- 15.Newman DK. Urinary incontinence, catheters, and urinary tract infections: An overview of CMS tag F315. Ostomy Wound Manag. 2006;52(12):34, 36, 38, 40–43. [PubMed] [Google Scholar]
- 16.Centers for Medicare & Medicaid Services . Nov 11, 2011. Quality measures. Available at: www.cms.gov/NursingHomeQualityInits/10_NHQIQualityMeasures.asp. Accessed February 6, 2012. [PubMed] [Google Scholar]
- 17.Watson NM, Brink CA, Zimmer JG, Mayer RD. Use of the Agency for Health Care Policy and Research Urinary Incontinence Guideline in nursing homes. J Am Geriatr Soc. 2003;51(12):1779–1786. doi: 10.1046/j.1532-5415.2003.51564.x. [DOI] [PubMed] [Google Scholar]
- 18.Levy R, Muller N. Urinary incontinence: Economic burden and new choices in pharmaceutical treatment. Adv Ther. 2006;23(4):556–573. doi: 10.1007/BF02850045. [DOI] [PubMed] [Google Scholar]
- 19.Hu TW, Wagner TH, Bentkover JD, et al. Costs of urinary incontinence and overactive bladder in the United States: A comparative study. Urology. 2004;63(3):461–465. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 20.Wilson L, Brown JS, Shin GP, et al. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 21.Cassells C, Watt E. The impact of incontinence on older spousal caregivers. J Adv Nurs. 2003;42(6):607–616. doi: 10.1046/j.1365-2648.2003.02664.x. [DOI] [PubMed] [Google Scholar]
- 22.DuBeau CE, Simon SE, Morris JN. The effect of urinary incontinence on quality of life in older nursing home residents. J Am Geriatr Soc. 2006;54(9):1325–1333. doi: 10.1111/j.1532-5415.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald ST, Palmer MH, Kirkland VL, et al. The impact of urinary incontinence in working women: A study in a production facility. Women Health. 2002;35(1):1–16. doi: 10.1300/J013v35n01_01. [DOI] [PubMed] [Google Scholar]
- 24.Bogner HR, Gallo JJ, Sammel MD, et al. Urinary incontinence and psychological distress in community-dwelling older adults. J Am Geriatr Soc. 2002;50(3):489–495. doi: 10.1046/j.1532-5415.2002.50115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson JF. Geriatric urological disorders. In: Koda Kimble MA, Young LL, editors. Applied Therapeutics: The Clinical Use of Drugs. 9th ed. Vancouver, Wash.: Applied Therapeutics, Inc.; 2009. pp. 101-1–101-32. [Google Scholar]
- 26.Gosing JA. The structure of the female lower urinary tract and pelvic floor. Urol Clin North Am. 1985;12:207–214. [PubMed] [Google Scholar]
- 27.Abrams P. Describing bladder storage function: Overactive bladder syndrome and detrusor overactivity. Urology. 2003;62:28–37. doi: 10.1016/j.urology.2003.09.050. [DOI] [PubMed] [Google Scholar]
- 28.Elbadawi A, Diokno A, Millard R. The aging bladder: Morphology and urodynamics. World J Urol. 1998;16(Suppl 1):S10–S34. doi: 10.1007/pl00014134. [DOI] [PubMed] [Google Scholar]
- 29.Chapple CR, Artibani W, Cardozo LD, et al. The role of urinary urgency and its measurement in the overactive bladder symptom syndrome: Current concepts and future prospects. BJU Int. 2005;95(3):335–340. doi: 10.1111/j.1464-410X.2005.05294.x. [DOI] [PubMed] [Google Scholar]
- 30.Anderson KE, Hedlund P. Pharmacologic perspective on the physiology of the lower urinary tract. Urology. 2002;605(Suppl 1):13–21. doi: 10.1016/s0090-4295(02)01786-7. [DOI] [PubMed] [Google Scholar]
- 31.Abrams P, Cardozo L, Fall M, et al. The standardization of terminology in lower urinary tract function: Report from the standardization sub-committee of the International Continence Society. Urology. 2003;61(1):37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 32.Resnick NM, Tadic SD, Yalla SV. Geriatric incontinence and voiding dysfunction. In: Wein AJ, Kavoussi LR, Novick AC, et al., editors. Campbell–Walsh Urology. 10th ed. Philadelphia: Elsevier Health Science, Inc.; 2011. pp. 409–414. [Google Scholar]
- 33.Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol. 1997;90(6):983–989. doi: 10.1016/s0029-7844(97)00537-1. [DOI] [PubMed] [Google Scholar]
- 34.Thom DH, Brown JS. Reproductive and hormonal risk factors for urinary incontinence in later life: A review of the clinical and epidemiologic literature. J Am Geriatr Soc. 1998;46(11):1411–1417. doi: 10.1111/j.1532-5415.1998.tb06009.x. [DOI] [PubMed] [Google Scholar]
- 35.Bump RC, McClish DM. Cigarette smoking and pure genuine stress incontinence of urine: A comparison of risk factors and determinants between smokers and nonsmokers. Am J Obstet Gynecol. 1994;170(2):579–582. doi: 10.1016/s0002-9378(94)70231-4. [DOI] [PubMed] [Google Scholar]
- 36.Kemmer H, Mathes AM, Dilk O, et al. Obstructive sleep apnea syndrome is associated with overactive bladder and urgency incontinence in men. Sleep. 2009;32(2):271–275. doi: 10.1093/sleep/32.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danforth KN, Townsend MK, Lifford K, et al. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194(2):339–345. doi: 10.1016/j.ajog.2005.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resnick NM, Yalla SV. Management of urinary incontinence in the elderly. N Engl J Med. 1985;313:800–804. doi: 10.1056/NEJM198509263131307. [DOI] [PubMed] [Google Scholar]
- 39.Factora R, Luciano M. When to consider normal pressure hydrocephalus in the patient with gait disturbance. Geriatrics. 2008;63(2):32–37. [PubMed] [Google Scholar]
- 40.DuBeau CE, Kuchel GA, Johnson T, et al. Incontinence in the frail elderly: Report from the 4th International Consultation on Incontinence. Neurourol Urodyn. 2010;29(1):165–178. doi: 10.1002/nau.20842. [DOI] [PubMed] [Google Scholar]
- 41.Sampselle CM, Harlow SD, Skurnick J, et al. Urinary incontinence predictors and life impact in ethnically diverse perimenopausal women. Obstet Gynecol. 2002;100(6):1230–1238. doi: 10.1016/s0029-7844(02)02241-x. [DOI] [PubMed] [Google Scholar]
- 42.Rondorf-Klyn LM, Colling J, Simonson W. Medication use by community-dwelling elderly with urinary incontinence. Urol Nurs. 1998;18(3):201–206. [PubMed] [Google Scholar]
- 43.Moore KN, Richardson VA. Pharmacology: Impact on bladder function. Ostomy Wound Manag. 1998;44(6):30–34. 36, 38. [PubMed] [Google Scholar]
- 44.Jeong SH, Kim JH, Ahn YM, et al. A 2-year prospective follow-up study of lower urinary tract symptoms in patients treated with clozapine. J Clin Psychopharmacol. 2008;28(6):618–624. doi: 10.1097/JCP.0b013e31818a6cfd. [DOI] [PubMed] [Google Scholar]
- 45.Dowling-Castronovo A, Specht JK. Assessment of transient urinary incontinence in older adults. Am J Nurs. 2009;109(2):62–71. doi: 10.1097/01.NAJ.0000345392.52704.6d. [DOI] [PubMed] [Google Scholar]
- 46.Rousseau P, Fuentevilla-Clifton A. Urinary incontinence in the aged, part 1: Patient evaluation. Geriatrics. 1992;47(6):22–26. 33–34. [PubMed] [Google Scholar]
- 47.Tannenbaum C, Perrin L, DuBeau C, et al. Diagnosis and management of urinary incontinence in the older patient. Arch Phys Med Rehabil. 2001;82:134–138. doi: 10.1053/apmr.2001.9392. [DOI] [PubMed] [Google Scholar]
- 48.Scientific Committee of the First International Consultation on Incontinence Assessment and treatment of urinary incontinence. Lancet. 2000;355:2153–2158. [PubMed] [Google Scholar]
- 49.Gray M. The importance of screening, assessing, and managing urinary incontinence in primary care. J Am Acad Nurse Pract. 2003;15(3):102–107. doi: 10.1111/j.1745-7599.2003.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 50.Abrams P, Chapple C, Khoury S, et al. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol. 2009;181(4):1779–1787. doi: 10.1016/j.juro.2008.11.127. [DOI] [PubMed] [Google Scholar]
- 51.Milsom I, Abrams P, Cardozo L. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87(9):760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 52.Dubeau CE. Therapeutic/pharmacological approaches to urinary incontinence in older adults. Clin Pharm Ther. 2009;85(1):98–102. doi: 10.1038/clpt.2008.230. [DOI] [PubMed] [Google Scholar]
- 53.Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. J Am Geriatr Soc. 2000;48(4):370–374. doi: 10.1111/j.1532-5415.2000.tb04692.x. [DOI] [PubMed] [Google Scholar]
- 54.Burgio KL, Locher JL, Goode PS, et al. Behavioral vs. drug treatment for urge urinary incontinence in older women: A randomized controlled trial. JAMA. 1998;280(23):1995–2000. doi: 10.1001/jama.280.23.1995. [DOI] [PubMed] [Google Scholar]
- 55.Peterson JA. Minimize urinary incontinence: Maximize physical activity in women. Urol Nurs. 2008;28(5):351–356. [PubMed] [Google Scholar]
- 56.Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: Randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med. 2008;148(6):459–473. doi: 10.7326/0003-4819-148-6-200803180-00211. [DOI] [PubMed] [Google Scholar]
- 57.Marshall LL, Baliey W. Urinary incontinence management in geriatric patients. Consult Pharm. 2008;23(9):681–694. doi: 10.4140/tcp.n.2008.681. [DOI] [PubMed] [Google Scholar]
- 58.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;360(5):481–490. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Townsend MK, Danforth KN, Rosner B. Body mass index, weight gain, and incident urinary incontinence in middle-aged women. Obstet Gynecol. 2007;110(2 Part 1):346–353. doi: 10.1097/01.AOG.0000270121.15510.57. [DOI] [PubMed] [Google Scholar]
- 60.Danforth KN, Shah AD, Townsend MK, et al. Physical activity and urinary incontinence among healthy, older women. Obstet Gynecol. 2007;109(3):721–727. doi: 10.1097/01.AOG.0000255973.92450.24. [DOI] [PubMed] [Google Scholar]
- 61.Kikuchi A, Niu K, Ikeda Y, et al. Association between physical activity and urinary incontinence in a community-based elderly population aged 70 years and over. Eur Urol. 2007;52(3):868–874. doi: 10.1016/j.eururo.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 62.Watson NM, Brink CA, Zimmer JG. Use of the Agency for Health Care Policy and Research Urinary Incontinence Guideline in nursing homes. J Am Geriatr Soc. 2003;51(12):1779–1786. doi: 10.1046/j.1532-5415.2003.51564.x. [DOI] [PubMed] [Google Scholar]
