Abstract
Fospropofol disodium (Lusedra) for anesthesia sedation
INTRODUCTION
Sedation is routinely used for various procedures as well as for mechanically ventilated, critically ill patients in an effort to decrease anxiety, reduce the patient’s memory of the event, improve outcomes, and make patients more comfortable.1,2 The preferred agents for procedural sedation include benzodiazepines alone or in combination with opioids.
Midazolam (Versed, Roche), the most commonly used benzodiazepine for procedural sedation, has a quick onset of action and a short duration of effect and possesses amnestic properties.2 However, it is metabolized by cytochrome P450 (CYP) 3A4, resulting in a high potential for drug–drug interactions, and it is associated with a long duration of cognitive recovery.1,3
Propofol lipid emulsion is considered an alternative to the benzodiazepines for procedural sedation as well as for sedation of critically ill patients receiving mechanical ventilation. Although the onset is rapid and recovery is faster, it also has the potential to cause hypotension, cardiac arrhythmias, hypertriglyceridemia, pancreatitis, and metabolic acidosis. Furthermore, midazolam is contraindicated in patients with an allergy to eggs or soybeans.1,3,4
Fospropofol disodium (Lusedra, Eisai), a sedative–hypnotic agent, was approved by the FDA on December 12, 2008, for monitored anesthesia-care sedation in patients who are undergoing diagnostic or therapeutic procedures. This article provides an overview of the pharmacokinetics, pharmacodynamics, clinical efficacy, and safety of fospropofol.
PHARMACOLOGY
Also known as Aquavan Injection (Guilford) and GPI 15715, fospropofol disodium is a water-soluble prodrug of propofol, chemically described as 2,6-diisopropylphenol methoxyphosphonic acid.5,6 Fospropofol disodium is hydrolyzed to propofol via endothelial cell phosphatases.7 Propofol, the active metabolite of fospropofol disodium, crosses the blood–brain barrier and binds to the gamma-aminobutyric acid-A (GABAA) receptor, resulting in enhanced GABA activity.7 It also inhibits the N-methyl-d-aspartate (NMDA) glutamate receptor, resulting in a sedative effect.5
PHARMACOKINETICS AND PHARMACODYNAMICS
Fospropofol disodium follows a two-compartment model, whereas the propofol liberated from fospropofol follows a three-compartment model.6,8 Fospropofol disodium has a molecular weight of 332.24 and a molecular weight ratio of fospropofol to propofol of 1.86:1.6 The molecular formula is C13H19O5PNa2. The structural formula is shown in Figure 1.
Figure 1.
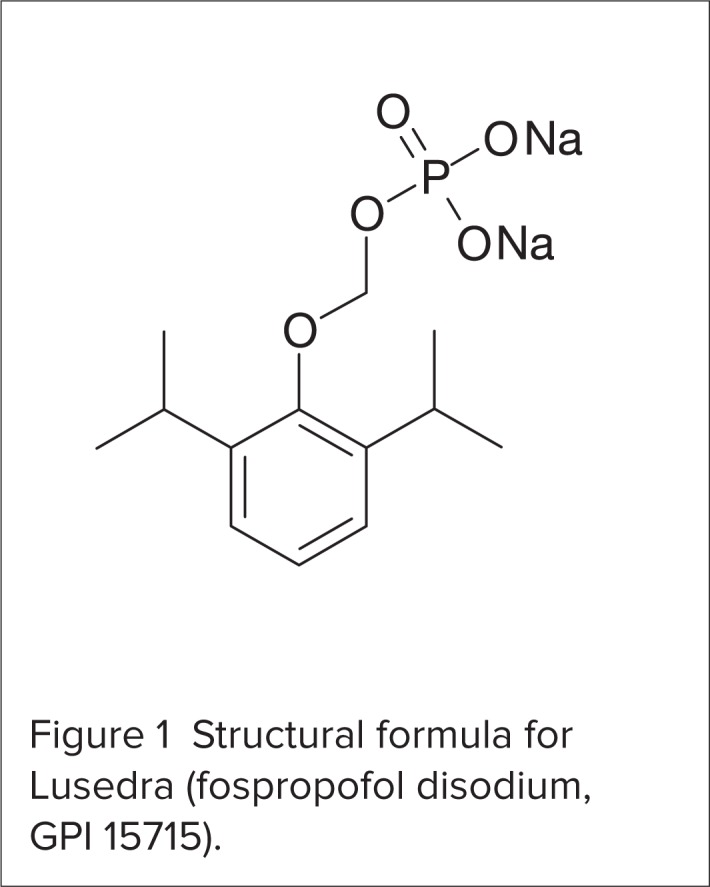
Structural formula for Lusedra (fospropofol disodium, GPI 15715).
