Abstract
Preterm births have increased by 27% over the last decade in the U.S. The main ingredient in Makena, 17P, is less expensive at compounding pharmacies, but this method is not FDA-approved. The authors discuss the quality, safety, and use of Makena and compounded 17P; the potential for liability; cost considerations; and recommended restrictions for the product’s use.
INTRODUCTION
Twelve percent of all births in the U.S. are premature.1 Preterm births have increased by 27% over the last decade in the U.S., accounting for 85% of all perinatal morbidity and mortality. Preterm delivery is defined as birth before 37 weeks of gestation. The steady increase in premature births is a public health concern because it is associated with increased infant mortality as well as long-term social and educational costs.
Predisposing risk factors for preterm delivery include a documented history of a previous singleton preterm delivery after less than 37 weeks of gestation, multiple gestations, short cervical length, body weight less than 50 kg (110 pounds), bleeding, and African-American ancestry.1 The most common risk factor is a history of preterm birth.1 According to the American College of Obstetricians and Gynecologists (ACOG), women with a singleton pregnancy and a documented history of spontaneous birth at less than 37 weeks or premature rupture of the amniotic membrane should be offered progesterone supplementation.2
CURRENT THERAPY
Two progesterone dosage forms are used to reduce pre-term labor––natural progesterone administered vaginally, and 17-alpha hydroxyprogesterone caproate (17P), a synthetic pro-gestin, administered via the intramuscular (IM) route. Natural progesterone has been shown to significantly reduce premature birth in women with a short cervix.3 Although patients may benefit from this alternative treatment, the ACOG does not recommend routine screening to measure cervical length.
As the result of a trial supported by the National Institute of Child Health and Human Development (NICHD),4 which concluded that 17P significantly reduces the rate of preterm delivery, injectable 17P is the only drug currently used as first-line therapy to reduce the risk of preterm birth. Although the mechanism by which 17P reduces the risk of recurrent preterm birth is unknown, the drug’s actions on the myometrium in pregnancy include relaxation of myometrial smooth muscle, oxytocin blockade, and inhibition of gap junction formation.5
17P is metabolized hepatically via extensive phase I reactions (reduction, hydroxylation, and conjugation) and phase II reactions (sulfation, glucuronidation, and acetylation), mediated by cytochrome P450 (CYP) 3A4 and 3A5, with retention of caproate. 17P binds extensively to plasma proteins, including albumin and corticosteroid-binding globulins, with a mean half-life of 7.8 (± 3.0) days. 17P is eliminated in feces (about 50%) and urine (about 30%) as free steroids and conjugated metabolites.6
For more than 40 years, 17P, under the trade name Delalutin (Bristol-Myers Squibb), was used to treat female hormone disturbances, to prevent recurrent or threatened abortion, and to treat uterine cancer, but the drug was withdrawn in 2000 because of the availability of superior treatments for these conditions. In 2003, the NICHD study was published,4 and the ACOG Committee on Obstetric Practice supported the treatment in a select group of women for the prevention of preterm labor.7 Since the NICHD study was published, 17P has been available through local and national compounding specialty pharmacies, although it was never approved by the FDA to prevent preterm labor. Thus, 17P has been the standard of care for the past 8 years. However, the drug is not without safety problems. In 2006, an FDA advisory committee discussed a possible association between 17P and second-trimester miscarriages.
Clinical head-to-head studies are not available for 17P, because it is the only drug indicated for reducing the risk of preterm labor. The landmark trial by the NICHD demonstrated the efficacy and safety of 17P in pregnant women who were at an increased risk of preterm labor.4 Women who received 17P prophylactically were more likely to carry their pregnancy to term.
A follow-up study demonstrated the safety of 17P in children born to mothers who received the drug.8 In another study, women with a previous history of more than one preterm delivery had a significantly higher incidence of spontaneous preterm labor before 34 weeks of gestation compared with women with a history of only one prior preterm delivery when receiving 17P prophylactically.9 Clinical studies of 17P are summarized in Table 1.
Table 1.