- 63.Brady L. Prompted voiding yields results: CNAs are key to the success of a pilot study that reduced urinary incontinence for residents of one Illinois facility. Provider. 2009;35(3):41–44. [PubMed] [Google Scholar]
- 64.Fink AH, Taylor BC, Tacklind JW, et al. Treatment interventions in nursing home residents with urinary incontinence: A systematic review of randomized trials. Mayo Clin Proc. 2008;83(12):1332–1343. doi: 10.1016/S0025-6196(11)60781-7. [DOI] [PubMed] [Google Scholar]
- 65.Eustice S, Roe B, Paterson J. Prompted voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev. 2000;(2):CD002113. doi: 10.1002/14651858.CD002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palmer MH. Effectiveness of prompted voiding for incontinent nursing home residents. In: Mazurek-Melnyk B, Fineout-Overholt E, editors. Evidence-Based Practice in Nursing and Healthcare: A Guide to the Best Practice. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. CD20–CD30. [Google Scholar]
- 67.Ostaszkiewicz J, Chestney T, Roe B. Habit retraining for the management of urinary incontinence in adults. Cochrane Database Syst Rev. 2004;(2):CD002801. doi: 10.1002/14651858.CD002801.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ostaszkiewicz J, Johnston L, Roe B. Timed voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev. 2004;(1):CD002802. doi: 10.1002/14651858.CD002802.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol. 1948;56(2):238–248. doi: 10.1016/0002-9378(48)90266-x. [DOI] [PubMed] [Google Scholar]
- 70.Aslan E, Komurcu N, Beji NK, Yalcin O. Bladder training and Kegel exercises for women with urinary complaints living in a rest home. Gerontology. 2008;54(4):224–231. doi: 10.1159/000133565. [DOI] [PubMed] [Google Scholar]
- 71.Choi H, Palmer MH, Park J. Meta-analysis of pelvic floor muscle training: Randomized controlled trials in incontinent women. Nurs Res. 2007;56(4):226–234. doi: 10.1097/01.NNR.0000280610.93373.e1. [DOI] [PubMed] [Google Scholar]
- 72.Schmidt AP, Sanches PR, Silva DP., Jr A new pelvic muscle trainer for the treatment of urinary incontinence. Int J Gynaecol Obstet. 2009;105(3):218–222. doi: 10.1016/j.ijgo.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 73.Harari D, Igbedioh C. Restoring continence in frail older people living in the community: What factors influence successful treatment outcomes? Age Ageing. 2009;38(2):228–233. doi: 10.1093/ageing/afn276. [DOI] [PubMed] [Google Scholar]
- 74.Forsberg J-G. A morphologist’s approach to the vagina: Age-related changes and estrogen sensitivity. Maturitas. 1995;22:S7–S15. doi: 10.1016/0378-5122(95)00957-4. [DOI] [PubMed] [Google Scholar]
- 75.Verelst M, Maltau J, Ørbo A. Computerised morphometric study of the paraurethral tissue in young and elderly women. Neurourol Urodyn. 2002;21:529–533. doi: 10.1002/nau.10089. [DOI] [PubMed] [Google Scholar]
- 76.Carlile A, Davies I, Rigby A, Brocklehurst J. Age changes in the human female urethra: A morphometric study. J Urol. 1988;139:532–535. doi: 10.1016/s0022-5347(17)42512-2. [DOI] [PubMed] [Google Scholar]
- 77.DeLancey J, Gosling J, Creed K, et al. Gross anatomy and cell biology of the lower urinary tract. In: Abrams PC, Khoury S, Wein A, editors. Incontinence: 2nd International Consultation on Incontinence. Plymouth, U.K.: Health Publications Ltd; 2002. pp. 19–82. [Google Scholar]
- 78.Kelleher C. Investigation and treatment of lower urinary tract dysfunction. Curr Obstet Gynaecol. 2003;13:342–349. [Google Scholar]
- 79.Romanzi LJ. Management of the urethral outlet in patients with severe prolapse. Curr Opin Urol. 2002;12(4):339–344. doi: 10.1097/00042307-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 80.Wassertheil-Smoller S, Hendrix S, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women’s Health Initiative: A randomized controlled trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 81.Kim DK, Chancellor MB. Is estrogen for urinary incontinence good or bad? Rev Urol. 2006;8(2):91–92. [PMC free article] [PubMed] [Google Scholar]
- 82.Steinauer JE, Waetjen LE, Vittinghoff E, et al. Postmenopausal hormone therapy: Does it cause incontinence? Obstet Gynecol. 2005;106(5 Part 1):940–945. doi: 10.1097/01.AOG.0000180394.08406.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grady D, Brown J, Vittinghoff E, et al. Postmenopausal hormones and incontinence: The Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001;97:116–120. doi: 10.1016/s0029-7844(00)01115-7. [DOI] [PubMed] [Google Scholar]
- 84.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293:935–948. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 85.Brown J, Nyberg L, Kusek J, et al. Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases International Symposium on epidemiologic issues in urinary incontinence in women. Am J Obstet Gynecol. 2003;188:S77–S88. doi: 10.1067/mob.2003.353. [DOI] [PubMed] [Google Scholar]
- 86.Andersson K-E, Amer A. Urinary bladder contraction and relaxation: Physiology and pathophysiology. Physiol Rev. 2004;84:935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 87.Zhu Q, Ritchie J, Marouf N, et al. Role of ovarian hormones in the pathogenesis of impaired detrusor contractility: Evidence in ovariectomized rodents. J Urol. 2001;166:1136–1141. [PubMed] [Google Scholar]
- 88.Zhu Q, Resnick N, Elbadawi A, Kuchel G. Estrogen and postnatal maturation increase caveolar number and caveolin-1 protein in bladder smooth muscle cells. J Urol. 2004;171:467–471. doi: 10.1097/01.ju.0000099480.18735.49. [DOI] [PubMed] [Google Scholar]
- 89.Jackson S, James M, Abrams P. The effect of oestradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. Br J Obstet Gynaecol. 2002;109:339–344. doi: 10.1111/j.1471-0528.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- 90.Keane D, Sims T, Abrams P, Bailey A. Analysis of collagen status in premenopausal nulliparous females with genuine stress incontinence. Br J Obstet Gynaecol. 1997;104:994–998. doi: 10.1111/j.1471-0528.1997.tb12055.x. [DOI] [PubMed] [Google Scholar]
- 91.Kuchel G, Tannenbaum C, Greenspan S, Resnick N. Can variability in the hormonal status of elderly women assist in the decision to administer estrogen? J Womens Health Gender Based Med. 2001;10:109–116. doi: 10.1089/152460901300039449. [DOI] [PubMed] [Google Scholar]
- 92.Bernier F, Sims TW. Management of clients with urinary disorders. In: Black JM, Hawks JH, editors. Medical-Surgical Nursing: Clinical Management for Positive Outcomes. 8th ed. St. Louis: Elsevier Saunders; 2009. pp. 727–778. [Google Scholar]
- 93.Brown JS, Grady D, Ouslander JG, et al. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Obstet Gynecol. 1999;94(1):66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 94.Cody JD, Richardson K, Moehrer B, et al. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev. 2009;(4):CD001405. doi: 10.1002/14651858.CD001405.pub2. [DOI] [PubMed] [Google Scholar]
- 95.Townsend MK, Curhan GC, Resnick NM, et al. Postmenopausal hormone therapy and incident urinary incontinence in middle-aged women. Am J Obstet Gynecol. 2009;200(1):86.e1–86.e5. doi: 10.1016/j.ajog.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Townsend MK, Curhan GC, Resnick NM. Oral contraceptive use and incident urinary incontinence in premenopausal women. J Urol. 2009;181(5):2170–2175. doi: 10.1016/j.juro.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 98.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 99.Women’s Health Initiative Steering Committee Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 100.Hays J, Ockene J, Brunner R, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 101.Grady D, Brown JS, Vittinghoff E. Postmenopausal hormones and incontinence: The Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001;97(1):116–120. doi: 10.1016/s0029-7844(00)01115-7. [DOI] [PubMed] [Google Scholar]
- 102.Steinauer JE, Waetjen LE, Vittinghoff E, et al. Postmenopausal hormone therapy: Does it cause incontinence? Obstet Gynecol. 2005;106(5 Part 1):940–945. doi: 10.1097/01.AOG.0000180394.08406.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waetjen LE, Dwyer PL. Estrogen therapy and urinary incontinence: What is the evidence and what do we tell our patients? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:541–545. doi: 10.1007/s00192-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 104.Moehrer B, Hextall A, Jackson S. Oestrogen for urinary incontinence in women. Cochrane Database Syst Rev. 2003;(2):CD001405. doi: 10.1002/14651858.CD001405. [DOI] [PubMed] [Google Scholar]
- 105.Bernier F, Jenkins P. The role of vaginal estrogen in the treatment of urogenital dysfunction in postmenopausal women. Urol Nurs. 1997;17(3):92–95. [PubMed] [Google Scholar]
- 106.Maloney C. Estrogen in urinary incontinence treatment: An anatomic and physiologic approach. Urol Nurs. 1997;17(3):88–91. [PubMed] [Google Scholar]
- 107.Makinen JI, Pitkanen YA, Salmi TA, et al. Transdermal estrogens for female stress urinary incontinence in postmenopause. Maturitas. 1995;22(3):233–238. doi: 10.1016/0378-5122(95)00944-g. [DOI] [PubMed] [Google Scholar]
- 108.Rovner ES, Wein AJ. Treatment options for stress urinary incontinence. Rev Urol. 2004;6(Suppl 3):S29–S47. [PMC free article] [PubMed] [Google Scholar]
- 109.Pantazis K, Freeman RM. Investigation and treatment of urinary incontinence. Curr Obstet Gynaecol. 2006;16:344–352. [Google Scholar]
- 110.Bump RC, Norton PA, Zinner NR, Yalcin I. Mixed urinary incontinence in women with predominant stress urinary incontinence symptoms: Urodynamic findings, incontinence severity measures, and duloxetine treatment response. Obstet Gynecol. 2003;102:76–83. doi: 10.1016/s0029-7844(03)00376-4. [DOI] [PubMed] [Google Scholar]