The metabolism of fospropofol results in the formation of propofol, formaldehyde, and phosphate.6–8 The times to hydrolysis of fospropofol disodium are reported as 7.1 minutes and 7.9 minutes.6 Propofol is further metabolized by CY-P2D6 to propofol glucuronide (34.8%), quinol-4-sulfate (4.6%), quinol-1-glucuronide (11.1%), and quinol-4-glucuronide (5.1%).7,8 Formaldehyde is further converted to formate, which is then eliminated via oxidation.7 Approximately 71% of fospropofol is excreted unchanged in the urine.8
The comparative pharmacokinetic characteristics of fospropofol disodium, propofol liberated from fospropofol, and propofol lipid emulsion are summarized in Table 1. The pharmacokinetic properties of fospropofol disodium do not appear to be affected by race, sex, age, or renal impairment. The effect of hepatic impairment on fospropofol pharmacokinetics is not known.9
Table 1.
Comparative Pharmacokinetics of Propofol Lipid Emulsion, Fospropofol Disodium, and Propofol Liberated From Fospropofol
| Propofol Lipid Emulsion | Fospropofol Disodium | Liberated Propofol | |
|---|---|---|---|
| Protein binding (%) | 97–99 | 95–97 | 95–97 |
| Steady-state volume of distribution (L/kg) | 4.5 | 0.33 | 5.8 |
| Half-life (hours) | 0.97 | 0.88 | 1.13 |
| Metabolism | Hydroxylation, conjugation | Hydroxylation, conjugation | Glucuronidation |
| Total body clearance (L/hours/kg) | 1.1 | 0.36 | 3.2 |
| Renal clearance | > 1% | < 0.02% | Not reported |
Although fospropofol and propofol lipid emulsion are similar in pharmacology, the two agents differ in the onset timing of the pharmacodynamic actions. The time to onset of effect for fospropofol disodium is 4 to 8 minutes compared with 2 minutes for the lipid emulsion.5 The propofol lipid emulsion and the inactive fospropofol are not equipotent. Equivalent doses of fospropofol disodium and propofol lipid emulsion result in a delayed onset of fospropofol effect and in decreased clinical efficacy compared with propofol lipid emulsion.5,6
CLINICAL TRIALS
Cohen et al.10
Cohen et al. conducted a double-blind, multicenter, dose-response study to evaluate fospropofol disodium as moderate sedation in patients undergoing colonoscopy. A total of 127 adults were randomly assigned to five treatment arms: 2 mg/kg, 5 mg/kg, 6.5 mg/kg, or 8 mg/kg of fospropofol disodium, or 0.02 mg/kg of midazolam. Most of the patients were Caucasian and younger than 65 years of age, with P1 or P2 status, according to the American Society of Anesthesiologists (ASA) Physical Classification System. Patient status is described as follows:11
P1 = patient is normal and healthy
P2 = patient has mild systemic disease that does not limit activities
P3 = patient has moderate or severe systemic disease that does not limit activities
P4 = patient has severe systemic disease that is a constant threat to life
P5 = patient is morbid and is at substantial risk of death within 24 hours, with or without a procedure
All patients were pretreated with intravenous (IV) fentanyl citrate injection 50 mcg before receiving the sedative. Supplemental doses of the study drug were allowed to be given for patients to achieve a score of 4 or less on the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale. Scores were as follows:12
5 = patient responds rapidly to name spoken in normal tone
4 = patient has a lethargic response to name spoken in normal tone
3 = patient responds only after name is called loudly or repeatedly
2 = patient responds only after mild prodding or shaking
1 = patient responds only after painful trapezius squeeze
0 = patient does not respond to painful trapezius squeeze
Administration of fospropofol brought about successful sedation in 24%, 35%, 69%, 96%, and 81% of patients who received 2 mg/kg, 5 mg/kg, 6.5 mg/kg, and 8 mg/kg, respectively, or midazolam. The differences in sedation success were statistically significant for groups of patients receiving fospropofol 6.5 mg/kg and 8 mg/kg and for the midazolam groups compared with groups receiving fospropofol 2 mg/kg and 5 mg/kg. A similar dose-dependent trend was seen in treatment success rates, which were defined as follows: 36%, 42%, 81%, and 96% for fospropofol 2 mg/kg, 5 mg/kg, 6.5 mg/kg, and 8 mg/kg, respectively, and 89% for midazolam.
