Abstract
Cyclin-dependent kinases (CDK) and their compulsory cofactors, the cyclins, are the two key classes of regulatory molecules that determine the eukaryotic cell's progress through the cell cycle by substrate phosphorylation. Cdk1 forms complexes with B-type cyclins and phosphorylates a number of substrates as cells prepare to enter mitosis. CYB-3 (Cyclin B3) is a B-type cyclin that has been recently shown to be required for the timely metaphase-to-anaphase transition, presumably by alleviating a spindle assembly checkpoint (SAC) block. Previously, we have shown that doubling the CYB-3 dosage suppresses sterility in the absence of the essential SAC component MDF-1/Mad1. Here we demonstrate the importance of the Mos1-mediated single-copy insertion method for understanding the effects of gene dosage by generating strains that have more (two or three) copies of the cyb-3 in wild-type and mdf-1(gk2) backgrounds to investigate dosage effect of CYB-3 on mitotic progression as well as development and fertility in the absence and the presence of the MDF-1 checkpoint component. We show that tripling the dosage of CYB-3 results in a significantly variable metaphase-to-anaphase transition, both in wild-type and mdf-1(gk2) mutant backgrounds. Although a majority of embryos initiate anaphase onset normally, a significant number of embryos initiate anaphase with a delay. We also show that tripling the dosage of CYB-3 has no effect on viability in the wild-type background; however, it does reduce the sterility caused by the absence of MDF-1. Together, these data reveal that proper dosage of CYB-3 is important for precision of timely execution of anaphase onset regardless of the presence of the MDF-1 checkpoint component.
Keywords: cyb-3 (Cyclin B3), dosage increase, MosSCI, anaphase onset variation, mdf-1/MAD1
Introduction
The progression through the stages of the eukaryotic cell cycle is temporally controlled by association of the cyclins to their corresponding cyclin-dependent kinases (CDK) (Sanchez and Dynlacht 2005; Satyanarayana and Kaldis 2009). Cyclins are expressed and most stable during the stages of the cell cycle when they are required. Thus, proper expression and degradation of cyclins are critical for controlled cell-cycle progression. Cyclin B levels, for example, rise in G2 phase of the cell cycle and decrease substantially at the metaphase-to-anaphase transition (Sanchez and Dynlacht 2005). In agreement with their expression profiles, activation of the CDK1 kinase by B-type cyclins triggers a cell to enter the M phase of the cell cycle, whereas inactivation of the CDK1 is required for exit from the M phase. In particular, B-type cyclins activate CDK1 to phosphorylate specific set of substrates, leading to proper chromosome condensation, centrosome maturation, and nuclear envelope breakdown (NEBD) as a cell prepares to partition the replicated genetic material to daughter cells (Blethrow et al. 2008).
Faithful segregation of chromosomes is ensured by the spindle assembly checkpoint (SAC), which monitors the status of kinetochore-microtubule attachment for proper chromosome attachment and tension state (May and Hardwick 2006; Musacchio and Salmon 2007). In the presence of improperly attached and tension-free chromosomes, the SAC is activated to delay anaphase onset by inhibiting the anaphase-promoting complex/cyclosome (APC/C), which thus stabilizes securin (May and Hardwick 2006; Musacchio and Salmon 2007). Once all the chromosomes have been properly attached to the spindle, the SAC needs to be silenced for timely anaphase onset to occur (Vanoosthuyse and Hardwick 2009). For instance, unattached kinetochores activate the SAC by recruiting the Mad2 component of the SAC to the kinetochores first (Waters et al. 1998; Essex et al. 2009). Once all of the kinetochores have achieved the proper attachment, the SAC is silenced by the minus-end–directed protein dynein, which “walks” away the Mad2 and other SAC components from kinetochores along mictotubules to centrosomes (Griffis et al. 2007; Howell et al. 2001; Schmidt et al. 2005; Sivaram et al. 2009). If the removal of the SAC components by dynein is compromised, the SAC remains activated even when the proper attachment is achieved, leading to unnecessary delay in anaphase onset due to the inhibition of APC/C activity.
In Caenorhabditis elegans, the SAC components (MAD1, MAD2, MAD3, BUB1, and BUB3) and the SAC regulation of the APC/C activity are conserved (Kitagawa and Rose 1999; Oegema et al. 2001; Nystul et al. 2003; Tarailo et al. 2007b; Kitagawa 2009a). Cyclin B is one of the key targets of the APC/C. In mammals, there are three B-type cyclins—B1, B2, and B3 (Gallant and Nigg 1994). Similarly, C. elegans has cyb-1, cyb-2.1/2.2, and cyb-3 (van der Voet et al. 2009) B-type cyclins, which were shown to have both overlapping and distinct functions in chromosome segregation (van der Voet et al. 2009; Deyter et al. 2010). In both systems, cyclins B1 and B2 were shown to be highly similar, whereas cyclin B3 displayed more sequence conservation among the B3 proteins from other species than with the B1 and B2 proteins from the same species (Nguyen et al. 2002; Nieduszynski et al. 2002; van der Voet et al. 2009). In C. elegans, CYB-3 plays an essential role because the absence of CYB-3 by RNAi depletion (van der Voet et al. 2009; Deyter et al. 2010) or a gene knockout (Tarailo-Graovac et al. 2010) results in lethality. In particular, CYB-3 depletion leads to persistent block in the anaphase onset initiation (van der Voet et al. 2009; Deyter et al. 2010). Recently, it was shown that inability of cyb-3(RNAi) embryos to initiate anaphase onset is due to the compromised dynein-dependent removal of the SAC components from the kinetochores (Deyter et al. 2010).