Summary of Clinical Trials of 17-alpha Hydroxyprogesterone (17P)
| Author | Sample Size | Intervention | Primary Outcome | Results | Conclusion |
|---|---|---|---|---|---|
| Meis et al.4 | 463 | Castor oil placebo vs. 17P injection (250 mg) | Preterm delivery before 37 weeks of gestation | Incidence of delivery at less than 37 weeks of gestation: 36.3% (17P) vs. 54.9% (placebo) RR, 0.66 (CI, 0.54–0.81) Incidence of delivery at less than 35 weeks of gestation: 20.6% (17P) vs. 30.7% (placebo) RR, 0.67 (CI, 0.48–0.93) Delivery at less than 32 weeks of gestation: 11.4% (17P) vs. 19.6% (placebo) RR, 0.58 (CI, 0.37–0.91) |
Women who received prophylactic 17P were more likely to carry their pregnancy to term, thereby reducing the rate of recurrent preterm delivery. 17P reduced the likelihood of enterocolitis, intraventricular hemorrhage, and the need for supplemental oxygen. |
| Northen et al.8 | 278 | Follow-up to study by Meis et al.4 | Health outcomes in children exposed to 17P in second and third trimesters | No significant differences between 17P and placebo
|
Use of 17P was safe in children born to mothers who received the drug. |
| Joy et al.9 | 1,177 | Formulation used in study by Meis et al. in outpatient setting at 16 to 26.9 weeks of gestation4 | Factors predisposing high-risk women receiving 17P to develop preterm labor (retrospective analysis) | No significant differences between women hospitalized and diagnosed with preterm labor at less than 34 weeks of gestation and those without preterm labor. Recurrent preterm labor occurred in 73.3% of women with preterm labor at less than 34 weeks. Maternal age, marital status, race, tobacco use, cervical cerclage (tracheloplasty), gestational age at start of 17P, and Medicaid status were similar between groups. |
Women with a history of more than one preterm delivery had a significantly higher incidence and rate of spontaneous preterm labor at less than 34 weeks compared with women with a history of only one previous preterm delivery when administered 17P prophylactically. |
CI = confidence interval; RR = relative risk.
To create a standardized formulation of 17P, the FDA approved Makena (KV Pharmaceutical Company), an orphan drug, on February 4, 2011. The approval was based on the premise that because 17P is a sterile injectable drug, a product approved according to FDA guidelines would provide “greater assurance of safety.”10 Makena was approved via an accelerated approval process based on improvement in the proportion of women who delivered after 37 weeks of gestation. No controlled trials have demonstrated a direct clinical benefit of Makena, such as improvements in neonatal mortality and morbidity.
The approved dosage of Makena is 250 mg (1 mL) weekly via IM injection, initiated at between 16 and 20 weeks of gestation and continued until week 37 or delivery, whichever occurs first.6,11
PHARMACOECONOMICS
Costs
The initial acquisition cost of Makena was set at $1,440 per injection; thus, for 18 to 20 injections, the total cost was about $30,000 per pregnancy. The drug’s initial cost was subsequently cut by 55%, to $690 per injection (about $15,000 per pregnancy) and was supplemented by Medicaid rebates and expanded patient-assistance programs. Because the cost of Makena is almost 50 times greater than that of the compounded form of 17P ($15 per injection, for a total cost of about $300 for 18 to 20 injections), the use of 17P from a compounding pharmacy offers a significant cost savings compared with Makena.
On March 30, 2011, in direct response to the pricing of Makena, the FDA released the following statement:12
In order to support access to this important drug, at this time and under this unique situation, FDA does not intend to take enforcement action against pharmacies that compound hydroxyprogesterone caproate based on a valid prescription for an individually identified patient unless the compounded products are unsafe, of substandard quality, or are not being compounded in accordance with appropriate standards for compounding sterile products.
The FDA has always been afforded proper deference in interpreting its enabling statute and possesses enforcement discretion with respect to violations of the Federal Food, Drug, and Cosmetic Act for compounded drug products.13 The FDA prioritizes enforcement actions related to compounded drugs using a risk-based approach, giving the highest enforcement priority to pharmacies that compound products that are causing harm or that amount to health fraud. The FDA does not ordinarily exercise its enforcement authority against a compounding pharmacy if the medication is dispensed within the confines of a pharmacist–prescriber–patient relationship; if the drug is adequately labeled to ensure proper use; and if the pharmacy adheres to the National Association of Boards of Pharmacy Good Compounding Practices or to equivalent state good-compounding regulations.