The average time to sedation was 12.4 minutes, 11.0 minutes, 6.5 minutes, and 4.7 minutes with fospropofol 2 mg/kg, 5 mg/kg, 6.5 mg/kg, and 8 mg/kg, respectively, and 5.0 minutes for midazolam. There was an inverse relationship between the fospropofol dose and the number of patients requiring an alternative sedative agent. There were no statistically significant differences in memory retention among the treatment groups.
Patients’ satisfaction scores were higher for all fospropofol groups, compared with the midazolam group. Patients receiving fospropofol 6.5 mg/kg reported the highest overall satisfaction.
Sixty-one percent of patients receiving fospropofol and 8% of patients receiving midazolam experienced treatment-related adverse events (AEs). The most commonly reported AEs were paresthesias (in 49% of fospropofol patients vs. 4% of midazolam patients) and pruritus (9% with fospropofol vs. none with midazolam). Hypotension (n = 2) and hypoxemia (n = 2) were reported in four patients who received fospropofol. All AEs were mild to moderate in severity and did not result in discontinuation of the study drug. The investigators did not observe a dose–response relationship in the rate of AEs among the fospropofol groups.
In this trial, fospropofol, in doses of 6.5 mg/kg or 8 mg/kg, was effective and well tolerated for the sedation of patients undergoing colonoscopy.
Silvestri et al.13,14
A phase 3, randomized, double-blind, dose-controlled study was designed to assess the efficacy and safety of fospropofol disodium injection for moderate sedation in 252 patients undergoing flexible bronchoscopy.13 The patients were randomly assigned to receive fospropofol 2 mg/kg (n = 102) or fospropofol 6.5 mg/kg (n = 150) in a 2:3 allocation ratio. The dose was reduced by 25% in patients 65 years of age or older and in those with ASA Physical Classification status P3 or P4.11 All patients were pretreated with IV fentanyl citrate 50 mcg. Supplemental doses of fospropofol disodium were allowed if the MOAA/S score12 was higher than 3 during the initiation of fospropofol or if it was 4 or higher in combination with purposeful movement during the procedure.
The primary endpoint was sedation success, defined as three consecutive MOAA/S scores of 4 or greater12 after fospropofol administration and after completion of the procedure without the need for either an alternative sedative or mechanical ventilation. Sedation success was achieved in 88.7% of patients who received fospropofol 6.5 mg/kg and in 27.5% of patients who received fospropofol 2 mg/kg (P < 0.0001).
Treatment success, defined as completion of the procedure without the need for either an alternative sedative or mechanical or manual ventilation, was achieved in 91.3% of patients who received fospropofol 6.5 mg/kg and in 41.2% of patients receiving fospropofol 2 mg/kg.
More patients receiving 2 mg/kg, compared with those receiving 6.5 mg/kg, required supplemental doses (93% vs. 44%, respectively) or alternative sedative agents (58.8% vs. 8.0% respectively).
The median time to achieve sedation was 4 minutes with fospropofol 6.5 mg/kg and 18 minutes with 2 mg/kg. The median time to full alertness from the end of the procedure was 5.5 minutes for those receiving 6.5 mg/kg and 3.0 minutes for those receiving 2 mg/kg. There was no significant difference between the two treatment groups in the median time to readiness for hospital discharge. Patient and physician satisfaction scores were higher with a dose of 6.5 mg/kg than with 2 mg/kg.