Previously, the power of genetic screens was exploited to discover genetic interactors of mdf-1/MAD1 and additional players in the SAC cascade by identifying suppressors (Tarailo et al. 2007a) and enhancers (Tarailo et al. 2007b) of the mdf-1(gk2) lethal phenotype. In C. elegans, the absence of MDF-1/Mad1–conserved SAC component leads to chromosome instability (CIN), accumulation of genetic errors, and ultimate death of mdf-1(gk2) worms in the F3 generation (Kitagawa and Rose 1999). So far, the majority of the mutants isolated from the suppressor screens proved to be lesions in the APC/C components that delayed anaphase onset and suppressed mdf-1(gk2) sterility (Kitagawa et al. 2002; Tarailo et al. 2007a). However, one of the cloned suppressors was shown to be due to doubling the CYB-3 dosage as a result of tandem duplication (Tarailo et al. 2007a; Zhao et al. 2008; Tarailo-Graovac et al. 2010). Interestingly, this was the first cloned suppressor of mdf-1(gk2) sterility that does not cause a constant delay in anaphase onset (Tarailo-Graovac et al. 2010). In this study, using the Mos1-mediated single-copy insertion method (MosSCI) (Frokjaer-Jensen et al. 2008), we created necessary strains and investigated dosage effect of CYB-3 on anaphase onset in strains that contain one, two, or three copies of the cyb-3 in wild-type and the mdf-1(gk2) backgrounds. We show that proper CYB-3 dosage is essential for the precision of anaphase onset.
Materials and Methods
Strains and culturing conditions
The following mutant alleles were used in this work: mdf-1(gk2), unc-46(e177), unc-119(ed3), ttTi5605, dotSi100, cxTi10882, dotSi110, cyb-3(gk195), such-4(h2168), nT1[qIs51], nT1[let-?(m435)], and ruIs32. The following strains were used in this work: N2 (Bristol strain as a wild-type), EG4322 [unc-119(ed3) III; ttTi5605 II]; EG6250 [unc-119(ed3) III; cxTi10882 IV], VC388 [cyb-3(gk195) V/nT1[qIs51] (IV;V)]; KR4233 [unc-46(e177) mdf-1(gk2) such-4(h2168)]; KR3627 [unc-46(e177) mdf-1(gk2) V/nT1[let-?(m435)] (IV;V)]. Additional strains used in this work were generated in this study using the standard genetic procedures. Strains were maintained using standard protocol on nematode growth media (NGM) plates seeded with OP50 bacteria (Brenner 1974). The strains were maintained at 20°, whereas the phenotypic analyses were performed at both 20° and 25° as noted in the Results section.
Generating stable single-gene duplications using MosSCI
cyb-3 locus was amplified using Phusion (NEB) high-fidelity DNA polymerase from the C. elegans N2 (Bristol) single worm lysates and cloned into the pCFJ178 vector (Frokjaer-Jensen et al. 2008). As described previously (Tarailo-Graovac et al. 2010), due to the toxic effect of the large amount of cyb-3, we co-injected 5 ng/μl of this targeting construct with the 50 ng/μL of Mos1 transposase pJL43.1 (Pglh-2::transposase), 5 ng/μL pGH8 (Prab-3::mCherry), 5 ng/μL pCFJ104 (Pmyo-3::mCherry), and 2.5 ng/μL (Pmyo-2::mCherry) into the gonad of 45 young adult P0 hermaphrodites of EG6250 [unc-119(ed3) III; cxTi10882 IV] strain. The plates that contained wild-type–looking mCherry–expressing worms were starved at 25° and then screened for stable integrants, as previously described (Frokjaer-Jensen et al. 2008). Once a stable line was obtained, it was confirmed by PCR and sequencing to contain a mutation-free wild-type copy of cyb-3 inserted into the cxTi10882 Mos1 site.
Phenotypic analysis
For each analysis, hermaphrodites at L4 stage were grown on fresh OP50 plates at 20° or 25°. The hermaphrodites were transferred to fresh plates every 12 hr. Brood size was calculated based on the total eggs laid by each hermaphrodite. Embryos that did not hatch were scored as embryonic arrests, and the embryos that hatched but did not grow to adult stage were scored as larval arrests. The embryos that developed into adults were analyzed for the presence of males in all the strains analyzed, and the worms in the mdf-1(gk2) background were also analyzed for the percentage of the adult progeny that were sterile by individually plating all the adult progeny and observing for the presence or absence of offspring.
To assess whether the dotSi110 could suppress the sterility of mdf-1(gk2) homozygotes, the F1 mdf-1(gk2) homozygotes segregated from KR3627 [unc-46(e177) mdf-1(gk2) V/nT1[let-?(m435)] (IV;V)] were mated to dotSi110 males. The wild-type–looking unc-46(e177) mdf-1(gk2)/ + +; dotSi110/ + were allowed to self-fertilize, and 43 Unc-46 progeny were plated individually. All of the worms that could be propagated for more than three generations were genotyped and confirmed to be homozygous for dotSi110 duplication of cyb-3, whereas none of the worms that could not be propagated for more than three generations were homozygous for the dotSi110 duplication.