- 111.Viktrup L. Addressing the need for a simpler algorithm for the management of women with urinary incontinence. Medscape Gen Med. 2005;7:3. Available at: www.medscape.com/viewarticle/506898. Accessed February 10, 2011. [PMC free article] [PubMed] [Google Scholar]
- 112.Urinary Incontinence in Adults: Acute and Chronic Management: 1996 Update––AHCPR Clinical Practice Guidelines, No. 2. Rockville, Md: Agency for Health Care Policy and Research; Mar, 1996. AHCPR Pub No 96-0682 Available at www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat6. chapter.9995. Accessed February 10, 2011. [Google Scholar]
- 113.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 114.Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder. Urology. 2002;60(Suppl 5A):7–12. doi: 10.1016/s0090-4295(02)01784-3. [DOI] [PubMed] [Google Scholar]
- 115.Poggesi A, Pracucci G, Chabriat H, et al. Urinary complaints in non-disabled elderly people with age-related white matter changes: The Leukoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc. 2008;56(9):1638–1643. doi: 10.1111/j.1532-5415.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 116.Sakakibara R, Uchiyama T, Yamanishi T, Kishi M. Dementia and lower urinary dysfunction: With a reference to anticholinergic use in elderly population. Int J Urol. 2008;15(9):778–788. doi: 10.1111/j.1442-2042.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- 117.Dmochowski RR, Gomelsky A. Update on the treatment of overactive bladder. Curr Opin Urol. 2011;21(4):286–290. doi: 10.1097/MOU.0b013e3283468da3. [DOI] [PubMed] [Google Scholar]
- 118.Wyman JF, Klutke C, Burgio K, et al. Effects of combined behavioral intervention and tolterodine on patient-reported outcomes. Can J Urol. 2010;17(4):5283–5290. [PubMed] [Google Scholar]
- 119.Burgio KL, Goode PS, Richter HE, et al. Combined behavioral and individualized drug therapy versus individualized drug therapy alone for urge urinary incontinence in women. J Urol. 2010;184(2):598–603. doi: 10.1016/j.juro.2010.03.141. [DOI] [PubMed] [Google Scholar]
- 120.Brown JH, Laiken N. Muscarinic receptor agonists and antagonists. In: Brunton L, Chabner BA, Chabner B, Knollman B, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011. pp. 219–237. [Google Scholar]
- 121.Lam S, Hilas O. Pharmacologic management of overactive bladder. Clin Interv Aging. 2007;2(3):337–345. [PMC free article] [PubMed] [Google Scholar]
- 122.Abrams P, Andersson K-E. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007;100(5):987–1006. doi: 10.1111/j.1464-410X.2007.07205.x. [DOI] [PubMed] [Google Scholar]
- 123.Yasuhiko I. Discussion: Functional role of M1, M2 and M3 muscarinic receptors in overactive bladder. Urology. 2000;55(5A):47–49. doi: 10.1016/s0090-4295(99)00493-8. [DOI] [PubMed] [Google Scholar]
- 124.Hegde SS. Muscarinic receptors in the bladder: From basic research to therapeutics. Br J Pharmacol. 2006;147(Suppl 2):S80–S87. doi: 10.1038/sj.bjp.0706560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mansfield KJ, Chandran JJ, Vaux KJ, et al. Comparison of receptor binding characteristics of commonly used muscarinic antagonists in human bladder detrusor and mucosa. J Pharmacol Exp Ther. 2009;328(3):893–899. doi: 10.1124/jpet.108.145508. [DOI] [PubMed] [Google Scholar]
- 126.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 127.Patel B, Bavendam T, Badlani G. Use of antimuscarinics in the elderly. Sci World J. 2009;9:459–465. doi: 10.1100/tsw.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Andersson K-E. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:46–53. doi: 10.1016/s1474-4422(03)00622-7. [DOI] [PubMed] [Google Scholar]
- 129.Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15–28. doi: 10.2165/00002512-199915010-00002. [DOI] [PubMed] [Google Scholar]
- 130.Power AE, McIntyre CK, Litmanovich A, et al. Cholinergic modulation of memory in the basolateral amygdala involves activation of both M1 and M2 receptors. Behav Pharmacol. 2003;14(3):207–213. doi: 10.1097/00008877-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 131.Martín-Merino E, García-Rodríguez LA, Massó-González EL. Do oral antimuscarinic drugs carry an increased risk of acute urinary retention? J Urol. 2009;182(4):1442–1448. doi: 10.1016/j.juro.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 132.Goode PS, Burgio KL. Pharmacological treatment of lower urinary tract dysfunction in geriatric patients. Am J Med Sci. 1997;14(4):262–267. doi: 10.1097/00000441-199710000-00010. 314; [DOI] [PubMed] [Google Scholar]
- 133.Meek PD, Evang SD, Tadrous M, et al. Overactive bladder drugs and constipation: A meta-analysis of randomized, placebo-controlled trials. Dig Dis Sci. 2011;56(1):7–18. doi: 10.1007/s10620-010-1313-3. [DOI] [PubMed] [Google Scholar]
- 134.Scheife R, Takeda M. Central nervous system safety of anticholinergic drugs for the treatment of overactive bladder in the elderly. Clin Ther. 2005;27(2):144–153. doi: 10.1016/j.clinthera.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 135.Gopal M, Haynes K, Bellamy SL, et al. Discontinuation rates of anticholinergic medications used for the treatment of lower urinary tract symptoms. Obstet Gynecol. 2008;112(6):1311–1318. doi: 10.1097/AOG.0b013e31818e8aa4. [DOI] [PubMed] [Google Scholar]
- 136.Sink KM, Thomas J, 3rd, Xu H, et al. Dual use of bladder anticholinergics and cholinesterase inhibitors: Long-term functional and cognitive outcomes. J Am Geriatr Soc. 2008;56:847–853. doi: 10.1111/j.1532-5415.2008.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chancellor MB, de Miguel F. Treatment of overactive bladder: Selective use of anticholinergic agents with low drug-drug interaction potential. Geriatrics. 2007;62(5):15–24. [PubMed] [Google Scholar]
- 138.Herbison P, Hay-Smith J, Bliss G, et al. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: Systemic review. BMJ. 2003;326:841. doi: 10.1136/bmj.326.7394.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fischer CP, Diokno A, Lapides J. The anticholinergic effects of dicyclomine HCl in uninhibited neurogenic bladder dysfunction. J Urol. 1978;120:328–329. doi: 10.1016/s0022-5347(17)57158-x. [DOI] [PubMed] [Google Scholar]
- 140.Briggs RS, Castleden CM, Asher MJ. The effect of flavoxate on uninhibited detrusor contractions and urinary incontinence in the elderly. J Urol. 1980;123:665–666. doi: 10.1016/s0022-5347(17)56078-4. [DOI] [PubMed] [Google Scholar]
- 141.Chapple CR. Muscarinic receptor antagonists in the treatment of overactive bladder. Urology. 2000;55(Suppl 5A):33–46. doi: 10.1016/s0090-4295(99)00492-6. [DOI] [PubMed] [Google Scholar]
- 142.Chapple C, Khullar V, Gabriel Z, Dooley JA. The effects of antimuscarinic treatments in overactive bladder: A systematic review and meta-analysis. Eur Urol. 2005;48:5–26. doi: 10.1016/j.eururo.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 143.Novara G, Galfano A, Secco S, et al. A systematic review and meta-analysis of randomized controlled trials with antimuscarinic drugs for overactive bladder. Eur Urol. 2008;54(4):740–763. doi: 10.1016/j.eururo.2008.06.080. [DOI] [PubMed] [Google Scholar]
- 144.Kessler TM, Bachmann LM, Minder C, et al. Adverse event assessment of antimuscarinics for treating overactive bladder: A network meta-analytic approach. PLoS One. 2011;6(2):e16718. doi: 10.1371/journal.pone.0016718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nabi G, Cody JD, Ellis G. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2006;(4):CD003781. doi: 10.1002/14651858.CD003781.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Erdem N, Chu FM. Management of overactive bladder and urge urinary incontinence in the elderly patient. Am J Med. 2006;119(3 Suppl 1):29–36. doi: 10.1016/j.amjmed.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 147.Staskin DR, MacDiarmid SA. Using anticholinergics to treat overactive bladder: The issue of treatment tolerability. Am J Med. 2006;119(3 Suppl 1):9–15. doi: 10.1016/j.amjmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 148.Yarker Y, Goa KL, Fitton A. Oxybutynin: A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in detrusor instability. Drugs Aging. 1995;6:243–262. doi: 10.2165/00002512-199506030-00007. [DOI] [PubMed] [Google Scholar]
- 149.Waldeck K, Larsson B, Andersson K-E. Comparison of oxybutynin and its active metabolite, N-desethyl-oxybutynin, in the human detrusor and parotid gland. J Urol. 1997;157:1093–1097. [PubMed] [Google Scholar]
- 150.Diokno AC, Appell RA, Sand PK, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: Results of the OPERA trial. Mayo Clin Proc. 2003;78:687–695. doi: 10.4065/78.6.687. [DOI] [PubMed] [Google Scholar]
- 151.Davila GW, Daugherty CA, Sanders SW. A short-term, multicenter, randomized double-blind dose titration study of the efficacy and anticholinergic side effects of transdermal compared to immediate release oral oxybutynin treatment of patients with urge urinary incontinence. J Urol. 2001;166:140–145. [PubMed] [Google Scholar]
- 152.Barkin J, Corcos J, Radomski S, et al. A randomized, double-blind, parallel-group comparison of controlled- and immediate-release oxybutynin chloride in urge urinary incontinence. Clin Ther. 2004;26:1026–1036. doi: 10.1016/s0149-2918(04)90174-9. [DOI] [PubMed] [Google Scholar]
- 153.Dmochowski RR, Sand PK, Zinner NR, et al. Comparative efficacy and safety of transdermal oxybutynin and oral tolterodine versus placebo in previously treated patients with urge and mixed urinary incontinence. Urology. 2003;62:237–242. doi: 10.1016/s0090-4295(03)00356-x. [DOI] [PubMed] [Google Scholar]
- 154.Anturol gel. Antares Pharma Available at: www.antarespharma.com/pipeline/anturol-gel/. Accessed February 17, 2012.