The most frequently reported AEs were paresthesias (47.6%) and pruritus (14.7%), both reported to be mild to moderate in severity. More patients receiving fospropofol 6.5 mg/kg experienced severe AEs compared with those receiving 2 mg/kg. Hypoxia was the most common sedation-related AE, occurring in 15.4% of the 6.5-mg/kg patients and in 12.6% of those receiving 2 mg/kg.
A subgroup analysis14 of this trial was performed to evaluate the effects of fospropofol disodium in patients 65 years of age or older compared with patients younger than 65 years of age. Elderly patients received fospropofol 4.88 mg/kg, whereas younger patients received fospropofol 6.5 mg/kg.
There were no statistically significant differences in mean MOAA/S scores, in required supplemental fospropofol doses, in time to full alertness, or in time to discharge between the two age groups. AEs occurred in 59.0% of elderly patients and in 77.5% of younger patients. The most common AEs were paresthesias (in 42.5% of elderly patients vs. 55.1% of younger patients) and pruritus (in 13.1% of elderly patients vs. 15.7% of younger patients). Hypotension occurred in 6.5% of elderly patients and in 6.7% of younger patients, and hypoxia was more also common in elderly patients (13.1% vs. 9.0%, respectively).
The authors concluded that fospropofol was an effective sedative that resulted in predictable moderate sedation with an acceptable safety profile in patients undergoing flexible bronchoscopy. Fospropofol also provided effective sedation in elderly patients, without a significant increase in AEs, when compared with younger patients.
Cohen et al.15
A multicenter, double-blind trial was conducted to evaluate the efficacy and safety of fospropofol for the sedation of patients undergoing elective colonoscopy. Following pretreatment with fentanyl, 314 patients (mean age, 52.9 years) were randomly assigned, in a 2:3:1 ratio, to receive fospropofol 2 mg/kg (n = 102), fospropofol 6.5 mg/kg (n = 160), or midazolam 0.02 mg/kg (n = 52). Most of the patients had ASA Physical Classification status P1 or P211 and were younger than 65 years of age.
Sedation success rates were 26% with fospropofol 2 mg/kg, 87% with fospropofol 6.5 mg/kg (P < 0.001 vs. fospropofol 2 mg/kg), and 69% with midazolam. Treatment success rates were 28% in patients receiving 2 mg/kg, 88% in patients treated with 6.5 mg/kg (P < 0.001 vs. fospropofol 2 mg/kg), and 79% in the midazolam group. Fewer patients required supplemental sedatives or analgesics with fospropofol 6.5 mg/kg (12% and 55%, respectively), compared with those receiving fospropofol 2 mg/kg (72% and 77%, respectively) (P < 0.001).
On average, memory retention was lower with fospropofol 6.5 mg/kg and with midazolam compared with fospropofol 2 mg/kg. All patients receiving the 2-mg/kg dose recalled being awake during the procedure, compared with 51% of patients receiving fospropofol 6.5 mg/kg (P < 0.05) and 60% of patients treated with midazolam. There were no significant differences in patient satisfaction scores among the three treatment groups.
The rates of treatment-related AEs were 75.5% for fospropofol 2 mg/kg, 78.5% for fospropofol 6.5 mg/kg, and 5.8% for midazolam.
The most common AEs were paresthesias (59.8%, 68.4%, and 0% for fospropofol 2 mg/kg, fospropofol 6.5 mg/kg, and midazolam, respectively) and pruritus (25.5%, 15.8%, and 0% with fospropofol 2 mg/kg, fospropofol 6.5 mg/kg, and midazolam, respectively). One patient who received fospropofol 6.5 mg/kg experienced hypotension. Most AEs were mild to moderate in severity.
The investigators concluded that fospropofol was effective and well tolerated for sedation during colonoscopy. The 6.5-mg/kg dose appeared to be more effective than the 2-mg/kg dose and as effective as midazolam. There were no significant differences in the safety profiles of the two fospropofol regimens. The potential for AEs was lower with midazolam than with fospropofol.