Time-lapse imaging of the embryos
One-day old gravid adult hermaphrodites were dissected, and embryos were mounted onto 3% agarose pads as described (Sulston et al. 1983). Quorum WaveFX Spinning Disk system mounted on Zeiss Axioplan microscope was used. Early embryonic cell division was recorded using time-lapse video microscopy at 400× with 200 ms fluorescent exposure, one image every 10 sec. Image acquisition and analysis was performed using Volocity software.
Results and Discussion
Mos1-mediated single-copy insertion method to investigate increased dosage of CYB-3 in Caenorhabditis elegans
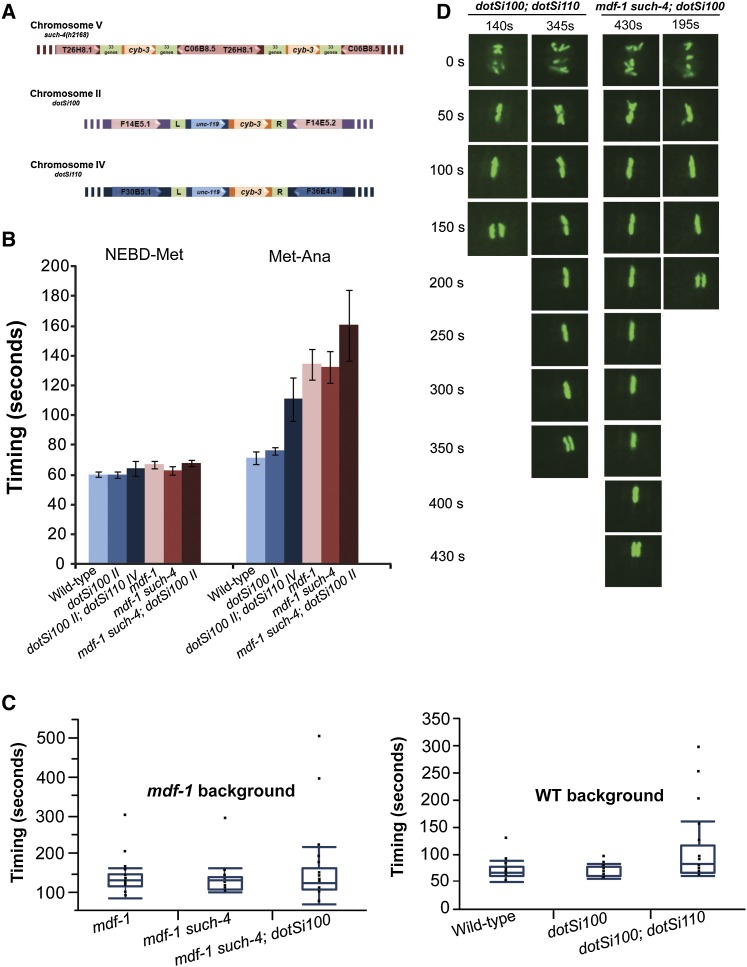
Our recent work has demonstrated that doubling the dosage of a cyb-3 gene (Cyclin B3) suppressed the sterility in the absence of an essential SAC component MDF-1/Mad1 and allowed for the mdf-1(gk2) homozygotes to be propagated well beyond the third generation (Tarailo-Graovac et al. 2010). This finding clearly showed that in addition to the complete absence of the CYB-3 due to cyb-3 gene knockout (Tarailo-Graovac et al. 2010) or RNAi depletion of the cyb-3 gene product (van der Voet et al. 2009; Deyter et al. 2010), dosage of the functional CYB-3 may play an important role in the SAC cascade. Phenotypic analysis of a strain that is homozygous for the cyb-3 duplication revealed that unlike other suppressors of the ∆mdf-1 sterility (Tarailo et al. 2007a), cyb-3 duplication did not result in any obvious phenotypes in the wild-type background (Tarailo-Graovac et al. 2010). However, the titrations of multiple different concentrations at which the cyb-3 constructs were injected into the gonads led us to conclude that high copy number of the cyb-3–containing constructs resulted in sterility, suggesting a dominant-negative effect of cyb-3 overexpression (Tarailo-Graovac et al. 2010). To further investigate the effect of increased dosage of CYB-3 in C. elegans and to reach better understanding of the mode of genetic interaction between the cyb-3 and mdf-1, we used the MosSCI method (Frokjaer-Jensen et al. 2008) to integrate a wild-type copy of the cyb-3 gene at multiple sites in the C. elegans genome. Previously, we created a strain that contains a wild-type copy of the cyb-3 gene integrated on chromosome II, in the ttTi5605 Mos1 integration site (Tarailo-Graovac et al. 2010). To assess the effect of three copies of the cyb-3 gene, we first inserted a copy of the cyb-3 gene on chromosome IV, in the cxTi10882 Mos1 integration site, and created the dotSi110 stable insertion in addition to the previously generated dotSi100 stable insertion on chromosome II (Tarailo-Graovac et al. 2010) (Figure 1A). Once confirmed by sequencing that the wild-type mutation-free copy of cyb-3 gene was inserted on chromosome IV, we assessed the functionality of cyb-3 from the dotSi110 by testing its ability to rescue the gk195 lethality. As we have shown previously, the gk195 knockout allele removes the majority of cyb-3 and results in larval arrest (Tarailo-Graovac et al. 2010). As with the stable integration of cyb-3 on chromosome II, we were able to construct the cyb-3(gk195) V; cyb-3(dotSi110) IV homozygotes and to show that dotSi110 fully rescues the larval arrest phenotype of the cyb-3(gk195) knockout allele, making cyb-3(gk195) V; cyb-3(dotSi110) IV homozygotes indistinguishable from the wild-type N2 strain at 20° and 25° (data not shown). This result confirms that the wild-type copy of cyb-3 integrated on chromosome IV is expressed and makes fully functional CYB-3. Next, we compared the development of dotSi110 duplication-containing worms to the dotSi100 duplication-containing worms for developmental defects, such as