17P continues to be available at a lower cost to patients at compounding pharmacies. On April 1, 2011, the ACOG and the Society for Maternal Fetal Medicine (SMFM) released a joint statement pertaining to the FDA’s decision about the use of compounded 17P:14
[While] there are clear benefits to having an FDA-approved version of 17P, there is no evidence that Makena is more effective or safer than the currently used compounded version. In fact, the evidence used to obtain FDA approval for Makena relied primarily on data obtained using the compounded product.
Subsequently, KV, the ACOG, and the SMFM issued a revised statement to clarify that the NICHD study was conducted using Makena and that compounded Makena and 17P are not identical. Although the compounded Makena and 17P contain the same active ingredient in the same concentration, with castor oil as an inactive ingredient, only Makena contains preservatives (benzyl benzoate and benzyl alcohol). Based on the active ingredient, however, compounded 17P is considered clinically interchangeable with Makena. The ACOG and the SMFM have reiterated that access to FDA-approved Makena for patients with the appropriate clinical indication is an important public health policy.
In October 2011, the manufacturer conducted testing regarding variability in the potency and purity of samples of bulk 17P active ingredients and compounded 17P products. Testing determined that eight out of 10 active ingredient samples and 16 out of 30 finished product vials failed to meet FDA standards for known impurities. One of 10 active ingredient samples and five of 30 finished product vials failed FDA potency standards. At that time, FDA issued the following statement, “…As with other approved drugs, greater assurance of safety and effectiveness is generally provided by the approved product than by a compounded product.”15 The International Academy of Compounding Pharmacists has stated its intention to conduct an independent analysis.
On November 9, 2011 the FDA announced it would review potency and purity data on the compounded versions of 17P. On June 15, 2012, FDA released its findings. All 16 samples of the active pharmaceutical ingredient passed the USP tests for potency and total purity used in the Makena NDA. However, all 16 failed to meet the limit for unidentified impurities used in the Makena application. While the impurities exceed the levels allowed in Makena, the FDA stated that they “do not raise safety concerns.”
The FDA also tested 13 samples of compounded 17P. All samples met the Makena standard for total purity, but one sample was found to be subpotent. Two samples failed to meet the standard for unidentified impurities.15 The FDA stated: “The FDA emphasizes that it is applying its normal enforcement policies for compounded drugs to compounded hydroxyprogesterone caproate. The compounding of any drug, including hydroxyprogesterone caproate, should not exceed the scope of traditional pharmacy compounding.”
The FDA also once again identified what it will not consider to be compounding: “Compounding large volumes of drugs that are copies of FDA-approved drugs circumvents important public health requirements, including the Federal Food, Drug, and Cosmetic Act.” Thus, although FDA will allow the continued compounding of 17P by pharmacies, its statement is vague.
Orphan Product Status
As an orphan product, Makena received 7 years of exclusivity. By statutory definition, orphan products must be clinically indicated for not more than 200,000 patients; otherwise, if the patient population exceeded 200,000, the manufacturer would be unlikely to recoup its investment in research and development (R&D). According to the March of Dimes, one in eight births in the U.S. is premature, which equates to the need for treatment of nearly 200,000 mothers.
KV claims that it spent more than $1 billion to bring Makena to market and $60 million for research. However, in addition to receiving orphan drug status and 7 years of exclusivity under the Orphan Drug Act, along with accelerated approval and expedited review, the manufacturer received considerable federal assistance in the form of government-funded research. The FDA clearly noted in its statement that the approval of Makena was based on such research. Some would argue that perhaps the Orphan Drug Act is too generous in allowing companies to designate readily available drugs, such as 17P or colchicine, as orphan drugs.
Availability
The FDA’s approval of Makena was also intended to increase access to the drug and to simplify its acquisition. However, KV limited the distribution of Makena to CuraScript, Inc., and CVS Caremark Corp., thereby restricting access and increasing the price for plan sponsors that did not have those companies within their networks. Moreover, it was anticipated that the original cost of the drug would make health care, in terms of prenatal care, less affordable for everyone with commercial insurance and would cause major issues for Medicaid budgets across the 50 states.