Candiotti et al.16
Candiotti and coworkers conducted a randomized, open-label study to evaluate the safety and tolerability of fospropofol in critically ill patients receiving mechanical ventilation. A total of 78 patients requiring mechanical ventilation for 2 to 12 hours were assigned to one of three study groups: fospropofol infusion/bolus (n = 25), fospropofol infusion p (n = 27), or propofol (n = 26). The fospropofol infusion/bolus group received fospropofol 25 mcg/kg per minute in addition to fospropofol bolus doses of 100 mg, as needed, for agitation. The fospropofol infusion group received 25 mcg/kg per minute without additional bolus doses. The propofol group received 5 mcg/kg per minute. All patients received morphine, fentanyl citrate, or hydromorphone for analgesia.
All continuous infusions were titrated to maintain scores of 2 to 5 on the Ramsay Sedation Scale (RSS). Scores are as follows:1
1 (awake) = patient is anxious and agitated or restless, or both
2 (awake) = patient is cooperative, oriented, and tranquil
3 (awake) = patient responds to commands only
4 (asleep) = patient has a brisk response to a light glabellar tap or a loud auditory stimulus
5 (asleep) = patient has a sluggish response to a light glabellar tap or a loud auditory stimulus
6 (asleep) = patient has no response to a light glabellar tap or a loud auditory stimulus
Mean RSS scores were 3.5 for the fospropofol infusion/bolus group, 3.3 for the fospropofol infusion group, and 3.2 for the propofol group. There were no significant differences in the percentage of time at target RSS score among the treatment groups: 91.6% of the time for fospropofol infusion/bolus, 95.5% of the time for fospropofol infusion, and 93.4% of the time for propofol.
Agitation was more common with fospropofol: 33.3% of patients in the fospropofol/bolus group and 35.0% of patients in the fospropofol infusion group, compared with 22.7% of patients in the propofol group. AEs occurred in 71.1% of the fospropofol groups and in 63.3% of the propofol group. The most common AEs were procedure-related pain (18.4% for fospropofol vs. 9.1% for propofol), infusion-site pain (5.3% for fospropofol vs. 13.6% for propofol), hyperglycemia (5.3% for fospropofol vs. 13.6% for propofol), and nausea (10.5% for fospropofol vs. 4.5% for propofol). Serious treatment-related AEs were more common with fospropofol (in 26.3%) than with propofol (in 13.6%).
The results suggested that fospropofol might have a role in the management of agitation. However, because of the small sample size and the lack of statistical analysis in this trial, it is difficult to conclude whether fospropofol has a clear advantage over propofol in the management of mechanically ventilated, critically ill patients.
Gan et al.17
Gan and colleagues conducted a phase 3, open-label, single-arm study to evaluate the safety of fospropofol disodium in 123 patients who were scheduled to undergo minor surgical procedures requiring sedation. Following pretreatment with fentanyl, all patients received an initial bolus dose of fospropofol 6.5 mg/kg. Supplemental doses of fospropofol were administered as needed to reach an MOAA/S score12 of 4 or less. Most of the patients were younger than 65 years of age with an ASA physical status11 of P1 or P2.
Treatment-related AEs occurred in 82.1% of the patients. The most common AEs were paresthesias (62.6%) and pruritus (27.6%), which usually occurred within 5 minutes of fospropofol administration. Patients also experienced nausea (4.1%), hypotension (3.3%), vomiting (3.3%), and headache (2.2%). Hypoxemia was reported in one patient (<1%). Most AEs were mild to moderate in severity.