developmental delay, increased incidence of embryonic or larval arrests, sterility and morphological defects at 20° and 25° (Table 1). Our analysis revealed no obvious difference between the two duplications (Table 1). Furthermore, this analysis confirmed our previous conclusions that, although overexpression of cyb-3 in high-copy extrachromosomal arrays is toxic, a duplication of cyb-3 does not have an obvious phenotype in an otherwise wild-type background in C. elegans. Finally, we compared the ability of the dotSi110 duplication to suppress lethality of the mdf-1(gk2) homozygotes. We observed that all of the 21 analyzed ∆mdf-1; dotSi110 homozygotes could be propagated indefinitely. Then we analyzed whether the dotSi110 duplication behaves the same in the mdf-1(gk2) background as the previously characterized such-4(h2168) tandem duplication (Tarailo et al. 2007a; Zhao et al. 2008; Tarailo-Graovac et al. 2010) and the dotSi100 duplication generated on chromosome II (Tarailo-Graovac et al. 2010). We observed that dotSi110 duplication has a very similar phenotype to the such-4(h2168) (Table 2) and dotSi100 duplications (Tarailo-Graovac et al. 2010). Together, these data suggest that increased dosage of CYB-3 has similar effect regardless of analyzed chromosomal positions of the duplicated cyb-3 gene, which makes the MosSCI a valuable tool for gene dosage studies (Figure 1A).
Figure 1 .
Increased variation in anaphase onset when dosage of CYB-3 is tripled using the MosSCI insertion method. (A) Schematic drawing of the cyb-3 duplication locations in the C. elegans genome. such-4(h2168) is a tandem duplication that affects the region between the T26H8.1 and C06B8.5 nearly identical transposons. dotSi100 is a cyb-3 duplication that was inserted into the ttTi5605 Mos1 insertion site on chromosome II between the F14E5.1 and the F14E5.2 genes. dotSi110 is a cyb-3 duplication that was inserted into the cxTi10882 Mos1 integration site on chromosome IV between the F30B5.1 and the F36E4.9 genes. (B) Summary of the timing measurements from NEBD-to-metaphase and metaphase-to-anaphase intervals given in seconds. Error bars represent SEM for n = 20 measurements for each strain. (C) Boxplots of the metaphase-to-anaphase interval timing data for the embryos with one, two, or three copies of the cyb-3 in the wild-type background (right) and the mdf-1(gk2) background (left). For each boxplot, all the data points (n = 20) were used. (D) dotSi100; dotSi110: An example of an embryo with normal timing of the anaphase onset (140 sec) and delayed anaphase onset (345 sec) in the wild-type background. mdf-1 such-4; dotSi100: An example of an embryo with normal timing of the anaphase onset (195 sec) and delayed anaphase onset (430 sec) in the mdf-1(gk2) background.
Table 1. cyb-3 dosage effect in the wild-type background.
| Genotype | cyb-3 Copies | T | Embryonic Arrest (%) | Larval Arrest (%) | Adult (%) | Male (%) |
|---|---|---|---|---|---|---|
| dotSi101 II [unc-119( +)] (n = 859) | One | 20° | 7.0 | 4.1 | 88.9 | 0.1 |
| dotSi100 II [T06E6.2 + unc-119( +)] (n = 4116) | Two | 20° | 1.8 | 0.8 | 97.4 | 0.0 |
| dotSi110 IV [T06E6.2 + unc-119( +)] (n = 3741) | Two | 20° | 1.4 | 0.7 | 97.9 | 0.2 |
| dotSi100 II; dotSi100 IV (n = 4599) | Three | 20° | 1.1 | 0.5 | 98.4 | 0.1 |
| dotSi101 II [unc-119( +)] (n = 859) | One | 25° | 7.8 | 1.3 | 90.9 | 0.0 |
| dotSi100 II [T06E6.2 + unc-119( +)] (n = 4116) | Two | 25° | 4.8 | 2.4 | 92.8 | 0.3 |
| dotSi110 IV [T06E6.2 + unc-119( +)] (n = 3741) | Two | 25° | 4.6 | 3.3 | 92.1 | 0.5 |
| dotSi100 II; dotSi100 IV (n = 4599) | Three | 25° | 2.0 | 3.5 | 94.5 | 0.5 |
Table 2. cyb-3 dosage effect on the mdf-1(gk2) lethality.
