Abstract
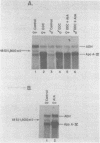
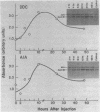
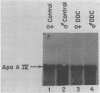
We have isolated cDNA clones for mRNAs that are induced by porphyria from a mouse liver library. Of the three inducible clones isolated, we have identified one as being apolipoprotein A-IV (apo A-IV) by its extensive homology with a rat apolipoprotein A-IV cDNA sequence. The level of liver apo A-IV mRNA increases rapidly in response to either of two porphyrogenic drugs. When the ferrochelatase-inhibited drug, 3,5-dicarbethoxy-1,4-dihydrocollidine (DDC) is used, a 6 and 28 fold induction of liver apo A-IV mRNA is observed in male and female mice, respectively. If the heme-destroying porphyrogenic drug, allylisopropylacetamide (AIA) is the inducing agent, liver apo A-IV mRNA levels increase 2-3 fold in both males and females. The level of apo A-IV mRNA reaches a maximum within 6-10 hr. after drug administration. Intestine apo A-IV mRNA levels do not change during either of these drug-induced porphyrias. RNA from acute-phase responsive liver or liver from mice treated with bilirubin, porphobilinogen, or protoporphyrin IX show no increase in apo A-IV mRNA. These results indicate that apo A-IV induction is tied to a disruption in porphyrin-heme biosynthesis but is not directly affected by several heme intermediates nor by the major heme degradation product, bilirubin.
Full text
PDF

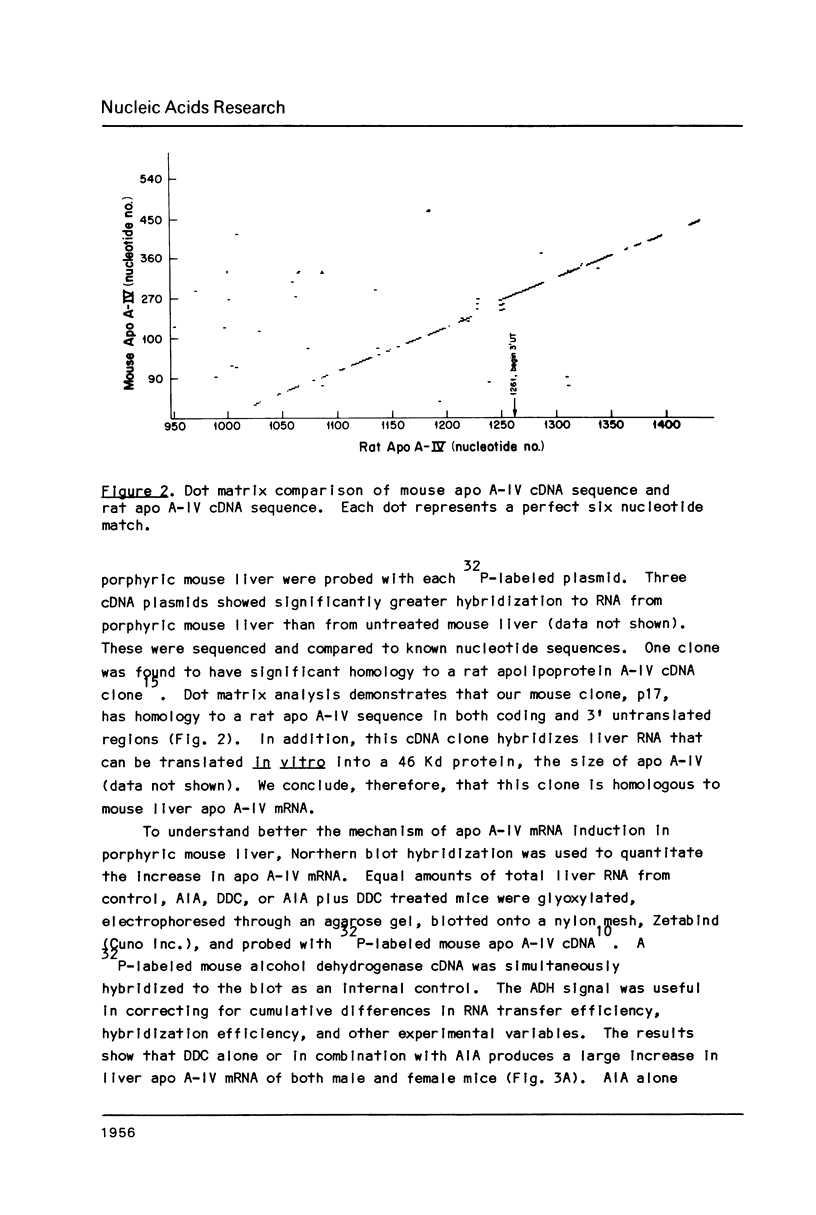
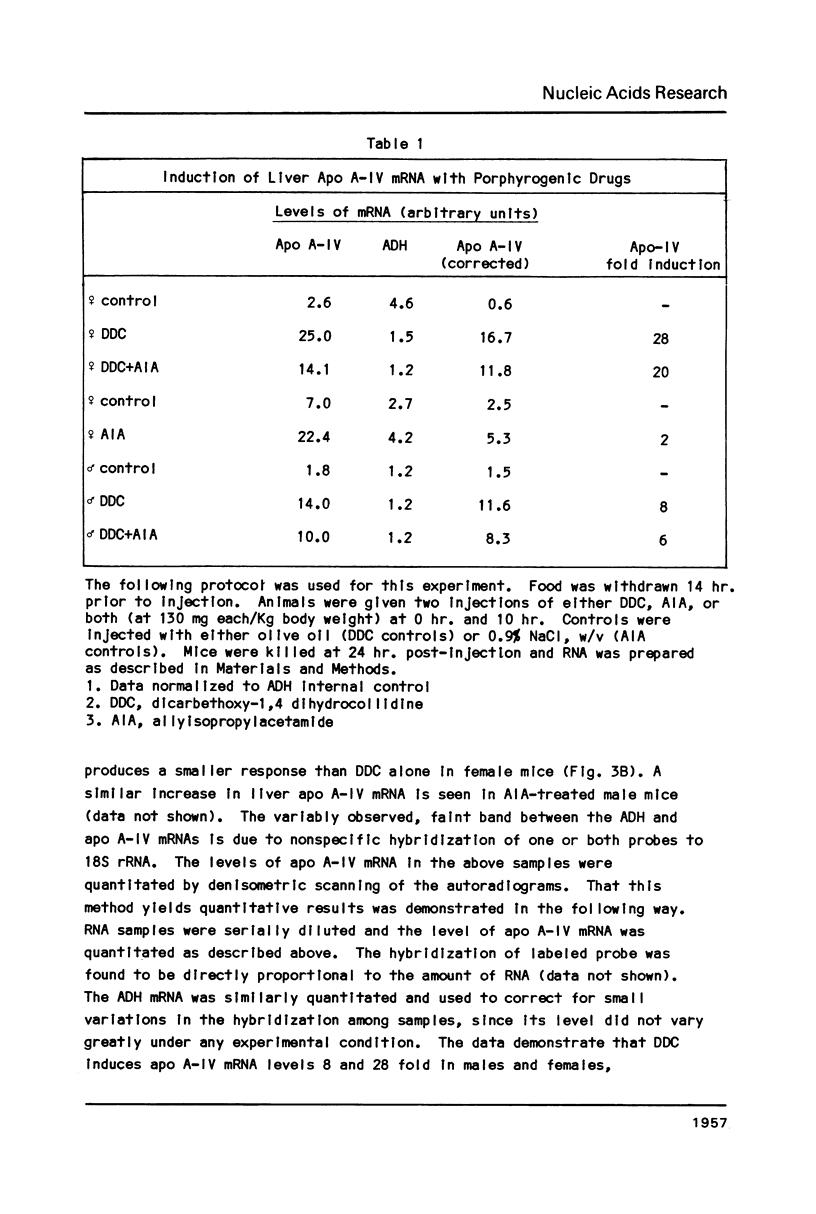
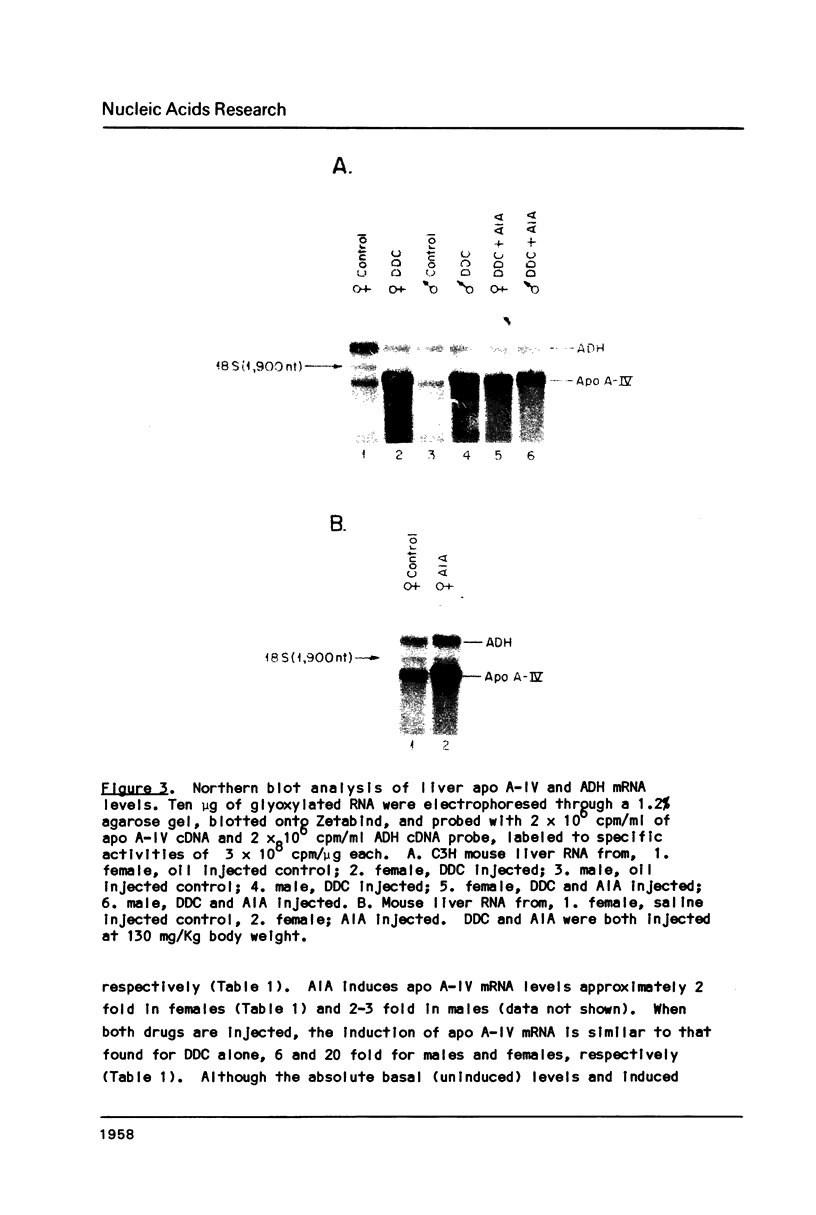
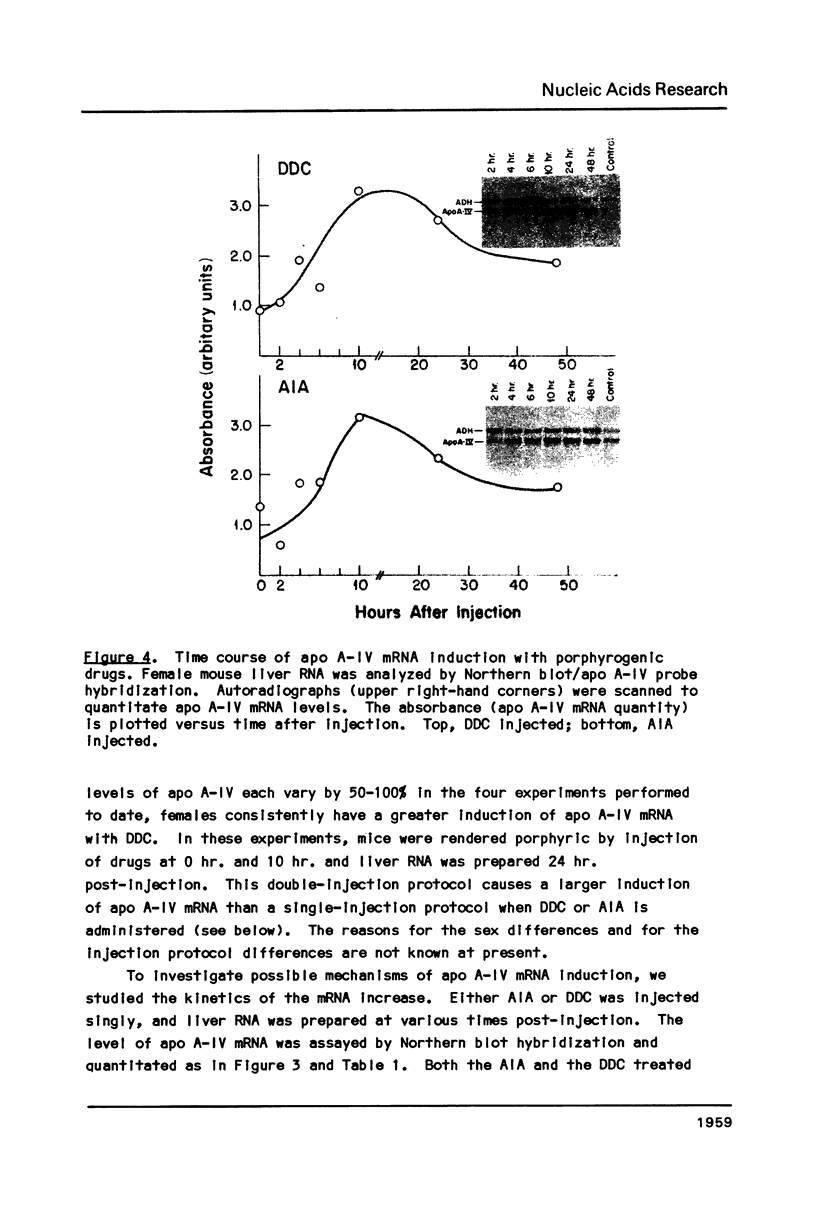




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Held W. A., Berger F. G. The acute phase response of mouse liver. Genetic analysis of the major acute phase reactants. J Biol Chem. 1984 Jan 10;259(1):566–573. [PubMed] [Google Scholar]