The findings from this study are consistent with those of other clinical trials. In other studies, the most commonly reported AEs associated with fospropofol were paresthesias and pruritus, which are believed to be caused by the phosphate ester component.7 At the dose approved by the FDA (6.5 mg/kg), the rates of paresthesias ranged from 47.6% to 68.4%, whereas the rates of pruritus ranged from 8.0% to 14.7%.10,11,13 Hypotension occurred in 2% to 4% of patients.10,11,13 Hypoxemia was classified as a sedation-related effect and occurred in 8% to 14% of patients in clinical trials.10,11 In most cases, these AEs were described as mild to moderate in severity. Fewer than 1% of patients discontinued therapy as a result of AEs.10,11,13
DRUG INTERACTIONS
According to the product labeling, fospropofol disodium may produce additive cardiorespiratory effects when given with other cardiorespiratory depressants, such as sedative–hypnotics and narcotic analgesics. The interaction of fospropofol with other highly protein-bound drugs has not been studied.8
In an in vitro study, the pharmacokinetic properties of fospropofol were not affected by the coadministration of fentanyl, meperidine (Demerol, Sanofi), morphine, or midazolam. Fospropofol is not a substrate for CYP enzymes. Whether fospropofol has the ability to induce or inhibit CYP enzymes is unknown.8,9
DOSAGE AND ADMINISTRATION
Fospropofol is supplied as a clear, colorless, sterile, aqueous solution containing 35 mg/mL of fospropofol disodium. The recommended initial dose of fospropofol is 6.5 mg/kg as an intravenous (IV) bolus injection, followed by supplemental doses of 1.6 mg/kg no more frequently than every 4 minutes, as needed, to re-establish sufficient sedation depth.8 This dose is limited by an upper (90-kg) and a lower (60-kg) weight limit. Patients weighing more than 90 kg should receive the dosage for 90 kg, and patients weighing less than 60 kg should receive the dosage for 60 kg.8 The drug may be administered via a peripheral line, which should be flushed before and after administration.7
No dosing adjustments are necessary for patients with a creatinine clearance (CrCl) of 30 mL/minute or greater. Limited data are available for patients with a CrCl of less than 30 mL/minute. Caution is advised in patients with hepatic impairment.8,15 The dose of fospropofol should be decreased by 25% in patients 65 years of age or older. Patients with severe systemic disease (ASA class P3 or P4)11 should receive a modified dosing regimen.8,9,14
COST
A search of the literature did not disclose any pharmacoeconomic evaluations of treatment with fospropofol disodium.
The average wholesale price (AWP) of fospropofol disodium 35 mg/mL, 30-mL single-use vial, is $43.20.18 Thus, if fospropofol disodium is given at 6.5 mg/kg (the dose recommended by the FDA), the AWP for one dose for a 70-kg patient would be approximately $18.72.
The AWP for midazolam 1 mg/mL, 2-mL single-use vial, is approximately $0.52 to $1.14.19 Therefore, the AWP for one dose of midazolam 0.02 mg/kg (the dose used in clinical trials for monitored anesthesia care) for a 70-kg patient would be approximately $0.36 to $0.70.
The AWP for propofol 10 mg/mL, 20-mL vial, is approximately $1.68 to $3.72, depending on the manufacturer.20 Because clinical trials of fospropofol sodium used midazolam as a comparator rather then propofol, it is difficult to compare the AWP of one dose of propofol with that of one dose of fospropofol.
P&T COMMITTEE CONSIDERATIONS
Fospropofol disodium is a water-soluble prodrug of propofol. Data from clinical trials suggest that fospropofol is as effective as midazolam for monitored anesthesia care when used in short procedures.10,15 No data are available on the efficacy of fospropofol disodium in longer procedures during which repeated dosing may be necessary.
We did not find any published clinical trials that compared the efficacy of fospropofol disodium with that of propofol for monitored anesthesia care. However, fospropofol disodium and propofol appear to be equally effective when used for the short-term sedation (less than 12 hours) of mechanically ventilated, critically ill patients.16
In clinical studies, more patients who received fospropofol disodium experienced treatment-related AEs compared with those receiving midazolam.10,15 Based on the results from one clinical trial,16 it appears that the administration of fospropofol disodium led to more AEs than propofol. The only advantages of fospropofol disodium over propofol were its lower rates of infusion-site pain and hyperglycemia.
Based on the available safety and efficacy data and on the higher price of fospropofol disodium, compared with that of midazolam and propofol,18–20 we recommend that fospropofol disodium not be added to hospital formularies as a first-line agent at the present time.
CONCLUSION
Fospropofol disodium, a sedative–hypnotic agent, was approved by the FDA for monitored anesthesia care in patients undergoing diagnostic or therapeutic procedures. In clinical studies, the most commonly reported AEs were mild-to-moderate paresthesias and pruritus. Further research is needed to establish an appropriate role for fospropofol in monitored anesthesia care and in the management of mechanically ventilated, critically ill patients.
Footnotes
Disclosure: The authors report that they have no financial or commercial/industrial relationships in regard to this article.