- 155.Lackner TE, Wyman JF, McCarthy TC, et al. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56(5):862–870. doi: 10.1111/j.1532-5415.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 156.Newman DK. The MATRIX study: Evaluating the data in older adults. Director. 2008;16(2):21–24. [PubMed] [Google Scholar]
- 157.Sahai A, Mallina R, Dowson C, et al. Evolution of transdermal oxybutynin in the treatment of overactive bladder. Int J Clin Pract. 2008;62(1):167–170. doi: 10.1111/j.1742-1241.2007.01623.x. [DOI] [PubMed] [Google Scholar]
- 158.Baldwin CM, Keating GM. Transdermal oxybutynion. Drugs. 2009;69(3):327–337. doi: 10.2165/00003495-200969030-00008. [DOI] [PubMed] [Google Scholar]
- 159.Staskin DR, Dmochowski RR, Sand PK, et al. Efficacy and safety of oxybutynin chloride topical gel for overactive bladder: A randomized, double-blind, placebo controlled, multicenter study. J Urol. 2009;181(4):1764–1772. doi: 10.1016/j.juro.2008.11.125. [DOI] [PubMed] [Google Scholar]
- 160.Kennelly MJ. A comparative review of oxybutynin chloride formulations: Pharmacokinetics and therapeutic efficacy in overactive bladder. Rev Urol. 2010;12(1):12–19. [PMC free article] [PubMed] [Google Scholar]
- 161.Staskin DR, Robinson D. Oxybutynin chloride topical gel: A new formulation of an established antimuscarinic therapy for overactive bladder. Exp Opin Pharmacother. 2009;10(18):3103–3111. doi: 10.1517/14656560903451682. [DOI] [PubMed] [Google Scholar]
- 162.Abrams P, Freeman R, Anderstrom C, et al. Tolterodine, a new anti-muscarinic agent: As effective but better tolerated than oxybutynin in patients with an overactive bladder. Br J Urol. 1998;81:801–810. doi: 10.1046/j.1464-410x.1998.00717.x. [DOI] [PubMed] [Google Scholar]
- 163.Nilvebrant L, Andersson K-E, Gillberg PG, et al. Tolterodine: A new bladder-selective antimuscarinic agent. Eur J Pharmacol. 1997;327:195–207. doi: 10.1016/s0014-2999(97)89661-6. [DOI] [PubMed] [Google Scholar]
- 164.Nilvebrant L, Glas G, Jonsson A, et al. The in vitro pharmacological profile of tolterodine: A new drug for the treatment of urinary incontinence. Neurourol Urodyn. 1994;13:433–435. [Google Scholar]
- 165.Andersson KE, Appell R, Cardozo L, et al. Pharmacological treatment of urinary incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 3rd International Consultation on Incontinence. Plymouth, U.K.: Health Publications Ltd.; 2005. pp. 809–855. [Google Scholar]
- 166.Guay D. Clinical pharmacokinetics of drugs used to treat urge incontinence. Clin Pharmacokinet. 2003;42(14):1243–1285. doi: 10.2165/00003088-200342140-00004. [DOI] [PubMed] [Google Scholar]
- 167.Klutke CG, Burgio KL, Wyman JF, et al. Combined effects of behavioral intervention and tolterodine in patients dissatisfied with overactive bladder medication. J Urol. 2009;181(6):2599–2607. doi: 10.1016/j.juro.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 168.Rogers R, Bachmann G, Jumadilova Z, et al. Efficacy of tolterodine on overactive bladder symptoms and sexual and emotional quality of life in sexually active women. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(11):1551–1557. doi: 10.1007/s00192-008-0688-6. [DOI] [PubMed] [Google Scholar]
- 169.Rogers RG, Omotosho T, Bachmann G, et al. Continued symptom improvement in sexually active women with overactive bladder and urgency urinary incontinence treated with tolterodine ER for 6 months. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(4):381–385. doi: 10.1007/s00192-008-0782-9. [DOI] [PubMed] [Google Scholar]
- 170.Rovner ES, Rackley R, Nitti VW, et al. Tolterodine extended release is efficacious in continent and incontinent subjects with overactive bladder. Urology. 2008;72(3):488–493. doi: 10.1016/j.urology.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 171.East Hanover, N.J.: Novartis; Jan, 2010. Enablex (darifenacin) Extended-Release Tablets, prescribing information. Available at: www.pharma.us.novartis.com/product/pi/pdf/enablex.pdf. Accessed February 8, 2012. [Google Scholar]
- 172.Deer-field, Ill.: Astellas Pharma US, Inc.; Jan, 2012. Vesicare (solifenacin succinate) Tablets, prescribing information. Available at: www.astel-las.us/docs/vesicare.pdf. Accessed February 8, 2012. [Google Scholar]
- 173.Irvine, Calif.: Allergan; Sep, 2011. Sanctura (trospium chloride) 20 mg Tablets, prescribing information. Available at: www.allergan.com/assets/pdf/sanctura_pi.pdf. Accessed February 8, 2012. [Google Scholar]
- 174.New York: Pfizer; Dec, 2011. Toviaz (fesoterodine fumarate), prescribing information. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=540. Accessed February 8, 2012. [Google Scholar]
- 175.Chapple C, Rechberger T, Al-Shukri S, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004;93(3):303–310. doi: 10.1111/j.1464-410x.2004.04606.x. [DOI] [PubMed] [Google Scholar]
- 176.Pak RW, Petrou SP, Staskin DR. Trospium chloride: A quaternary amine with unique pharmacologic properties. Curr Urol Rep. 2003;4:436–440. doi: 10.1007/s11934-003-0023-1. [DOI] [PubMed] [Google Scholar]
- 177.Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. Eur Urol. 2004;45:420–429. doi: 10.1016/j.eururo.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 178.Kershen RT, Hsieh M. Preview of new drugs for overactive bladder and incontinence: Darifenacin, solifenacin, trospium, and duloxetine. Curr Urol Rep. 2004;5(5):359–367. doi: 10.1007/s11934-004-0083-x. [DOI] [PubMed] [Google Scholar]
- 179.Witte LP, Mulder WM, de la Rosette JJ, et al. Muscarinic receptor antagonists for overactive bladder treatment: Does one fit all? Curr Opin Urol. 2009;19(1):13–19. doi: 10.1097/MOU.0b013e32831a6ff3. [DOI] [PubMed] [Google Scholar]
- 180.Biastre K, Burnakis T. Trospium chloride treatment of overactive bladder. Ann Pharmacother. 2009;43(2):283–295. doi: 10.1345/aph.1L160. [DOI] [PubMed] [Google Scholar]
- 181.Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride is undetectable in the older human central nervous system. J Am Geriatr Soc. 2010;58(8):1618–1619. doi: 10.1111/j.1532-5415.2010.02988.x. [DOI] [PubMed] [Google Scholar]
- 182.Todorova A, Vonderheid-Guth B, Dimpfel W. Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system. J Clin Pharmacol. 2001;41(6):636–644. doi: 10.1177/00912700122010528. [DOI] [PubMed] [Google Scholar]
- 183.Pietzko A, Dimpfel W, Schwantes U, Topfmeier P. Influences of trospium chloride and oxybutynin on quantitative EEG in healthy volunteers. Eur J Clinic Pharmacol. 1994;47:337–343. doi: 10.1007/BF00191165. [DOI] [PubMed] [Google Scholar]
- 184.Rovner ES. Trospium chloride in the management of overactive bladder. Drugs. 2004;64(21):2433–2446. doi: 10.2165/00003495-200464210-00005. [DOI] [PubMed] [Google Scholar]
- 185.Fusgen I, Iiauri D. Trospium chloride: An effective option for medical treatment of bladder overactivity. Int J Clin Pharmacol Ther. 2000;38:223–234. doi: 10.5414/cpp38223. [DOI] [PubMed] [Google Scholar]
- 186.Madersbacher H, Stöhrer, Richter R, et al. Trospium chloride versus oxybutynin: A randomized, double-blind, multicentre trial in the treatment of detrusor hyper-reflexia. Br J Urol. 1995;75:452–456. doi: 10.1111/j.1464-410x.1995.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 187.Singh-Franco D, Machado C, Tuteja S, et al. Trospium chloride for the treatment of overactive bladder with urge incontinence. Clin Ther. 2005;27(5):511–530. doi: 10.1016/j.clinthera.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 188.Staskin DR. Trospium chloride: Distinct among other anticholinergic agents available for the treatment of overactive bladder. Urol Clin North Am. 2006;33(4):465–473. doi: 10.1016/j.ucl.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 189.Beckmann-Knopp S, Rietbrock S, Weyenmeyer R, et al. Inhibitory effects of trospium chloride on cytochrome P540 enzymes in human liver microsomes. Pharmacol Toxicol. 1999;85(6):299–304. doi: 10.1111/j.1600-0773.1999.tb02026.x. [DOI] [PubMed] [Google Scholar]
- 190.Dmochowski RR, Sand PK, Zinner NR, Staskin DR. Trospium 60 mg once daily (QD) for overactive bladder syndrome: Results from a placebo-controlled interventional study. Urology. 2008;71(3):449–454. doi: 10.1016/j.urology.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 191.Macdiarmid SA, Ellsworth PI, Ginsberg DA. Safety and efficacy of once-daily trospium chloride extended-release in male patients with overactive bladder. Urology. 2011;77(1):24–29. doi: 10.1016/j.urology.2010.07.469. [DOI] [PubMed] [Google Scholar]
- 192.Sand PK, Johnson Li TM, Rovner ES, et al. Trospium chloride once daily extended release is efficacious and tolerated in elderly subjects (aged ≥75 years) with overactive bladder syndrome. BJU Int. 2011;107(4):612–620. doi: 10.1111/j.1464-410X.2010.09519.x. [DOI] [PubMed] [Google Scholar]
- 193.Staskin DR, Rosenberg MT, Sand PK. Trospium chloride once-daily extended release is effective and well tolerated for the treatment of overactive bladder syndrome: An integrated analysis of two randomised, phase III trials. Int J Clin Pract. 2009;63(12):1715–1723. doi: 10.1111/j.1742-1241.2009.02189.x. [DOI] [PubMed] [Google Scholar]
- 194.Brunton S, Kuritzky L. Recent developments in the management of overactive bladder: Focus on the efficacy and tolerability of once daily solifenacin succinate 5 mg. Curr Med Res Opin. 2005;21:71–80. doi: 10.1185/030079904x20268. [DOI] [PubMed] [Google Scholar]
- 195.Doroshyenko O, Fuhr U. Clinical pharmacokinetics and pharmacodynamics of solifenacin. Clin Pharmacokinet. 2009;48(5):281–302. doi: 10.2165/00003088-200948050-00001. [DOI] [PubMed] [Google Scholar]
- 196.Eilber KS. Is solifenacin succinate safe and effective for the treatment of overactive bladder? Nat Clin Pract Urol. 2008;5(3):128–129. doi: 10.1038/ncpuro1040. [DOI] [PubMed] [Google Scholar]
- 197.Pelman RS, Capo JP, Jr, Forero-Schwanhaeuser S. Solifenacin at 3 years: A review of efficacy and safety. Postgrad Med. 2008;120(2):85–91. doi: 10.3810/pgm.2008.07.1795. [DOI] [PubMed] [Google Scholar]
- 198.Chapple C, Wyndaele JJ, Gronen S. Solifenacin provided statistically significant and clinically relevant reductions in urgency, a defining symptom of overactive bladder (Abstract) Neurourol Urodyn. 2004;23:316. [Google Scholar]
- 199.Haab F, Cardozo L, Chapple C, Ridder AM. Long-term open-label solifenacin treatment associated with persistence with therapy in patients with overactive bladder syndrome. Eur Urol. 2005;47:376–384. doi: 10.1016/j.eururo.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 200.Cardozo L, Lisec M, Millard R, et al. Randomized, double-blind, placebo-controlled trial of the once-daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004;172:1919–1924. doi: 10.1097/01.ju.0000140729.07840.16. [DOI] [PubMed] [Google Scholar]
- 201.Karram MM, Toglia MR, Serels SR, et al. Treatment with solifenacin increases warning time and improves symptoms of overactive bladder: Results from VENUS, a randomized, double-blind, placebo-controlled trial. Urology. 2009;73(1):14–18. doi: 10.1016/j.urology.2008.08.485. [DOI] [PubMed] [Google Scholar]
- 202.Kaplan SA, Goldfischer ER, Steers WD, et al. Solifenacin treatment in men with overactive bladder: Effects on symptoms and patient-reported outcomes. Aging Male. 2010;13(2):100–107. doi: 10.3109/13685530903440408. [DOI] [PubMed] [Google Scholar]
- 203.Schaefer W. Solifenacin in the treatment of urgency and other symptoms of overactive bladder: Results from a randomized, double-blind, placebo-controlled, rising-dose trial. BJU Int. 2009;103(4):554–555. doi: 10.1111/j.1464-410X.2009.08395_4.x. [DOI] [PubMed] [Google Scholar]
- 204.Vardy MD, Mitcheson HD, Samuels TA, et al. Effects of solifenacin on overactive bladder symptoms, symptom bother and other patient-reported outcomes: Results from VIBRANT: A double-blind, placebo-controlled trial. Int J Clin Pract. 2009;63(12):1702–1714. doi: 10.1111/j.1742-1241.2009.02209.x. [DOI] [PubMed] [Google Scholar]
- 205.Cardozo L, Hessdörfer E, Milani R, et al. Solifenacin in the treatment of urgency and other symptoms of overactive bladder: Results from a randomized, double-blind, placebo-controlled, rising-dose trial. BJU Int. 2008;102(9):1120–1127. doi: 10.1111/j.1464-410X.2008.07939.x. [DOI] [PubMed] [Google Scholar]