| Genotype | cyb-3 Copies | Embryonic Arrest (%) | Larval Arrest (%) | Adult (%) | Fertile (%) | Male (%) |
|---|---|---|---|---|---|---|
| unc-46(e177) mdf-1(gk2) such-4(h2168) (n = 927) | Two | 43.8 | 27.9 | 28.3 | 52.8 | 1.5 |
| unc-46(e177) mdf-1(gk2); dotSi110 (n = 1010) | Two | 52.5 | 26.3 | 21.2 | 54.5 | 2.1 |
| unc-46(e177) mdf-1(gk2) such-4(h2168); dotSi100 (n = 2460) | Three | 43.0 | 26.5 | 30.5 | 77.3 | 5.5 |
| unc-46(e177) mdf-1(gk2); dotSi100; dotSi110 (n = 726) | Three | 41.4 | 26.3 | 32.3 | 71.9 | 4.7 |
An effect of further increase in the cyb-3 gene number on development in the presence and the absence of the functional MDF-1/Mad1 checkpoint component
The MosSCI (Frokjaer-Jensen et al. 2008) method allows us to construct the strains with the desired number of copies of the gene of interest and follow the consequences of increased dosage on development of the whole organism in different genetic backgrounds. Once we confirmed the functionality of both of the MosSCI-engineered duplications in vivo, we constructed a strain that has three copies of the cyb-3 gene: an endogenous cyb-3 located on chromosome V, dotSi100 integrated copy of cyb-3 located on chromosome II, and the cyb-3 integrated on chromosome IV (dotSi110 IV) (Figure 1A). To assess the effect of three copies of the cyb-3 gene, we first investigated the development of these worms at 20° and 25° (Table1). Our analysis revealed that the animals that have three copies of the cyb-3 gene develop normally at these temperatures, as they do not display any obvious difference from the wild-type or duplication strains (Table 1). Thus, our results suggest that there may be a threshold to the amount of CYB-3 dosage increase at which animals that bear too many cyb-3 gene copies become sterile and uncoordinated, the phenotype observed when concentration of the cyb-3–containing constructs is greater than 5 ng/μL. However, a 3-fold increase in the CYB-3 dosage, like the 2-fold increase (Tarailo-Graovac et al. 2010), is well tolerated throughout the development in the wild-type background.
The such-4 suppressor of the mdf-1(gk2) sterility is the weakest suppressor isolated to date (Tarailo et al. 2007a). Similar to the cyb-3–containing tandem duplication (Figure 1A), the MosSCI-engineered cyb-3 duplications are very weak suppressors of mdf-1(gk2) sterility (Table 2). To investigate whether three copies of the cyb-3 display any difference in suppressing the mdf-1(gk2) lethal phenotype, we introduced the dotSi100 and dotSi110 single-copy cyb-3 stable integrations into the mdf-1(gk2) background using standard genetic crosses followed by genotyping procedures. Phenotypic analysis of the unc-46(e177) mdf-1(gk2); dotSi100; dotSi110 strain revealed 41.1% of embryonic arrests, 26.3% of larval arrests, and 32.3% of progeny that developed into adults (Table 2). These results are very similar to the percentages of developmental arrests and adult progeny that we observed when analyzing either a tandem duplication such-4(h2168) or a cyb-3 duplication located on either chromosome IV (Table 2) or II (Tarailo-Graovac et al. 2010), suggesting that tripling the CYB-3 dosage does not affect mdf-1(gk2) lethality. However, we did observe a difference in percentage of adult progeny that were fertile. Whereas in the strains that contained duplicated cyb-3 in the mdf-1(gk2) background, some 50% of fertile adult progeny was fertile, in the unc-46(e177) mdf-1(gk2); dotSi100; dotSi110 strain, the number of fertile progeny was 71.9% (Table 2). To ensure that the observed increase in the fertile adult progeny was due to an extra cyb-3 copy, we constructed two additional strains: unc-46(e177) mdf-1(gk2) such-4(h2168); dotSi100 and unc-46(e177) mdf-1(gk2) such-4(h2168); dotSi110. In both strains, the percentage of fertile progeny was over 70% (Table 2 and data not shown), which is greater than in the strains that contained cyb-3 duplications in the mdf-1(gk2) background (Table 2; Tarailo-Graovac et al. 2010). Thus, these data suggest that increasing the cyb-3 copy number from two to three suppresses the sterility of the mdf-1(gk2) homozygotes further by increasing the percentage of adult progeny that are fertile.
An effect of three copies of cyb-3 gene on metaphase-to-anaphase transition in the presence and the absence of the functional MDF-1/Mad1 checkpoint component
To date, the such-4 suppressor is the only cloned suppressor that allows indefinite propagation of the mdf-1(gk2) homozygotes without having any obvious anaphase onset delays (Tarailo-Graovac et al. 2010). An additional three suppressors whose molecular identity is currently unknown belong to this class (Tarailo et al. 2007a). To further investigate the effect of the CYB-3 dosage on anaphase onset timing, we decided to analyze the cell-cycle progression in the strains that contain three copies of the cyb-3 gene. We reasoned that further increase in the CYB-3 dosage might result in more obvious alteration of anaphase onset. Similar to the analysis of the duplication strains, we introduced all of the analyzed strains into a ruIs32 background (Table 3), which is an integrated histone-GFP transgene that marks mitotic chromosome behavior (Praitis et al. 2001). Next, we used time-lapsed fluorescence microscopy of one-cell–stage embryos to measure the time it takes an embryo to progress from complete nuclear envelope breakdown (NEBD) to anaphase onset (Table 3). As expected, we did not observe any significant difference in the anaphase onset timing between wild-type (131.6 sec, n = 20) and dotSi110cyb-3 duplication-containing embryos (135.0 sed, n = 10) (Table 3). We also observed the similar NEBD-to-anaphase onset progression in the control embryos (dotSi101), which have the unc-119 rescue construct integrated without the wild-type copy of the cyb-3 gene and the dotSi100cyb-3 duplication embryos (Table 3). These results agree well with our previously published analysis (Tarailo-Graovac et al. 2010). Unlike the timing in the strains that contain two copies of the cyb-3 gene, the strain that had three copies had a very variable anaphase onset (Figure 1, C and D; Table 3). In these embryos, the anaphase onset timing ranged from 120 to 450 sec, which is very different from the wild-type strain, in which the range was very small (110 to 190 sec). Although the majority of the embryos that contained three copies of the cyb-3 had normal anaphase onset timing, the 25% of embryos that divided with the delay (Figure 1, C and D) made an overall difference in the NEBD-to-anaphase onset progression significant (175.2 sec, n = 20; P = 0.0291) (Table 3).