On March 30, 2011, the Center for Medicaid, CHIP (Children’s Health Insurance Program) and Survey & Certification (CMCS) announced that compounded 17P would be covered by state Medicaid plans. The announcement said that the states might choose to pay for the extemporaneously compounded hydroxyprogesterone caproate as an active pharmaceutical ingredient that can be covered under the “medical supplies, equipment, and appliances suitable for use in the home” portion of home health.16
The International Academy of Compounding Pharmacists locator service (www.iacprx.org) was suggested as a resource to identify compounding pharmacies.
Even at the reduced price of $690 per injection for Makena, the March of Dimes ended its 10-year relationship with KV. The ACOG stated, “This lower price still remains prohibitively high for a safe and effective treatment that is currently available at a much lower price in the form of compounded 17P.”14
The previous statement from the FDA specified that it would not undertake enforcement against compounding of the FDA-approved product (Makena) “in order to support access to this important drug at this time and under this unique situation.”11
FORMULARY CONSIDERATIONS
Health Care Plans
Health care plans currently offer four options: (1) they cover compounded 17P only; (2) they cover Makena with step therapy, requiring that compounded 17P be used first; (3) they cover Makena and compounded 17P equally; or (4) they cover Makena only.
Most health plans recognize that 17P, the active ingredient in both 17P and Makena, is present in the same concentration in both formulations and should have the same efficacy when 17P is compounded in a compounding pharmacy that complies with applicable regulations. As such, health plans have placed the compounded 17P on their formularies as first-line therapy, with precertification or prior approval required for Makena. Thus, most plans reqiring prior authorization have been covering both Makena and compounded 17P for years.17 Precertification helps to ensure that the treatment is both medically necessary and cost-effective.
Some plans are continuing to cover compounded 17P without prior authorization when billed with Healthcare Common Procedure Coding System (HCPCS) code J3490 (unclassified drugs) or J2675 (progesterone injection). 17P is also being covered under HCPCS code Q2042 (hydroxyprogesterone caproate injection) with the Current Procedural Terminology (CPT) code 96372 for therapeutic, prophylactic, or diagnostic injection.
Several health plans (e.g., Express Scripts) cover Makena only. For health plans with 200 members who require 17P annually, for example, this translates to about $3 million in plan costs. Even though 17P does not completely prevent all preterm births and the costs associated with resultant preterm infants (e.g., the neonatal intensive-care unit), plans that deny coverage for compounded 17P or Makena run the risk of incurring such costs for women who do not take the medication and who go into preterm labor. In addition, the Institute of Medicine estimated that the average direct cost of medical care for a preterm infant in the U.S. is approximately $33,000.18
Most health plans are limiting the use of Makena to FDA-approved indications and permit use of the drug only when all of the following criteria have been met: (1) a singleton spontaneous preterm birth (prior to 37 weeks of gestation) has been documented, (2) the member is pregnant with a single fetus, and (3) the prescribing physician is a specialist in obstetrics and gynecology.
For the most part, state Medicaid programs have also chosen the option in which patients must use compounded 17P first. Makena may be authorized based on the availability of compounding facilities or if the prescriber can document compelling and decisive clinical factors necessitating Makena.
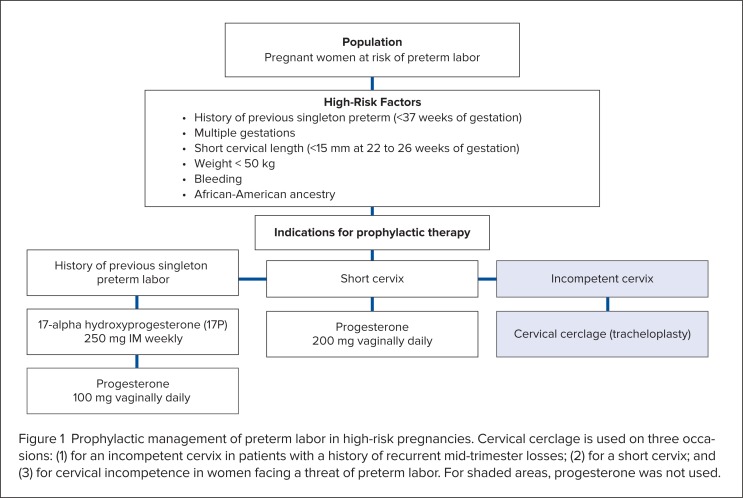
Examples of the therapeutic limitations that have been placed on Makena are shown in Table 2 and Figure 1.19,20
Table 2.