- 206.Chapple CR, Martinez-Garcia R, Selvaggi L, et al. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: Results of the STAR trial. Eur Urol. 2005;48:464–470. doi: 10.1016/j.eururo.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 207.Herschorn S, Stothers L, Carlson K, et al. Tolerability of 5 mg solifenacin once daily versus 5 mg oxybutynin immediate release 3 times daily: Results of the VECTOR trial. J Urol. 2010;183(5):1892–1898. doi: 10.1016/j.juro.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 208.Chancellor MB, Zinner N, Whitmore K, et al. Efficacy of solifenacin in patients previously treated with tolterodine extended release 4 mg: Results of a 12-week, multicenter, open-label, flexible-dose study. Clin Ther. 2008;30(10):1766–1781. doi: 10.1016/j.clinthera.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 209.Swift SE, Siami P, Forero-Schwanhaeuser S. Diary and patient-reported outcomes in patients with severe overactive bladder switching from tolterodine extended release 4 mg/day to solifenacin treatment: An open-label, flexible-dosing, multicentre study. Clin Drug Investig. 2009;29(5):305–316. doi: 10.2165/00044011-200929050-00003. [DOI] [PubMed] [Google Scholar]
- 210.Asajima H, Sekiguchi Y, Matsushima S, et al. QT prolongation and torsade de pointes associated with solifenacin in an 81-year-old woman. Br J Clin Pharmacol. 2008;66(6):896–897. doi: 10.1111/j.1365-2125.2008.03298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ikeda K, Kobayashi S, Suzuki M, et al. M(3) receptor antagonism by the novel antimuscarinic agent solifenacin in the urinary bladder and salivary gland. Arch Pharmacol. 2002;366:97–103. doi: 10.1007/s00210-002-0554-x. [DOI] [PubMed] [Google Scholar]
- 212.Chapple CR. Darifenacin: A novel M3 muscarinic selective receptor antagonist for the treatment of overactive bladder. Exp Opin Invest Drugs. 2004;13(11):1493–1500. doi: 10.1517/13543784.13.11.1493. [DOI] [PubMed] [Google Scholar]
- 213.Newgreen DT, Anderson DW, Carter AJ. Darifenacin: A novel bladder-selective agent for the treatment of urge incontinence. Neurourol Urodyn. 1995;14:95–99. [Google Scholar]
- 214.Braverman AS, Ruggieri MR, Pontari MA. The M2 muscarinic receptor subtype mediates cholinergic bladder contractions in patients with neurogenic bladder dysfunction. J Urol. 2001;165:36. doi: 10.1152/ajpregu.00391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yoshida A, Fujino T, Maruyama S, et al. The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: Bladder selectivity based on in vivo drug-receptor binding characteristics of antimuscarinic agents for treatment of overactive bladder. J Pharmacol Sci. 2010;112(2):142–150. doi: 10.1254/jphs.09r14fm. [DOI] [PubMed] [Google Scholar]
- 216.Foote JE. Darifenacin, a highly M3 selective muscarinic receptor antagonist, is effective and well tolerated by elderly patients with overactive bladder. J Am Geriatr Soc. 2004;52:S126. [Google Scholar]
- 217.Zinner N, Tuttle J, Marks L. Efficacy and tolerability of darifenacin, a muscarinic M3 selective receptor antagonist (M3 SRA), compared with oxybutynin in the treatment of patients with overactive bladder. World J Urol. 2005;23(4):248–252. doi: 10.1007/s00345-005-0507-3. [DOI] [PubMed] [Google Scholar]
- 218.Khullar V. Darifenacin, an M3 selective receptor antagonist, reduces the frequency of nocturnal awaking, an important symptom of overactive bladder (Abstract) J Urol. 2004;171(4 Suppl):131. [Google Scholar]
- 219.Chancellor MB, Kianifard F, Beamer E, et al. Comparison of the efficacy of darifenacin alone vs. darifenacin plus a behavioural modification programme upon the symptoms of overactive bladder. Int J Clin Pract. 2008;62(4):606–613. doi: 10.1111/j.1742-1241.2008.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zinner N, Kobashi KC, Ebinger U, et al. Darifenacin treatment for overactive bladder in patients who expressed dissatisfaction with prior extended-release antimuscarinic therapy. Int J Clin Pract. 2008;62(11):1664–1674. doi: 10.1111/j.1742-1241.2008.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Green L, Kerney D. Patient experience with darifenacin: Results of a short-term community-based survey in managing overactive bladder. Curr Med Res Opin. 2011;27(2):431–437. doi: 10.1185/03007995.2010.541432. [DOI] [PubMed] [Google Scholar]
- 222.Malhotra B, Darsey E, Crownover P, et al. Comparison of pharmacokinetic variability of fesoterodine vs tolterodine extended release in cytochrome P450 2D6 extensive and poor metabolizers. Br J Clin Pharmacol. 2011;2:226–234. doi: 10.1111/j.1365-2125.2011.03948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Malhotra B, Sachse R, Wood N. Evaluation of drug–drug interactions with fesoterodine. Eur J Clin Pharmacol. 2009;65(6):551–560. doi: 10.1007/s00228-009-0648-1. [DOI] [PubMed] [Google Scholar]
- 224.Gomelsky A, Dmochowski RR. Fesoterodine fumarate. Drugs Today (Barc) 2010;46(2):81–90. doi: 10.1358/dot.2010.46.2.1437712. [DOI] [PubMed] [Google Scholar]
- 225.Tzefos M, Dolder C, Olin JL. Fesoterodine for the treatment of overactive bladder. Ann Pharmacother. 2009;43(12):1992–2000. doi: 10.1345/aph.1M308. [DOI] [PubMed] [Google Scholar]
- 226.Ellsworth P, Berriman SJ, Brodsky M. Fesoterodine: A new agent for treating overactive bladder. Am J Manag Care. 2009;15(4 Suppl):S115–S117. [PubMed] [Google Scholar]
- 227.Corcos J, Angulo JC, Garely AD. Effect of fesoterodine 4mg on bladder diary and patient-reported outcomes during the first week of treatment in subjects with overactive bladder. Curr Med Res Opin. 2011;27(5):1059–1065. doi: 10.1185/03007995.2011.565044. [DOI] [PubMed] [Google Scholar]
- 228.Dmochowski RR, Peters KM, Morrow JD, et al. Randomized, double-blind, placebo-controlled trial of flexible-dose fesoterodine in subjects with overactive bladder. Urology. 2010;75(1):62–68. doi: 10.1016/j.urology.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 229.Kraus SR, Ruiz-Cerdá JL. Efficacy and tolerability of fesoterodine in older and younger subjects with overactive bladder. Urology. 2010;76(6):1350–1357. doi: 10.1016/j.urology.2010.03.097. [DOI] [PubMed] [Google Scholar]
- 230.Kelleher CJ, Tubaro A, Wang JT, Kopp Z. Impact of fesoterodine on quality of life: Pooled data from two randomized trials. BJU Int. 2008;102(1):56–61. doi: 10.1111/j.1464-410X.2008.07710.x. [DOI] [PubMed] [Google Scholar]
- 231.Scarpero H, Sand PK, Kelleher CJ. Long-term safety, tolerability, and efficacy of fesoterodine treatment in men and women with overactive bladder symptoms. Curr Med Res Opin. 2011;27(5):921–930. doi: 10.1185/03007995.2011.559581. [DOI] [PubMed] [Google Scholar]
- 232.Khullar V, Rovner ES, Dmochowski R, et al. Fesoterodine dose response in subjects with overactive bladder syndrome. Urology. 2008;71(5):839–843. doi: 10.1016/j.urology.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 233.Wyndaele JJ, Goldfischer ER, Morrow JD, et al. Effects of flexible-dose fesoterodine on overactive bladder symptoms and treatment satisfaction: An open-label study. Int J Clin Pract. 2009;63(4):560–567. doi: 10.1111/j.1742-1241.2009.02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chapple C, Van Kerrebroeck P, Tubaro A, et al. Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol. 2007;52(4):1204–1212. doi: 10.1016/j.eururo.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 235.Chapple CR, Van Kerrebroeck PE, Jünemann KP, et al. Comparison of fesoterodine and tolterodine in patients with overactive bladder. BJU Int. 2008;102(9):1128–1132. doi: 10.1111/j.1464-410X.2008.07907.x. [DOI] [PubMed] [Google Scholar]
- 236.Herschorn S, Swift S, Guan Z. Comparison of fesoterodine and tolterodine extended release for the treatment of overactive bladder: A head-to-head placebo-controlled trial. BJU Int. 2010;105(1):58–66. doi: 10.1111/j.1464-410X.2009.09086.x. [DOI] [PubMed] [Google Scholar]
- 237.Kaplan SA, Schneider T, Foote JE, et al. Superior efficacy of fesoterodine over tolterodine extended release with rapid onset: A prospective, head-to-head, placebo-controlled trial. BJU Int. 2011;107(9):1432–1440. doi: 10.1111/j.1464-410X.2010.09640.x. [DOI] [PubMed] [Google Scholar]
- 238.Cardozo L, Thorpe A, Warner J, et al. The cost-effectiveness of solifenacin vs. fesoterodine, oxybutynin immediate-release, propiverine, tolterodine extended-release, and tolterodine immediate-release in the treatment of patients with overactive bladder in the U.K. National Health Service. BJU Int. 2010;106(4):506–514. doi: 10.1111/j.1464-410X.2009.09160.x. [DOI] [PubMed] [Google Scholar]
- 239.Anger JT, Weinberg A, Suttorp MJ, et al. Outcomes of intravesical botulinum toxin for idiopathic overactive bladder symptoms: A systematic review of the literature. J Urol. 2010;183(6):2258–2264. doi: 10.1016/j.juro.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of on-abotulinumtoxinA for idiopathic overactive bladder: A double-blind, placebo controlled, randomized, dose ranging trial. J Urol. 2010;184(6):2416–2422. doi: 10.1016/j.juro.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 241.Vasavada PS, Rackley RR. Electrical stimulation in storage and emptying failure. In: Wein AJ, Kavoussi LR, Novick AC, et al., editors. Campbell-Walsh Urology. 10th ed. Philadelphia: Elsevier Health Science, Inc.; 2011. pp. 377–381. [Google Scholar]
- 242.Homma Y, Yamaguchi T, Yamaguchi O. A randomized, double-blind, placebo-controlled phase II dose-finding study of the novel anti-muscarinic agent imidafenacin in Japanese patients with overactive bladder. Int J Urol. 2008;15(9):809–815. doi: 10.1111/j.1442-2042.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 243.Frenkl TL, Zhu H, Reiss T, et al. A multicenter, double-blind, randomized, placebo-controlled trial of a neurokinin-1 receptor antagonist for overactive bladder. J Urol. 2010;184(2):616–622. doi: 10.1016/j.juro.2010.03.147. [DOI] [PubMed] [Google Scholar]
- 244.Tyagi P, Tyagi V. Mirabegron, a β3-adrenoceptor agonist for the potential treatment of urinary frequency, urinary incontinence or urgency associated with overactive bladder. IDrugs. 2010;13(10):713–722. [PubMed] [Google Scholar]
- 245.Mushkat Y, Bukovsky I, Lanfer Female urinary stress incontinence: Does it have familial prevalence? Am J Obstet Gynecol. 1996;174:617–619. doi: 10.1016/s0002-9378(96)70437-4. [DOI] [PubMed] [Google Scholar]
- 246.Viktrup L, Koke S, Burgio KL, Ouslander JG. Stress urinary incontinence in active elderly women. South Med J. 2005;98:79–89. doi: 10.1097/01.SMJ.0000146587.06656.98. [DOI] [PubMed] [Google Scholar]
- 247.Urinary Incontinence in Women. Bethesda, Md.: National Kidney and Urologic Diseases Clearinghouse; Oct, 2007. NIH Pub. No. 02-4132. Available at: http://kidney.niddk.nih.gov/KUDiseases/pubs/uiwomen/index.aspx. Accessed February 10, 2012. [Google Scholar]
- 248.Dugan E, Cohen S, Bland DR, et al. The association of depressive symptoms and urinary incontinence among older adults. J Am Geriatr Soc. 2000;48:413–416. doi: 10.1111/j.1532-5415.2000.tb04699.x. [DOI] [PubMed] [Google Scholar]
- 249.Melville JL, Walker E, Katon W, et al. Prevalence of comorbid psychiatric illness and its impact on symptom perception, quality of life, and functional status in women with urinary incontinence. Am J Obstet Gynecol. 2002;187:80–87. doi: 10.1067/mob.2002.124839. [DOI] [PubMed] [Google Scholar]
- 250.Brubaker L, Stoddard A, Richter H, et al. Mixed incontinence: Comparing definitions in women having stress incontinence surgery. Neurourol Urodyn. 2009;28(4):268–273. doi: 10.1002/nau.20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Campbell SC, Siegel SW. Female urinary incontinence: Current evaluation and management. Consultant. 1992;32(10):45–52. [Google Scholar]
- 252.Artibani W, Andersen JT, Gajewski JB, et al. Imaging and other investigations. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 2nd International Consultation on Incontinence. Plymouth, U.K.: Health Publications Ltd.; 2002. pp. 425–477. [Google Scholar]
- 253.Lingam K. Genuine stress incontinence. Curr Obstet Gynaecol. 2001;11:353–358. [Google Scholar]
- 254.Dorey G. A clinical overview of the treatment of post-prostatectomy incontinence. Br J Nurs. 2007;16:1194–1199. doi: 10.12968/bjon.2007.16.19.27357. [DOI] [PubMed] [Google Scholar]
- 255.Smith IA. Postprostatectomy stress urinary incontinence. Aust Fam Phys. 2009;38(6):399–404. [PubMed] [Google Scholar]
- 256.Jackson SL, Scholes D, Boyko EJ, et al. Predictors of urinary incontinence in a prospective cohort of postmenopausal women. Obstet Gynecol. 2006;108(4):855–862. doi: 10.1097/01.AOG.0000236446.17153.21. [DOI] [PubMed] [Google Scholar]
- 257.Heit M, Mudd K, Culligan P. Prevention of childbirth injuries to the pelvic floor. Curr Womens Health Rep. 2001;1:72–80. [PubMed] [Google Scholar]
- 258.Wilson PD, Bø K, Hay-Smith J, et al. Conservative treatment in women. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence: 2nd International Consultation on Incontinence. Plymouth, U.K.: Health Publications Ltd; 2002. pp. 571–624. [Google Scholar]
- 259.American Academy of Family Physicians Kegel exercises for your pelvic muscles. Aug, 2010. Available at: http://familydoctor.org/handouts/642.html. Accessed February 10, 2012.