Table 3. cyb-3 dosage effect on anaphase onset in wild-type and mdf-1(gk2) backgrounds.
| Genotype | cyb-3 Copies | NEBD-to-Anaphase Onset sec ± SEM (n) |
|---|---|---|
| Wild-type background | ||
| unc-119(ed3); ruIs32(pie-1::H2B-GFP) | One | 131.6 ± 4.3 (20) |
| dotSi101 II [unc-119( +)]; ruIs32 | One | 136.8 ± 4.6 (10) |
| dotSi100 II [T06E6.2 + unc-119( +)]; ruIs32 | Two | 135.6 ± 2.7 (20) |
| dotSi110 IV [T06E6.2 + unc-119( +)]; ruIs32 | Two | 135.0 ± 3.0 (10) |
| dotSi100; dotSi110; ruIs32 | Three | 175.2 ± 18.7a (20) |
| mdf-1 background | ||
| F2 unc-46(e177) mdf-1(gk2); ruIs32 | One | 201.3 ± 10.7a (20) |
| unc-46(e177) mdf-1(gk2) such-4(h2168); ruIs32 | Two | 195.2 ± 11.3a (21) |
| unc-46(e177) mdf-1(gk2) such-4(h2168); dotSi100; ruIs32 | Three | 226.5 ± 22.9a (20) |
Significant difference from the measurements in the unc-119(ed3); ruIs32(pie-1::H2B-GFP) background was determined using the unpaired Student t-test.
Next, we asked whether the same variability in the anaphase onset timing is also observed when there are three copies of the cyb-3 gene in the mdf-1(gk2) background (Table 3). In agreement with our previous results, we observed a constant, significant delay in the embryos that lacked MDF-1 compared with the wild-type embryos (Tarailo-Graovac et al. 2010) (Table 3). Whereas the cyb-3 duplication strains analyzed in the mdf-1(gk2) background did not progress through anaphase onset with any additional delays, when compared with the mdf-1(gk2) embryos alone, the strain that contained three copies of the cyb-3 displayed a highly variable progression through anaphase onset (Figure 1, C and D; Table 3). Similar to the embryos analyzed in the wild-type background, the anaphase onset timing of the embryos that had three copies of the cyb-3 in the mdf-1(gk2) background displayed a large range (144 to 570 sec) (Figure 1, C and D; Table 3), which is very different from the range observed in the mdf-1(gk2) homozygotes (150 to 368 sec) (Figure 1, C and D; Table 3). Similar to the analysis in the wild-type background, the majority of the embryos (75%) that contained three copies of the cyb-3 in the mdf-1(gk2) background progressed to anaphase with the timing similar to the mdf-1(gk2) homozygotes; however, 25% of embryos divided with the significant delay. To confirm that the highly variable anaphase onset is due to three copies of the cyb-3 gene, we constructed the unc-46(e177) mdf-1(gk2); dotSi100; dotSi110; ruIs32 and unc-46(e177) mdf-1(gk2) such-4(h2168); dotSi110; ruIs32 strains. In both of these strains, we also observed the similarly variable anaphase onset as that observed in the unc-46(e177) mdf-1(gk2) such-4(h2168); dotSi100; ruIs32 strain (data not shown). In summary, these results show that increasing the dosage of CYB-3 3-fold results in highly variable NEBD-to-anaphase onset progression that is independent of the presence or the absence of the SAC component MDF-1.
The observed variability in NEBD-to-anaphase onset progression may be due to a delay in alignment of chromosomes on the metaphase plate or due to a delay in initiation of anaphase onset. To distinguish between the two, we measured the intervals from NEBD-to-metaphase plate formation and from metaphase-to-anaphase transition (Figure 1B). In all the embryos analyzed in either the wild-type or the mdf-1(gk2) backgrounds, we observed little variation in the formation of the metaphase plates (Figure 1B). Unlike the time it takes for the chromosomes to align at the metaphase plate, the metaphase-to-anaphase interval in the strains that had three copies of the cyb-3 gene was very variable, regardless of the presence or the absence of the MDF-1 checkpoint component (Figure 1, B and D). To assess a significance of the observed variation in metaphase-to-anaphase transition, we compared the boxplots of the timing results between different strains (Figure 1C). These boxplots clearly show the tendency of time for the anaphase onset in the strains that contain triple the amount of CYB-3, regardless of the presence of the MDF-1 checkpoint component, to be skewed toward the longer anaphase onset (Figure 1C). To further test whether the observed variation in metaphase-to-anaphase transition was significant, we used Bartlett's test to probe the variances from the strains analyzed. We determined that the variances in anaphase onset timing between a duplication strain and the wild-type strain were not significantly different (P = 0.0572). We also calculated that variances in anaphase onset timing between a duplication strain in the mdf-1(gk2) background and the mdf-1(gk2) homozygotes were not significantly different (P = 0.575). Importantly, the anaphase onset timing of the strains that contained three copies of the cyb-3 in either the wild-type (P = 8.46E−07) or mdf-1(gk2) background (P = 7.84E−04) was significantly more variable than in the wild-type or mdf-1(gk2) homozygous strain alone. Therefore, our data show that increasing the copy number of cyb-3 to three resulted in significantly variable anaphase onset that was independent of the presence of the functional MDF-1 SAC component. Whereas in the wild-type background the observed variation in anaphase onset does not affect viability of the strain, in the mdf-1(gk2) background, the delay in anaphase onset in 25% of the embryos might actually be the reason for the observed increase in fertility.