- Beisiegel U., Utermann G. An apolipoprotein homolog of rat apolipoprotein A-IV in human plasma. Isolation and partial characterisation. Eur J Biochem. 1979 Feb 1;93(3):601–608. doi: 10.1111/j.1432-1033.1979.tb12860.x. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Elshourbagy N., Taylor J. M., Gordon J. I. Rat apolipoprotein A-IV contains 13 tandem repetitions of a 22-amino acid segment with amphipathic helical potential. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5021–5025. doi: 10.1073/pnas.81.16.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker J. D., May B. K., Elliott W. H. Synthesis of delta-aminolaevulinate synthase in vitro using hepatic mRNA from chick embryos with induced porphyria. Eur J Biochem. 1980 May;106(1):17–24. doi: 10.1111/j.1432-1033.1980.tb05992.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S. R., Hutton J. J. Induction of hepatic delta-aminolevulinic acid synthetase activity in strains of inbred mice. J Biol Chem. 1971 Feb 10;246(3):606–614. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Gielen J., Packman S., Ikawa Y., Leder P. Globin gene expression in cultured erythroleukemic cells. J Mol Biol. 1974 Aug 25;87(4):697–714. doi: 10.1016/0022-2836(74)90079-5. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Wu A. L. Biosynthesis of plasma apolipoproteins by rat small intestine without dietary or biliary fat. J Biol Chem. 1981 Mar 25;256(6):3012–3016. [PubMed] [Google Scholar]
- Yamamoto M., Hayashi N., Kikuchi G. Translational inhibition by heme of the synthesis of hepatic delta-aminolevulinate synthase in a cell-free system. Biochem Biophys Res Commun. 1983 Aug 30;115(1):225–231. doi: 10.1016/0006-291x(83)90993-2. [DOI] [PubMed] [Google Scholar]