- 260.Bø K, Talseth T, Holme I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ. 1999;318:487–493. doi: 10.1136/bmj.318.7182.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210–213. doi: 10.1007/BF01901606. [DOI] [PubMed] [Google Scholar]
- 262.Weber AM, Walters MD. Burch procedure compared with sling for stress urinary incontinence: A decision analysis. Obstet Gynecol. 2000;96:867–873. doi: 10.1016/s0029-7844(00)01013-9. [DOI] [PubMed] [Google Scholar]
- 263.Hilton P. Trials of surgery for stress incontinence: Thoughts on the ‘Humpty Dumpty principle’. Br J Obstet Gynaecol. 2002;109:1081–1088. doi: 10.1111/j.1471-0528.2002.02011.x. [DOI] [PubMed] [Google Scholar]
- 264.Oliphant SS, Wang L, Bunker CH, Lowder JL. Trends in stress urinary incontinence inpatient procedures in the United States, 1979–2004. Am J Obstet Gynecol. 2009;200(5):521e1–521e6. doi: 10.1016/j.ajog.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Waetjen LE, Subak LL, Shen H, et al. Stress urinary incontinence surgery in the United States. Obstet Gynecol. 2003;101(4):671–676. doi: 10.1016/s0029-7844(02)03124-1. [DOI] [PubMed] [Google Scholar]
- 266.Bombieri L, Freeman RM. Do bladder neck position and amount of elevation influence the outcome of colposuspension? Br J Obstet Gynaecol. 2003;110(2):197–200. [PubMed] [Google Scholar]
- 267.Hilton P, Mohammed KA, Ward K, et al. Postural perineal pain associated with perforation of the lower urinary tract due to insertion of a tension-free vaginal tape. Br J Obstet Gynaecol. 2003;110(1):79–82. [PubMed] [Google Scholar]
- 268.Harding CK, Thorpe AC. The surgical treatment of female stress urinary incontinence. Indian J Urol. 2010;26(2):257–262. doi: 10.4103/0970-1591.65401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Oreskovi S, Kalafati D, Grsi HL, et al. Operative complications and results of the ‘SPARC’ procedure for stress urinary incontinence. Coll Antropol. 2009;33(1):201–204. [PubMed] [Google Scholar]
- 270.Shin JH, Lim JS, Song KH, et al. Prospective study comparing the supra-pubic arc (Sparc) procedure and the transobturator (Monarc) procedure for treating female stress urinary incontinence. LUTS. 2010;2(1):37–42. doi: 10.1111/j.1757-5672.2010.00061.x. [DOI] [PubMed] [Google Scholar]
- 271.Lee JH, Yoon HJ, Lee SJ, et al. Modified transobturator tape (canal transobturator tape) surgery for female stress urinary incontinence. J Urol. 2009;181(6):2616–2621. doi: 10.1016/j.juro.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 272.Schierlitz L, Dwyer PL, Rosamilia A. Effectiveness of tension-free vaginal tape compared with transobturator tape in women with stress urinary incontinence and intrinsic sphincter deficiency: A randomized controlled trial. Obstet Gynecol. 2008;112:1253–1261. doi: 10.1097/AOG.0b013e31818db391. [DOI] [PubMed] [Google Scholar]
- 273.Peeker I, Peeker R. Early diagnosis and treatment of genuine stress urinary incontinence in women after pregnancy: Midwives as detectives. J Midwifery Womens Health. 2003;48(1):60–66. doi: 10.1016/s1526-9523(02)00365-3. [DOI] [PubMed] [Google Scholar]
- 274.Pantazis K, Freeman RM. Investigation and treatment of urinary incontinence. Curr Obstet Gynaecol. 2006;16:344–352. [Google Scholar]
- 275.Sasso KM. The Colpexin sphere: A new conservative management option for pelvic organ prolapse. Urol Nurs. 2006;26(6):433–440. [PubMed] [Google Scholar]
- 276.Woldringh C, van den Wijngaart M, Albers-Heitner P. Pelvic floor muscle training is not effective in women with UI in pregnancy: A randomised controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):383–390. doi: 10.1007/s00192-006-0175-x. [DOI] [PubMed] [Google Scholar]
- 277.Mørkved S, Bø K, Schei B, Salvesen KA. Pelvic floor muscle training during pregnancy: A single-blind randomized controlled trial. Obstet Gynecol. 2003;101(2):313–319. doi: 10.1016/s0029-7844(02)02711-4. [DOI] [PubMed] [Google Scholar]
- 278.Culligan PJ, Heit M. Urinary incontinence in women: Evaluation and management. Am Fam Physician. 2000;62(11):2433–2444. 2447, 2452. [PubMed] [Google Scholar]
- 279.Mørkved S, Salvesen KA, Bø K, Eik-Nes S. Pelvic floor muscle strength and thickness in continent and incontinent nulliparous pregnant women. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(6):384–389. doi: 10.1007/s00192-004-1194-0. [DOI] [PubMed] [Google Scholar]
- 280.Salvesen KA, Mørkved S. Randomised controlled trial of pelvic floor muscle training during pregnancy. BMJ. 2004;329(7462):378–380. doi: 10.1136/bmj.38163.724306.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yoo EH, Kim YM, Kim D. Factors predicting the response to biofeedback-assisted pelvic floor muscle training for urinary incontinence. Int J Gynaecol Obstet. 2011;112(3):179–181. doi: 10.1016/j.ijgo.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 282.Zaccardi JE, Wilson L, Mokrzycki ML. The effect of pelvic floor reeducation on comfort in women having surgery for stress urinary incontinence. Urol Nurs. 2010;30(2):137–146. 148. [PubMed] [Google Scholar]
- 283.Imamura M, Abrams P, Bain C, et al. Systematic review and economic modeling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence. Health Technol Assess. 2010;14(40):1–188. iii–iv. doi: 10.3310/hta14400. [DOI] [PubMed] [Google Scholar]
- 284.Sand PK, Richardson DA, Staskin DR. Pelvic floor electrical stimulation in the treatment of genuine stress incontinence: A multicenter, placebo-controlled trial. Am J Obstet Gynecol. 1995;173(1):72–79. doi: 10.1016/0002-9378(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 285.Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108(1):93–99. doi: 10.1097/01.AOG.0000222903.38684.cc. [DOI] [PubMed] [Google Scholar]
- 286.McIntosh L. The role of the nurse in the use of vaginal pessaries to treat pelvic organ prolapse and/or urinary incontinence: A literature review. Urol Nurs. 2005;25(1):41–48. [PubMed] [Google Scholar]
- 287.Richter HE, Burgio KL, Brubaker L. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: A randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617. doi: 10.1097/AOG.0b013e3181d055d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Appell RA. Transurethral collagen denaturation for women with stress urinary incontinence. Curr Urol Rep. 2008;9(5):373–379. doi: 10.1007/s11934-008-0065-5. [DOI] [PubMed] [Google Scholar]
- 289.Kim JH, Nam D, Park MK, et al. Randomized controlled trial of hand acupuncture for female stress urinary incontinence. Acupunct Electrother Res. 2008;33(3–4):179–192. doi: 10.3727/036012908803861122. [DOI] [PubMed] [Google Scholar]
- 290.Westfall TC, Westfall DP. Adrenergic receptor agonists and antagonists. In: Brunton L, Chabner BA, Chabner B, Knollman B, editors. Goodman & Gilman’s The Pharmacolgical Basis of Therapeutics. 12th ed. New York: 2011. p. 277.p. 333. [Google Scholar]
- 291.Collste L, Lindskog M. Phenylpropanolamine in the treatment of female stress urinary incontinence: Double-blind placebo controlled study in 24 patients. Urology. 1987;30(4):398–403. doi: 10.1016/0090-4295(87)90314-1. [DOI] [PubMed] [Google Scholar]
- 292.Stier BG, Hennekens CH. Phenylpropanolamine and hemorrhagic stroke in the Hemorrhagic Stroke Project: A reappraisal in the context of science, the Food and Drug Administration, and the law. Ann Epidemiol. 2006;16(1):49–52. doi: 10.1016/j.annepidem.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 293.Zinner NR, Koke SC, Viktrup L. Pharmacotherapy for stress urinary incontinence: Present and future options. Drugs. 2004;64(14):1503–1516. doi: 10.2165/00003495-200464140-00001. [DOI] [PubMed] [Google Scholar]
- 294.Millard RJ, Moore K, Rencken R, et al. Duloxetine vs placebo in the treatment of stress urinary incontinence: A four-continent randomized clinical trial. BJU Int. 2004;93:311–318. doi: 10.1111/j.1464-410x.2004.04607.x. [DOI] [PubMed] [Google Scholar]
- 295.Lin AT, Sun MJ, Tai HL, et al. Duloxetine versus placebo for the treatment of women with stress predominant urinary incontinence in Taiwan: A double-blind, randomized, placebo-controlled trial (Abstract) BMC Urol. 2008;25(8):2. doi: 10.1186/1471-2490-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bump RC, Voss S, Beardsworth A, et al. Long-term efficacy of duloxetine in women with stress urinary incontinence. BJU Int. 2008;102(2):214–218. doi: 10.1111/j.1464-410X.2008.07577.x. [DOI] [PubMed] [Google Scholar]
- 297.Vella M, Duckett J, Basu M. Duloxetine 1 year on: The long-term outcome of a cohort of women prescribed duloxetine. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(7):961–964. doi: 10.1007/s00192-008-0564-4. [DOI] [PubMed] [Google Scholar]
- 298.Schagen van Leeuwen JH, Lange RR. Efficacy and safety of duloxetine in elderly women with stress urinary incontinence or stress-predominant mixed urinary incontinence. Maturitas. 2008;60(2):138–147. doi: 10.1016/j.maturitas.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 299.Cornu JN, Merlet B, Ciofu C, et al. Duloxetine for mild to moderate post-prostatectomy incontinence: Preliminary results of a randomised, placebo-controlled trial. Eur Urol. 2011;59(1):148–154. doi: 10.1016/j.eururo.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 300.Erdinc A, Gurates B, Celik H, et al. The efficacy of venlafaxine in the treatment of women with stress urinary incontinence. Arch Gynecol Obstet. 2009;279(3):343–348. doi: 10.1007/s00404-008-0729-x. [DOI] [PubMed] [Google Scholar]
- 301.Lee ML. Management of benign prostatic hyperplasia. In: DiPiro JT, Talbert RL, Yee G, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York: McGraw-Hill; 2011. pp. 1455–1466. [Google Scholar]
- 302.Lepor H. Pathophysiology of lower urinary tract symptoms in the aging male population. Rev Urol. 2005;7(Suppl 7):S3–S11. [PMC free article] [PubMed] [Google Scholar]
- 303.Meigs JB, Mohr B, Barry MJ, et al. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy aging men. J Clin Epidemiol. 2001;54:935–944. doi: 10.1016/s0895-4356(01)00351-1. [DOI] [PubMed] [Google Scholar]
- 304.American Urological Association Management of Benign Prostatic Hyperplasia (BPH) (Revised, 2010) Chapter 1: Guideline on the management of benign prostatic hyperplasia (BPH). Available at: www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines/main-reports/bph-management/chap_1_GuidelineManagementof(BPH).pdf. Accessed February 13, 2012.