In contrast to the mammalian SAC components, absence of a functional SAC component in C. elegans due to either RNAi depletion or gene knockout does not cause precocious anaphase onset (Encalada et al. 2005; Tarailo et al. 2007a; Tarailo-Graovac et al. 2010). In contrast, absence of BUB-1 and MDF-1 was shown to extend the metaphase-to-anaphase transition (Encalada et al. 2005; Tarailo-Graovac et al. 2010). Furthermore, albeit the SAC components were shown to be required to delay anaphase onset in the presence of microtubule depolymerizing drug nocodazole (Encalada et al. 2005), the SAC-dependent anaphase onset delay is very small compared with the delays experienced by the mammalian cells in the presence of compromised attachment of kinetochores to the spindle (Waters et al. 1998). Whereas the anaphase onset may be delayed up to several hours in mammals (Waters et al. 1998), the delays observed using the early C. elegans embryonic cells were relatively small, 2- to 3-fold, even in the presence of the severe spindle damage after nocodazole treatment (Kitagawa and Rose 1999; Encalada et al. 2005; Essex et al. 2009; Kitagawa 2009b). One of the reasons for such a difference may be the absolute requirement of the asynchrony of cell division for proper development and cell fate specification. It is likely that in C. elegans longer than 2- to 3-fold delays in M phase would interfere with asynchrony of cell division and would ultimately lead to lethality. Thus, one would expect the cell to balance between the time required for a cell experiencing aberrant attachment of chromosomes to the spindle to correct the defects and the time that cannot be exceeded for the asynchrony of the cell division to be maintained.
Recently, it was shown that in CYB-3–depleted embryos the SAC-dependent anaphase onset delay is extended well beyond the previously observed 2- to 3-fold delays due to the inability of the CYB-3–depleted embryos to silence the activated SAC signal (Deyter et al. 2010). In this article, we document an exciting discovery that tripling the CYB-3 dosage resulted in significant variation in anaphase onset skewed toward the delay in anaphase onset. In the 25% of embryos that divided with delayed anaphase onset, the delay did not exceed the previously reported 2- to 3-fold increase (Figure 1D), which may explain the lack of lethality in the wild-type background (Table 1). On the other hand, the observed delays may explain the increase in the percentage of fertile progeny in the absence of the MDF-1 checkpoint component when the dosage of CYB-3 is increased from 2-fold to 3-fold (Table 2). Furthermore, an interesting discovery is that the observed delays in the 25% of embryos were not dependent on the presence of the functional SAC component MDF-1, which may suggest that the observed delays might be caused by activation of another SAC component. In agreement with this hypothesis, Schumacher and colleagues reported that in the absence of MDF-1, the prolonged metaphase in the cyb-3 (RNAi) embryos was abolished, resulting in anaphase onset; however, the anaphase onset was nonetheless delayed (Deyter et al. 2010).
Conclusion
The MosSCI represents an invaluable tool for studying the consequence of altered gene dosage on development of a multicellular organism in different genetic backgrounds. Using this method, we were able to create necessary strains and compare the effects of one, two, and three copies of the cyb-3 gene on timely progression through mitosis and development in the wild-type and mdf-1(gk2) backgrounds. Our analysis revealed significant variation in the anaphase onset in the strains that had three copies of the cyb-3. The observed variation was independent of the functional MDF-1 SAC component and was skewed toward delayed anaphase onset. The increased CYB-3 dosage did not seem to affect proper development in the wild-type background, presumably because, even though significant, the observed delays did not extend the 2- to-3-fold increase in timing, which would not interfere with asynchrony of cell division and proper development. However, in the mdf-1(gk2) background, the increased CYB-3 dosage results in increased fitness.
Acknowledgments
We thank Ann M. Rose for critically reading the manuscript, the C. elegans Gene Knockout Consortium for generating the deletion mutant, and Erik M. Jorgensen for reagents. We would also like to thank Chen Lab members Jun Wang, Christian Frech, and Tao Luo for technical support in the study and the Caenorhabditis Genetics Center (CGC) for providing the strains. Most of all, we are grateful for funding sources: the Canadian Institutes for Health Research (CIHR) and Fanconi Anemia Fellowship to M.T.-G., and Natural Science and Engineering Research Council (NSERC) of Canada Discovery Grant to N.C. N.C. is a Michael Smith Foundation for Health Research (MSFHR) scholar and a CIHR new investigator.