Guidelines for Health Plan Coverage of Makena
|
Figure 1.
Prophylactic management of preterm labor in high-risk pregnancies. Cervical cerclage is used on three occasions: (1) for an incompetent cervix in patients with a history of recurrent mid-trimester losses; (2) for a short cervix; and (3) for cervical incompetence in women facing a threat of preterm labor. For shaded areas, progesterone was not used.
Health Care Systems
The FDA’s approval of the 17P commercial product put hospital P&T committees in an untenable position, in that an equivalent non-approved formulation, compounded 17P, as used in the past, was now pitted against an FDA-approved product, Makena, at many times the price. Complicating matters further, alternative equivalent therapy for recurrent preterm labor is not available, because 17P is the only FDA-approved, first-line drug for preventing recurrent preterm labor in high-risk women.
Because extemporaneous compounding of injectable 17P would not be in violation of the law, would health system resources be better allocated by purchasing the active 17P powder (a formula is provided by the U.S. Pharmacopeia) or by outsourcing the compounding of 17P to a compounding pharmacy?
Should Makena be considered for addition to the formulary or only if other means of obtaining 17P are exhausted?
Health care systems throughout the U.S. are grappling with these questions. Our P&T committee experience at New York–Presbyterian, the largest hospital in New York City, is discussed next.
The addition of Makena to New York–Presbyterian Hospital’s formulary was requested because the non–FDA-approved 17P could not be identified by the pharmacy. Thus, staff personnel, including physicians, registered nurses, and nurse practitioners, could not administer the drug because hospital policy does not allow the use of externally acquired medications for inpatient use. New York–Presbyterian Hospital’s policy prevents patients from bringing in their own supply of 17P to be administered during their hospital stay. Physicians often have difficulty obtaining medications for administration, especially if they are not on the formulary.
The requestor anticipated the use of Makena for 15 to 20 patients per month, with an average treatment duration of 20 weeks per patient, and noted that the use of Makena would be restricted to the antepartum service. The estimated cost of Makena to the hospital was projected at $3,120,000 per year (20 patients per month × 12 months = 240 patients per year × average duration of 20 weeks = 4,800 injections × $650 per injection = $3,120,000 per year).
Many topics were debated when the possibility of adding Makena was introduced to the P&T committee members. These issues included the quality of compounded 17P, the drug’s cost-effectiveness, legal and social liabilities, health insurance coverage, and the ease of obtaining and administering the treatment to patients. Although outside vendors can compound 17P, quality assurance was considered variable compared with that of FDA-approved Makena, which undergoes strict quality-testing procedures. If the hospital contracted with an outside vendor to provide compounded 17P in the presence of an FDA-approved product, the hospital would be liable for a possible lawsuit if the compounded version failed to prevent preterm delivery and/or resulted in an adverse outcome to either the neonate or the mother. This liability was a disadvantage, because neither compounded 17P nor Makena has proved effective in eliminating preterm labor completely. The potential cost of liability far outweighed the cost of adding Makena to New York–Presbyterian Hospital’s formulary.
In addition to the potential legal costs of not providing Makena, societal costs were considered as well. Premature infants are at risk for many complications, both at birth and throughout their life cycle, which has the potential to cost the hospital more than the price of the drug itself, ultimately consuming resources that could possibly benefit other patients. It was decided that the time spent by hospital personnel in acquiring the drug via a third-party vendor would be better spent in patient care and safety, adding yet another reason to approve the formulary-addition request––not to mention the cost to society if the premature neonate developed potential learning disabilities when mature, which would require additional resources, personnel, and money for education and work-related help.
A concern raised during the formulary review of Makena was based on a misinterpretation of the need to acquire the multidose vial for single-patient use. Makena is available in only one size, 5 mL––enough for five injections. It was originally presumed that if a health care practitioner needed only one injection, most of the vial’s contents (worth about $3,000) would be wasted. It was thought that because of the forms provided, each vial had to be registered for use in one patient, thus resulting in increased costs and potential waste if the patient did not need the five doses provided per vial. However, because this is a multidose vial, pharmacies have the option of dispensing multiple patient doses of 1 mL in a single vial.