- 305.MacDiarmid SA, Peters KM, Chen A, et al. Efficacy and safety of extended-release oxybutynin in combination with tamsulosin for treatment of lower urinary tract symptoms in men: Randomized, double-blind, placebo-controlled study. Mayo Clin Proc. 2008;83(9):1002–1010. doi: 10.4065/83.9.1002. [DOI] [PubMed] [Google Scholar]
- 306.Han E, Black LK, Lavelle JP. Incontinence related to management of benign prostatic hypertrophy. Am J Geriatr Pharmacother. 2007;5(4):324–334. doi: 10.1016/j.amjopharm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 307.Gnanapragasam VJ, Kumar V, Langton D, et al. Outcome of transurethral prostatectomy for the palliative management of lower urinary tract symptoms in men with prostate cancer. Int J Urol. 2006;13(6):711–715. doi: 10.1111/j.1442-2042.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 308.Mattiasson A, Wagrell L, Schelin S, et al. Five-year follow-up of feedback microwave thermotherapy versus TURP for clinical BPH: A prospective randomized multicenter study. Urology. 2007;69(1):91–97. doi: 10.1016/j.urology.2006.08.1115. [DOI] [PubMed] [Google Scholar]
- 309.Fried NM. New laser treatment approaches for benign prostatic hyperplasia. Curr Urol Rep. 2007;8(1):47–52. doi: 10.1007/s11934-007-0020-x. [DOI] [PubMed] [Google Scholar]
- 310.Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP): Incidence, management, and prevention. Eur Urol. 2006;50:969–979. doi: 10.1016/j.eururo.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 311.Madersbacher S, Lackner J, Brössner C, et al. Reoperation, myocardial infarction and mortality after transurethral and open prostatectomy: A nation-wide, long-term analysis of 23,123 cases. Eur Urol. 2005;47:499–504. doi: 10.1016/j.eururo.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 312.Riehmann M, Knes JM, Heisey D, et al. Transurethral resection versus incision of the prostate: A randomized, prospective study. Urology. 1995;45:768–775. doi: 10.1016/S0090-4295(99)80081-8. [DOI] [PubMed] [Google Scholar]
- 313.Eaton AC, Francis RN. The provision of transurethral prostatectomy on a day-case basis using bipolar plasma kinetic technology. BJU Int. 2002;89:534–537. doi: 10.1046/j.1464-410x.2002.02673.x. [DOI] [PubMed] [Google Scholar]
- 314.Fung BT, Li SK, Yu CF, et al. Prospective randomized controlled trial comparing plasmakinetic vaporesection and conventional transurethral resection of the prostate. Asian J Surg. 2005;28:24–28. doi: 10.1016/S1015-9584(09)60253-0. [DOI] [PubMed] [Google Scholar]
- 315.Dunsmuir WD, McFarlane JP, Tan A, et al. Gyrus bipolar electrovaporization vs transurethral resection of the prostate: A randomized prospective single-blind trial with 1 y follow-up. Prostate Cancer Prostatic Dis. 2003;6:182–186. doi: 10.1038/sj.pcan.4500631. [DOI] [PubMed] [Google Scholar]
- 316.Kaya C, Ilktac A, Gokmen E, et al. The long-term results of transurethral vaporization of the prostate using plasmakinetic energy. BJU Int. 2007;99:845–848. doi: 10.1111/j.1464-410X.2006.06683.x. [DOI] [PubMed] [Google Scholar]
- 317.Wendt-Nordahl G, Häcker A, Reich O, et al. The Vista system: A new bipolar resection device for endourological procedures. Comparison with conventional resectoscope. Eur Urol. 2004;46:586–590. doi: 10.1016/j.eururo.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 318.Rassweiler J, Schulze M, Stock C, et al. Bipolar transurethral resection of the prostate: Technical modifications and early clinical experience. Minim Invasive Ther Allied Technol. 2007;16:11–21. doi: 10.1080/13645700601159410. [DOI] [PubMed] [Google Scholar]
- 319.Gupta N, Sivaramakrishna, Kumar R, et al. Comparison of standard transurethral resection, transurethral vapour resection and holmium laser enucleation of the prostate for managing benign prostatic hyperplasia of >40 g. BJU Int. 2006;97:85–89. doi: 10.1111/j.1464-410X.2006.05862.x. [DOI] [PubMed] [Google Scholar]
- 320.Kuntz RM, et al. Transurethral holmium laser enucleation of the prostate versus transurethral electrocautery resection of the prostate: A randomized prospective trial in 200 patients. J Urol. 2004;172:1012–1016. doi: 10.1097/01.ju.0000136218.11998.9e. [DOI] [PubMed] [Google Scholar]
- 321.Montorsi F, Naspro R, Salonia A, et al. Holmium laser enucleation versus transurethral resection of the prostate: Results from a 2-center, prospective, randomized trial in patients with obstructive benign prostatic hyperplasia. J Urol. 2004;172:1926–1929. doi: 10.1097/01.ju.0000140501.68841.a1. [DOI] [PubMed] [Google Scholar]
- 322.Tan AH, Gilling PJ, Kennett KM, et al. A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams) J Urol. 2003;170:1270–1274. doi: 10.1097/01.ju.0000086948.55973.00. [DOI] [PubMed] [Google Scholar]
- 323.Peterson MD, Matlaga BR, Kim SC, et al. Holmium laser enucleation of the prostate for men with urinary retention. J Urol. 2005;174:998–1001. doi: 10.1097/01.ju.0000170230.26743.e4. [DOI] [PubMed] [Google Scholar]
- 324.Pedraza R, Samadi A, Eshqhi M. Holmium laser enucleation of the prostate in critically ill patients with technique modification. J Endourol. 2004;18:795–798. doi: 10.1089/end.2004.18.795. [DOI] [PubMed] [Google Scholar]
- 325.Elzayat E, Habib E, Elhilali M. Holmium laser enucleation of the prostate in patients on anticoagulant therapy or with bleeding disorders. J Urol. 2006;75:1428–1432. doi: 10.1016/S0022-5347(05)00645-2. [DOI] [PubMed] [Google Scholar]
- 326.D’Ancona FC. Nonablative minimally invasive thermal therapies in the treatment of symptomatic benign prostatic hyperplasia. Curr Opin Urol. 2008;18:21–27. doi: 10.1097/MOU.0b013e3282f20157. [DOI] [PubMed] [Google Scholar]
- 327.Baumert H, Ballaro A, Dugardin F, Kaisary AV. Laparoscopic versus open simple prostatectomy: A comparative study. J Urol. 2006;175:1691–1694. doi: 10.1016/S0022-5347(05)00986-9. [DOI] [PubMed] [Google Scholar]
- 328.Porpiglia F, Terrone C, Renard J, et al. Transcapsular adenomectomy (Millin): A comparative study, extraperitoneal laparoscopy versus open surgery. Eur Urol. 2006;49:120–126. doi: 10.1016/j.eururo.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 329.Sotelo R, Spaliviero M, Garcia-Sequi A, et al. Laparoscopic retropubic simple prostatectomy. J Urol. 2005;173:757–760. doi: 10.1097/01.ju.0000152651.27143.b0. [DOI] [PubMed] [Google Scholar]
- 330.Vela-Navarrete R, Gonzalez-Enguita C, Garcia-Cardoso JV, et al. The impact of medical therapy on surgery for benign prostatic hyperplasia: A study comparing changes in a decade (1992–2002) BJU Int. 2005;96:1045–1048. doi: 10.1111/j.1464-410X.2005.05735.x. [DOI] [PubMed] [Google Scholar]
- 331.Souverein PC, Erkens JA, de la Rosette JJ, et al. Drug treatment of benign prostatic hyperplasia and hospital admission for BPH-related surgery. Eur Urol. 2003;43(5):528–534. doi: 10.1016/s0302-2838(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 332.Lowe FC. Role of the newer alpha1-adrenergic-receptor antagonists in the treatment of benign prostatic hyperplasia-related lower urinary tract symptoms. Clin Ther. 2004;26(11):1701–1713. doi: 10.1016/j.clinthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 333.Saseen JJ, Maclaughlin EJ. Hypertension. In: DiPiro JT, Talbert RL, Yee G, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York: McGraw-Hill; 2011. pp. 101–135. [Google Scholar]
- 334.American Urological Association Practice Guidelines Committee AUA guidelines on management of benign prostatic hyperplasia (2003). Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–547. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 335.Oades GM, Eaton JD, Kirby RS. The clinical role of alpha-blockers in the treatment of benign prostatic hyperplasia. Curr Urol Rep. 2000;1(2):97–102. doi: 10.1007/s11934-000-0043-z. [DOI] [PubMed] [Google Scholar]
- 336.Roehrborn CG. Efficacy of alpha-adrenergic receptor blockers in the treatment of male lower urinary tract symptoms. Rev Urol. 2009;11(Suppl 1):S1–S8. [PMC free article] [PubMed] [Google Scholar]
- 337.Lepor H. The evolution of alpha-blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2006;8(Suppl 4):S3–S9. [PMC free article] [PubMed] [Google Scholar]
- 338.Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2007;9(4):181–190. [PMC free article] [PubMed] [Google Scholar]
- 339.Clifford GM, Farmer RD. Drug or symptom-induced depression in men treated with alpha 1-blockers for benign prostatic hyperplasia? A nested case-control study. Pharmacoepidemiol Drug Saf. 2002;11(1):55–61. doi: 10.1002/pds.671. [DOI] [PubMed] [Google Scholar]
- 340.Roehrborn CG, Rosen RC. Medical therapy options for aging men with benign prostatic hyperplasia: Focus on alfuzosin 10 mg once daily. Clin Interv Aging. 2008;3(3):511–524. doi: 10.2147/cia.s3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Nix JW, Carson CC. Medical management of benign prostatic hypertrophy. Can J Urol. 2007;14(Suppl 1):53–57. [PubMed] [Google Scholar]
- 342.Schilit S, Benzeroual KE. Silodosin: A selective alpha1A-adrenergic receptor antagonist for the treatment of benign prostatic hyperplasia. Clin Ther. 2009;31(11):2489–2502. doi: 10.1016/j.clinthera.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 343.Blouin MC, Blouin J, Perreault S, et al. Intraoperative floppy-iris syndrome associated with alpha1-adrenoreceptors: Comparison of tamsulosin and alfuzosin. J Cataract Refract Surg. 2007;33(7):1227–1234. doi: 10.1016/j.jcrs.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 344.Chatziralli IP, Sergentanis TN. Risk factors for intraoperative floppy iris syndrome: A meta-analysis. Ophthalmology. 2011;118:730–735. doi: 10.1016/j.ophtha.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 345.Cantrell MA, Bream-Rouwenhorst HR, Steffensmeier A, et al. Intra-operative floppy iris syndrome associated with alpha1-adrenergic receptor antagonists. Ann Pharmacother. 2008;42(4):558–563. doi: 10.1345/aph.1K679. [DOI] [PubMed] [Google Scholar]
- 346.ALLHAT Collaborative Research Group Major cardiovascular events in hypertensive patients randomized to doxazosin vs. chlorthalidone: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 347.Schulman CC. Tamsulosin modified release and oral controlled absorption system in the management of lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Exp Opin Drug Metab Toxicol. 2008;4(6):771–782. doi: 10.1517/17425255.4.6.771. [DOI] [PubMed] [Google Scholar]