Footnotes
Communicating editor: M. C. Zetka
Literature Cited
- Blethrow J. D., Glavy J. S., Morgan D. O., Shokat K. M., 2008. Covalent capture of kinase-specific phosphopeptides reveals Cdk1-cyclin B substrates. Proc. Natl. Acad. Sci. USA 105: 1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyter G. M., Furuta T., Kurasawa Y., Schumacher J. M., 2010. Caenorhabditis elegans cyclin B3 is required for multiple mitotic processes including alleviation of a spindle checkpoint-dependent block in anaphase chromosome segregation. PLoS Genet. 6: e1001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encalada S. E., Willis J., Lyczak R., Bowerman B., 2005. A spindle checkpoint functions during mitosis in the early Caenorhabditis elegans embryo. Mol. Biol. Cell 16: 1056–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex A., Dammermann A., Lewellyn L., Oegema K., Desai A., 2009. Systematic analysis in Caenorhabditis elegans reveals that the spindle checkpoint is composed of two largely independent branches. Mol. Biol. Cell 20: 1252–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C., Davis M. W., Hopkins C. E., Newman B. J., Thummel J. M., et al. , 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40: 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P., Nigg E. A., 1994. Identification of a novel vertebrate cyclin: cyclin B3 shares properties with both A- and B-type cyclins. EMBO J. 13: 595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis E. R., Stuurman N., Vale R. D., 2007. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J. Cell Biol. 177: 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B. J., McEwen B. F., Canman J. C., Hoffman D. B., Farrar E. M., et al. , 2001. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 155: 1159–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa R., 2009a Key players in chromosome segregation in Caenorhabditis elegans. Front. Biosci. 14: 1529–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa R., 2009b The spindle assembly checkpoint in Caenorhabditis elegans: one who lacks Mad1 becomes mad one. Cell Cycle 8: 338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa R., Rose A. M., 1999. Components of the spindle-assembly checkpoint are essential in Caenorhabditis elegans. Nat. Cell Biol. 1: 514–521 [DOI] [PubMed] [Google Scholar]
- Kitagawa R., Law E., Tang L., Rose A. M., 2002. The Cdc20 homolog, FZY-1, and its interacting protein, IFY-1, are required for proper chromosome segregation in Caenorhabditis elegans. Curr. Biol. 12: 2118–2123 [DOI] [PubMed] [Google Scholar]
- May K. M., Hardwick K. G., 2006. The spindle checkpoint. J. Cell Sci. 119: 4139–4142 [DOI] [PubMed] [Google Scholar]
- Musacchio A., Salmon E. D., 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8: 379–393 [DOI] [PubMed] [Google Scholar]
- Nguyen T. B., Manova K., Capodieci P., Lindon C., Bottega S., et al. , 2002. Characterization and expression of mammalian cyclin b3, a prepachytene meiotic cyclin. J. Biol. Chem. 277: 41960–41969 [DOI] [PubMed] [Google Scholar]
- Nieduszynski C. A., Murray J., Carrington M., 2002. Whole-genome analysis of animal A- and B-type cyclins. Genome Biol. 3: RESEARCH0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystul T. G., Goldmark J. P., Padilla P. A., Roth M. B., 2003. Suspended animation in C. elegans requires the spindle checkpoint. Science 302: 1038–1041 [DOI] [PubMed] [Google Scholar]
- Oegema K., Desai A., Rybina S., Kirkham M., Hyman A. A., 2001. Functional analysis of kinetochore assembly in Caenorhabditis elegans. J. Cell Biol. 153: 1209–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praitis V., Casey E., Collar D., Austin J., 2001. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157: 1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez I., Dynlacht B. D., 2005. New insights into cyclins, CDKs, and cell cycle control. Semin. Cell Dev. Biol. 16: 311–321 [DOI] [PubMed] [Google Scholar]
- Satyanarayana A., Kaldis P., 2009. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28: 2925–2939 [DOI] [PubMed] [Google Scholar]
- Schmidt D. J., Rose D. J., Saxton W. M., Strome S., 2005. Functional analysis of cytoplasmic dynein heavy chain in Caenorhabditis elegans with fast-acting temperature-sensitive mutations. Mol. Biol. Cell 16: 1200–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaram M. V., Wadzinski T. L., Redick S. D., Manna T., Doxsey S. J., 2009. Dynein light intermediate chain 1 is required for progress through the spindle assembly checkpoint. EMBO J. 28: 902–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N., 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119 [DOI] [PubMed] [Google Scholar]
- Tarailo M., Kitagawa R., Rose A. M., 2007a Suppressors of spindle checkpoint defect (such) mutants identify new mdf-1/MAD1 interactors in Caenorhabditis elegans. Genetics 175: 1665–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarailo M., Tarailo S., Rose A. M., 2007b Synthetic lethal interactions identify phenotypic “interologs” of the spindle assembly checkpoint components. Genetics 177: 2525–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarailo-Graovac M., Wang J., Tu D., Baillie D. L., Rose A. M., et al. , 2010. Duplication of cyb-3 (cyclin B3) suppresses sterility in the absence of mdf-1/MAD1 spindle assembly checkpoint component in Caenorhabditis elegans. Cell Cycle 9: 4858–4865 [DOI] [PubMed] [Google Scholar]
- van der Voet M., Lorson M. A., Srinivasan D. G., Bennett K. L., van den Heuvel S., 2009. C. elegans mitotic cyclins have distinct as well as overlapping functions in chromosome segregation. Cell Cycle 8: 4091–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoosthuyse V., Hardwick K. G., 2009. Overcoming inhibition in the spindle checkpoint. Genes Dev. 23: 2799–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J. C., Chen R. H., Murray A. W., Salmon E. D., 1998. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141: 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Tarailo-Graovac M., O'Neil N. J., Rose A. M., 2008. Spectrum of mutational events in the absence of DOG-1/FANCJ in Caenorhabditis elegans. DNA Repair (Amst.) 7: 1846–1854 [DOI] [PubMed] [Google Scholar]