After much deliberation, the P&T committee added Makena to the formulary, but its use was restricted to the antepartum service, specifically to women considered at high risk for preterm delivery, as defined by ACOG guidelines. It was agreed that restriction and authorization would act as a cost-containment method and would thus make Makena available to the potential 240 patients per year at risk for recurrent pre-term labor.
Most of the recent requests for formulary additions have the potential to add millions of dollars to the pharmacy drug-purchasing budget. The P&T committee’s responsibility to critically evaluate these requests remains very important, and each formulary-addition request receives extra scrutiny, especially when less expensive alternatives are available or when efficacy or safety is a concern. The addition of Makena proved controversial, not because another drug could have been used as an alternative but because the same drug could have been used as an alternative, albeit at a lower cost and via an independent distributor. The only difference between compounded 17P and Makena, in terms of final outcomes, was the quality assurance provided by an FDA-approved product compared with the potential variability in quality associated with compounding practices of pharmacists who are trained to “compound and dispense” medications.
The question is whether having an FDA-approved product actually improved efficacy or patient safety or whether patient safety was never in jeopardy; thus, did the introduction of Makena simply provide another profit avenue for pharmaceutical companies and undermine compounding pharmacies as compounders and dispensers of quality medications? The answer is not yet known.
Other Liability Issues
It is well established that using a compounded drug instead of an FDA-approved medication exposes physicians to liability if the patient has an untoward outcome. Quality-control procedures in compounding pharmacies are rarely as stringent or as comprehensive as the good manufacturing practices required by the FDA for approved products. In the case of a therapeutic failure of compounded 17P when Makena is available, the physician would be exposed to legal liabilities because the FDA does not test or approve compounded drugs.
Several studies have reported quality problems with compounded medications, including subpotency, superpotency, and contamination. The final compounded formulation is not an FDA-approved medication because it has not been fully tested for efficacy, safety, potency, sterility, dosage, or even stability. The FDA has stated that “the drugs that pharmacists compound are rarely FDA-approved and thus lack an FDA finding of safety and efficacy.”21 There can be further liability if the patient is not fully informed about the risk of using the compounded substitute.
In the past, if a physician prescribed a compounded product knowing that there was an equivalent medication on the market, that itself was an FDA violation. Compounding is typically used to prepare medications that are not commercially available or that provide an alternative for patients who are allergic to an ingredient in a mass-produced product.22 The FDA has taken the unusual step of announcing that it would allow pharmacies to continue to produce less expensive versions of 17P (at $10 to $20 per dose)––a move aimed at blunting the harsh criticism from Congress and professional groups that erupted after the manufacturer priced the drug at $1,500 per dose and then warned compounders to essentially “cease and desist” via a letter.12
The FDA does not usually recommend that patients use compounded versions of FDA-approved drugs. In fact, the FDA’s statement flies in the face of its own Compliance Policy Guide on compounding.23 The FDA Modernization Act is even clearer, stating that “the pharmacist or physician compounding … may not compound … any drug products that are essentially copies of a commercially available drug product.”24
It is possible that the manufacturer will sue the FDA, arguing that it is disobeying its own regulations and is therefore in violation of the Administrative Procedures Act. Moreover, several state administrative codes or Pharmacy Practice Acts specify that compounding excludes the preparation pursuant to a prescription of drugs or devices that are commercially available.25 The issue may be decided if the FDA analyzes the sterility and potency of the compounded 17P and either corroborates or refutes the data that KV provided.
On November 2, 2011, a class action shareholder lawsuit was filed in the U.S. District Court for the Eastern District of Missouri, alleging that KV Pharmaceutical Company had issued false or misleading statements concerning the exclusive distribution rights for Makena and had falsely claimed that the FDA would enforce those rights. The complaint also alleges that the defendants failed to disclose that the drug’s $1,500 price would reduce the availability of Makena to “low-income and other at-risk groups.”
No class has been certified in this action.26 KV Pharmaceuticals’ stock soared after the FDA’s announcement on November 9, 2011, that it would review the potency and sterility of compounded 17P.