REFERENCES
- 1.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein DR, Jagannath S, Baron TH, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68(5):815–826. doi: 10.1016/j.gie.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96(4):1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Gan TJ. Pharmacokinetic and pharmaco-dynamic characteristics of medications used for moderate sedation. Clin Pharmacokinet. 2006;45(9):855–869. doi: 10.2165/00003088-200645090-00001. [DOI] [PubMed] [Google Scholar]
- 5.Bengalorkar GM, Bhuvana K, Sarala N, Kumar T. Fospropofol: Clinical pharmacology. J Anaesthesiol Clin Pharmacol. 2011;27(1):79–83. [PMC free article] [PubMed] [Google Scholar]
- 6.Fechner J, Schwilden H, Schuttler J. Pharmacokinetics and pharmacodynamics of GPI 15715 or fospropofol (Aquavan injection): A water-soluble propofol prodrug. Handbook Exp Pharmacol. 2008;182:253–266. doi: 10.1007/978-3-540-74806-9_12. [DOI] [PubMed] [Google Scholar]
- 7.Moore GD, Walker AM, MacLaren R. Fospropofol: A new sedative–hypnotic agent for monitored anesthesia care. Ann Pharmacother. 2009;43(11):1802–1808. doi: 10.1345/aph.1M290. [DOI] [PubMed] [Google Scholar]
- 8.Lusedra (fospropofol disodium) Injection, prescribing information. Woodcliff Lake, N.J: Eisai Inc; Oct, 2009. Available at: www.lusedra.com/downloads/PrescribingInformation.pdf. Accessed November 11, 2011. [Google Scholar]
- 9.Garnock-Jones KP, Scott LJ. Fospropofol. Drugs. 2010;70(4):469–477. doi: 10.2165/11204450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Cohen LB. Clinical trial: A dose–response study of fospropofol disodium for moderate sedation during colonoscopy. Aliment Pharmacol Ther. 2008;27(7):597–608. doi: 10.1111/j.1365-2036.2008.03598.x. [DOI] [PubMed] [Google Scholar]
- 11.Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2(3):281–284. [Google Scholar]
- 12.Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the observer’s assessment of alertness/sedation scale: Study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244–251. [PubMed] [Google Scholar]
- 13.Silvestri GA, Vincent BD, Wahidi MM, et al. A phase 3, randomized, double-blind study to assess the efficacy and safety of fospropofol disodium injection for moderate sedation in patients undergoing flexible bronchoscopy. Chest. 2009;135(1):41–47. doi: 10.1378/chest.08-0623. [DOI] [PubMed] [Google Scholar]
- 14.Silvestri GA, Vincent BD, Wahidi MM. Fospropofol disodium for sedation in elderly patients undergoing flexible bronchoscopy. J Bronchol Interv Pulmonol. 2011;18(1):15–22. doi: 10.1097/LBR.0b013e3182074892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen LB, Cattau E, Goetsch A, et al. A randomized, double-blind, phase 3 study of fospropofol disodium for sedation during colonoscopy. J Clin Gastroenterol. 2010;44(5):345–353. doi: 10.1097/MCG.0b013e3181c2987e. [DOI] [PubMed] [Google Scholar]
- 16.Candiotti KA, Gan TJ, Young C, et al. A randomized, open-label study of the safety and tolerability of fospropofol for patients requiring intubation and mechanical ventilation in the intensive care unit. Anesth Analg. 2011;113(3):550–556. doi: 10.1213/ANE.0b013e31821d7faf. [DOI] [PubMed] [Google Scholar]
- 17.Gan TJ, Berry BD, Ekman EF, et al. Safety evaluation of fospropofol for sedation during minor surgical procedures. J Clin Anesth. 2010;22(4):260–267. doi: 10.1016/j.jclinane.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Fospropofol Red Book Online, version 5.1. Greenwood Village, Colo: Thomson Healthcare; 2011. Accessed October 20, 2011. [Google Scholar]
- 19.Midazolam Red Book Online, version 5.1. Greenwood Village, Colo: Thomson Healthcare; 2011. Accessed October 20, 2011. [Google Scholar]
- 20.Propofol Red Book Online, version 5.1. Greenwood Village, Colo: Thomson Healthcare; 2011. Accessed October 20, 2011. [Google Scholar]