- 348.Noble AJ, Chess-Williams R, Couldwell C, et al. The effects of tamsulosin, a high affinity antagonist at functional alpha1A and alpha1D-adrenoceptor subtypes. Br J Pharmacol. 1997;120:231–238. doi: 10.1038/sj.bjp.0700907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.MacDonald R, Wilt TJ. Alfuzosin for treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia: A systemic review of efficacy and adverse effects. Urology. 2005;66(4):780–788. doi: 10.1016/j.urology.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 350.Nickel JC. Comparision of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(Suppl 9):S31–S39. [PMC free article] [PubMed] [Google Scholar]
- 351.Kumar VL, Wahane VD. Current status of 5 alpha-reductase inhibitors in the treatment of benign hyperplasia of prostate. Indian J Med Sci. 2008;62(4):167–175. [PubMed] [Google Scholar]
- 352.Sandhu JS, Te AE. The role of 5-alpha-reductase inhibition as monotherapy in view of the MTOPS data. Curr Urol Rep. 2004;5(4):274–279. doi: 10.1007/s11934-004-0050-6. [DOI] [PubMed] [Google Scholar]
- 353.Anderson JT, Nickel JC, Marshall VR, et al. Finasteride significantly reduces acute urinary retention and the need for surgery in patients with symptomatic benign prostatic hyperplasia. Urology. 1997;49:839–845. doi: 10.1016/s0090-4295(97)00185-4. [DOI] [PubMed] [Google Scholar]
- 354.Lepor H, Willford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostate hyperplasia. N Engl J Med. 1996;335:533–540. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 355.Yang Y, Zhao XF, Li HZ, et al. Efficacy and safety of combined therapy with terazosin and tolteradine for patients with lower urinary tract symptoms associated with benign prostatic hyperplasia: A prospective study. Chin Med J. 2007;120(5):370–374. [PubMed] [Google Scholar]
- 356.Naslund MJ, Miner M. A review of the clinical efficacy and safety of 5-alpha-reductase inhibitors for the enlarged prostate. Clin Ther. 2007;29(1):17–25. doi: 10.1016/j.clinthera.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 357.Scott LJ. Dutasteride: A review of its use in the management of prostate disorders. Drugs. 2008;68(4):463–485. doi: 10.2165/00003495-200868040-00008. [DOI] [PubMed] [Google Scholar]
- 358.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 359.Greco KA, McVary KT. The role of combination medical therapy in benign prostatic hyperplasia. Int J Impot Res. 2008;20(Suppl 3):S33–S43. doi: 10.1038/ijir.2008.51. [DOI] [PubMed] [Google Scholar]
- 360.Roehrborn CG, Barkin J, Siami P. Clinical outcomes after combined therapy with dutasteride plus tamsulosin or either monotherapy in men with benign prostatic hyperplasia (BPH) by baseline characteristics: 4-year results from the randomized, double-blind Combination of Avodart and Tamsulosin (CombAT) trial. BJU Int. 2011;107(6):946–954. doi: 10.1111/j.1464-410X.2011.10124.x. [DOI] [PubMed] [Google Scholar]
- 361.McVary KT. A review of combination therapy in patients with benign prostatic hyperplasia. Clin Ther. 2007;29(3):387–398. doi: 10.1016/s0149-2918(07)80077-4. [DOI] [PubMed] [Google Scholar]
- 362.Gann PH. Prostate cancer: A closer look at the initial results from the REDUCE trial. Nat Rev Urol. 2010;7(10):535–537. doi: 10.1038/nrurol.2010.144. [DOI] [PubMed] [Google Scholar]
- 363.Andriole G, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 364.Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 365.Assessment of dutasteride (Avodart) in extending the time to progression of low-risk, localized prostate cancer in men. Jun 27, 2011. Available at: http://clinicaltrials.gov/ct2/show/NCT0036311. Accessed February 13, 2012.
- 366.Fleshner N, Gomella LG, Cookson MS. Delay in the progression of low-risk prostate cancer: Rationale and design of the Reduction by Dutasteride of Clinical Progression Events in Expectant Management (REDEEM) trial. Contemp Clin Trials. 2007;28(6):763–769. doi: 10.1016/j.cct.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 367.Andriole GL. Overview of pivotal studies for prostate cancer risk reduction, past and present. Urology. 2009;73(5 Suppl):S36–S43. doi: 10.1016/j.urology.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 368.Roehrborn CG, Kaplan SA, Jones JS, et al. Tolterodine extended release with or without tamsulosin in men with lower urinary tract symptoms including overactive bladder symptoms: Effects of prostate size. Eur Urol. 2009;55(2):472–479. doi: 10.1016/j.eururo.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 369.Chapple C. Antimuscarinics in men with lower urinary tract symptoms suggestive of bladder outlet obstruction due to benign prostatic hyperplasia. Curr Opin Urol. 2010;20(1):43–48. doi: 10.1097/MOU.0b013e3283330862. [DOI] [PubMed] [Google Scholar]
- 370.Chapple C, Herschorn S, Abrams P, et al. Tolterodine treatment improves storage symptoms suggestive of overactive bladder in men treated with alpha-blockers. Eur Urol. 2009;56:534–543. doi: 10.1016/j.eururo.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 371.Kaplan SA, Roehrborn CG, Abrams P. Antimuscarinics for treatment of storage lower urinary tract symptoms in men: A systematic review. Int J Clin Pract. 2011;65(4):487–507. doi: 10.1111/j.1742-1241.2010.02611.x. [DOI] [PubMed] [Google Scholar]
- 372.Kaplan SA, Roehrborn CG, Chancellor M, et al. Extended-release tolterodine with or without tamsulosin in men with lower urinary tract symptoms and overactive bladder: Effects on urinary symptoms assessed by the International Prostate Symptom Score. BJU Int. 2008;102(9):1133–1139. doi: 10.1111/j.1464-410X.2008.07761.x. [DOI] [PubMed] [Google Scholar]
- 373.Rovner ES, Kreder K, Sussman DO, et al. Effect of tolterodine extended release with or without tamsulosin on measures of urgency and patient reported outcomes in men with lower urinary tract symptoms. J Urol. 2008;180(3):1034–1041. doi: 10.1016/j.juro.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 374.Masumori N, Tsukamoto T, Yanase M, et al. The add-on effect of solifenacin for patients with remaining overactive bladder after treatment with tamsulosin for lower urinary tract symptoms suggestive of benign prostatic obstuction. Adv Urol. 2010 Oct 26; doi: 10.1155/2010/205251. (online). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Govonlu S, Wooding FG. Pharmacological management of benign prostatic hypertrophy with phosphodiesterase-5 inhibitors. Consult Pharm. 2009;24(10):769–771. doi: 10.4140/tcp.n.2009.769. [DOI] [PubMed] [Google Scholar]
- 376.Andersson K-E, de Groat WC, McVary KT, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: Pathophysiology and mechanism(s) of action. Neurourol Urodyn. 2011;30(3):292–301. doi: 10.1002/nau.20999. [DOI] [PubMed] [Google Scholar]
- 377.Egerdie RB, Auerbach S, Roehrborn CG, et al. Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: Results of a randomized, placebo-controlled, double-blind study. J Sex Med. 2012;9(1):271–281. doi: 10.1111/j.1743-6109.2011.02504.x. [DOI] [PubMed] [Google Scholar]
- 378.Liguori G, Trombetta C, De Giorgi G, et al. Efficacy and safety of combined oral therapy with tadalafil and alfuzosin: An integrated approach to the management of patients with lower urinary tract symptoms and erectile dysfunction: Preliminary report. J Sex Med. 2009;6(2):544–552. doi: 10.1111/j.1743-6109.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 379.Porst H, Kim ED, Casabé AR. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60(5):1105–1113. doi: 10.1016/j.eururo.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 380.Lee JY, Park SY, Jeong TY. Combined tadalafil and {alpha}-blocker therapy for benign prostatic hyperplasia in patients with erectile dysfunction: A multicenter, prospective study. J Androl. 2011 Aug 25; doi: 10.2164/jandrol.111.013185. (online). [DOI] [PubMed] [Google Scholar]
- 381.Chuang YC, Chiang PH, Yoshimura N. Sustained beneficial effects of intraprostatic botulinum toxin type A on lower urinary tract symptoms and quality of life in men with benign prostatic hyperplasia. BJU Int. 2006;98(5):1033–1037. doi: 10.1111/j.1464-410X.2006.06479.x. [DOI] [PubMed] [Google Scholar]
- 382.Ogushi T, Takahashi S. Effect of Chinese herbal medicine on overactive bladder. Hinyokika Kiyo. 2007;53(12):857–862. [PubMed] [Google Scholar]
- 383.Barry MJ. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: A randomized trial. JAMA. 2011;306(12):1344–1351. doi: 10.1001/jama.2011.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kawachi Y, Sakurai T, Sugimura S. Long-term treatment and prognostic factors of alpha 1-blockers for lower urinary tract symptoms associated with benign prostatic hyperplasia: A pilot study comparing naftopidil and tamsulosin hydrochloride. Scand J Urol Nephrol. 2010;44(1):38–45. doi: 10.3109/00365590903335221. [DOI] [PubMed] [Google Scholar]
- 385.Idan A, Griffiths KA, Harwood DT, et al. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease: A randomized, placebo-controlled trial. Ann Intern Med. 2010;153(10):621–632. doi: 10.7326/0003-4819-153-10-201011160-00004. [DOI] [PubMed] [Google Scholar]
- 386.Mahajan ST, Elkadry EA, Kenton KS, et al. Patient-centered surgical outcomes: The impact of goal achievement and urge incontinence on patient satisfaction one year after surgery. Am J Obstet Gynecol. 2006;194(3):722–728. doi: 10.1016/j.ajog.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 387.Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: Variation in prevalence estimates and risk factors. Obstet Gynecol. 2008;111(2 Part 1):324–331. doi: 10.1097/01.AOG.0000267220.48987.17. [DOI] [PubMed] [Google Scholar]
- 388.Rovner ES, Wyman J, Lackner T, et al. Urinary incontinence. In: DiPiro JT, Talbert RL, Yee G, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York: McGraw-Hill; 2011. pp. 1467–1485. [Google Scholar]
- 389.Thüroff JW, Abrams P, Andersson K-E, et al. EAU guidelines on urinary incontinence. Eur Urol. 2011;59:387–400. doi: 10.1016/j.eururo.2010.11.021. [DOI] [PubMed] [Google Scholar]