The entire controversy regarding injectable progesterone may become moot if a vaginal progesterone product receives FDA approval for the prevention of preterm birth. A gel product is currently approved for women undergoing fertility treatments and for those with secondary amenorrhea, but the gel was recently denied approval for preterm birth (Progest Gel 8%, Watson/Columbia).27 The ACOG guidelines offer only general recommendations for progesterone use and are not specific to 17P.1 The preferred formulation for progesterone remains unknown, and the ACOG guidelines do not endorse a specific formulation.
Studies continue to substantiate the efficacy of vaginal progesterone. In February 2012, a meta-analysis of five studies confirmed that routine screening with ultrasound of the cervix and treatment with vaginal progesterone can greatly reduce the rate of preterm birth in women with a short cervix and can reduce the complications of prematurity in their infants.28 Vaginal progesterone at a dosage of between 90 and 200 mg daily reduced preterm delivery before week 28 by 50%. This treatment also reduced mortality in premature infants by 43%, respiratory distress syndrome by 52%, a weight of less than 3.5 pounds by 45%, the need for intensive care by 25%, and the need for mechanical ventilation by 34%.26 If vaginal progesterone prevents prematurity at rates that are comparable with or better than those of 17P, then 17P might be abandoned once again.
CONCLUSION
The conflict between defending licensed manufacturers’ exclusivity, which is costly but encourages future research, and not doing so, which may be less expensive initially but may risk future commercially funded research, is especially important in obstetrics and, specifically, in the diseases of pregnancy, for which no new medications have been introduced in the U.S. for decades. Atosiban, an oxytocin receptor antagonist licensed in Europe for uncomplicated preterm labor, exemplifies this conflict. Because of the cost of this drug, many hospitals have declined to add it to their formularies. Instead, less expensive sympathomimetics and off-label nifedipine (e.g., Adalat, Bayer; Procardia, Pfizer) are being used, with hospitals risking liability for using an off-label but less expensive drug.29 The similarity to the present situation with 17P is that the compounded progesterone product is less expensive and is not FDA-approved; the difference is that Makena is not a new drug, and the research was not commercially funded. The distinction is important.
Pharmaceutical companies and Congress need to recognize when the line is blurred between profitable R&D incentives and price gouging. The FDA’s approval of Makena allows an ease of procurement in the presence of an equally safe and efficacious alternative––compounded 17P. The FDA has exempted compounding pharmacies from the restriction to compound manufactured products because of the orphan drug status of Makena and to “support the access to this important drug.”4 This solution avoids excessive drug-related acquisition costs and provides medical personnel with easy access to the drug. Liability issues have surfaced, however, and the manufacturer is making a major effort to have the FDA retract its position.
Current practice with 17P allows women at risk for preterm labor to receive the standard of care. The use of Makena should be limited to the antepartum service, particularly to women who are at high risk for preterm delivery, as defined by the NICHD study4 and ACOG guidelines.2,7
Footnotes
Disclosure: The authors report that they have no financial or commercial relationships in regard to this article.
REFERENCES
- 1.Society for Maternal Fetal Medicine Publications Committee ACOG Committee Opinion No. 419 October 2008 (replaces No. 291, November 2003): Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963–965. doi: 10.1097/AOG.0b013e31818b1ff6. [DOI] [PubMed] [Google Scholar]
- 2.Preventing Preterm Birth: The Role of 17α-Hydroxyprogesterone Caproate. Albany, N.Y: American College of Obstetricians and Gynecologists; Jan, 2009. District II. Available at: http://mail.ny.acog.org/website/17PResourceGuide.pdf. Accessed November 9, 2011. [Google Scholar]
- 3.Da Fonseca E, Celik E, Parra M, et al. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 4.Meis PJ, Klebonoff M, Thorn E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 5.Lexicomp Online. Hydroxyprogesterone caproate. Available at: www.lexi.com; http://online.lexi.com. Accessed February 9, 2011.
- 6.Makena (hydroxyprogesterone caproate injection), prescribing information. St. Louis, Mo: Ther-Rx Corp; Feb, 2011. Available at: www.makena.com/media/PDFs/full-pi.pdf. Accessed February 9, 2011. [Google Scholar]
- 7.ACOG Committee Opinion Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102(5 Part 1):1115–1116. doi: 10.1016/j.obstetgynecol.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Northen AT, Normal GS, Anderson K, et al. Follow-up of children exposed in utero to 17α-hydroxyprogesterone caproate compared with placebo. Obstet Gynecol. 2007;110:865–872. doi: 10.1097/01.AOG.0000281348.51499.bc. [DOI] [PubMed] [Google Scholar]
- 9.Joy S, Rhea DJ, Istwan NB, et al. The risk for preterm labor in women receiving 17 alpha-hydroxyprogesterone caproate prophylaxis for preterm birth prevention. Am J Perinatol. 2010;27(4):343–348. doi: 10.1055/s-0029-1243306. [DOI] [PubMed] [Google Scholar]
- 10.FDA FDA approves drug to reduce risk of preterm birth in at-risk pregnant women. Feb 4, 2011. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm242234.htm. Accessed November 9, 2011.
- 11.Armstrong J. Unintended consequences: The cost of preventing preterm births after FDA approval of a branded version of 17OHP. N Engl J Med. 2011;364:1689–1691. doi: 10.1056/NEJMp1102796. [DOI] [PubMed] [Google Scholar]
- 12.FDA statement on Makena. Mar 30, 2011. Available at www.fda.gov/newsEvents/Newsroom/PressAnnouncements/ucm249025.htm. Accessed November 9, 2011.
- 13.Food, Drug, and Cosmetic Act of 1938, Pub. L. No. 75-717, 52 Stat. 1040 §201(j).
- 14.American College of Obstetricians and Gynecologists Makena price reduction is inadequate. Apr 1, 2011. Available at: www.acog.org/About_ACOG/News_Room/News_Releases/2011/Makena_Price_Reduction_Is_Inadequate. Accessed February 28, 2012.
- 15.Updated FDA Statement on Compounded Versions of Hydroxyprogesterone caproate (the active ingredient in Makena®) Jun 15, 2012. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm308546.htm. Accessed June 15, 2012.
- 16.Center for Medicaid, CHIP, and Survey & Certification (CMCS) CMCS Informational Bulletin. Mar 30, 2011. Makena. Available at: https://www.cms.gov/CMCSBulletins/downloads/Makena-CMCS-Info-Bulletin-03-30-2011.pdf. Accessed November 28, 2011.
- 17.Maas A. FDA OKs compounding of 17P: Plans say they will still cover it. Specialty Pharm News. 2011;8(4):6–7. [Google Scholar]
- 18.Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington, D.C: National Academy Press; 2006. [PubMed] [Google Scholar]
- 19.Farine D, Dodd J, Basso M, et al. The use of progesterone for prevention of preterm birth. J Obstet Gynaecol Can. 2008;30(1):67–71. doi: 10.1016/S1701-2163(16)32716-5. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham FG, Leveno KJ, Bloom SL, et al. Preterm birth. In: Cunningham FG, Williams JW, Leveno KJ, editors. Williams Obstetrics. 23rd ed. New York: McGraw-Hill; 2011. pp. 804–831. chapter 36. [Google Scholar]
- 21.FDA Statement on Makena. Mar 30, 2011. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm249025.htm. Accessed June 15, 2012.
- 22.Western States, 535 U.S. at 360-66
- 23.FDA Inspections, Compliance, Enforcement, and Criminal Investigations. Section 460.200: Pharmacy Compounding. May 29, 2002. Available at www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm116791.htm. Accessed November 9, 2011.
- 24.Food and Drug Modernization and Accountability Act of 1997. Act. 21 U.S.C. § 353a(b)(1)(D).
- 25.Florida Administrative Code Ann. 64B16-27.700(1).
- 26.Reuters. Law offices of Howard G. Smith announces class action lawsuit against KV Pharmaceutical Company. Nov 2, 2011. Available at: www.reuters.com/article/2011/11/03/idUS24830+03-Nov-2011+BW20111103. Accessed November 9, 2011.
- 27.Progesterone Vaginal Gel 8% (Watson Pharmaceuticals/Columbia Laboratories). Available at: http://ir.watson.com/phoenix.zhtml?c=65778&p=irol-newsArticle&ID=1665690&highlight. Accessed June 15, 2012.
- 28.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neo natal morbidity: A systematic review and meta-analysis of individual patient data. Am J Obstet Gynecol. 2012;206:124.e1–124.e19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornton J. The drugs we deserve. Br J Obstet Gynecol. 2003;110:969–970. [PubMed] [Google Scholar]