Abstract
Introduction
Vitamin D is a sectosteroid that functions through Vitamin D receptor (VDR), a transcription factor, which controls the transcription of many targets genes. Vitamin D deficiency has been linked with cardiovascular diseases, including heart failure and coronary artery disease. Suppressor of cytokine signaling (SOCS)3 regulates different biological processes such as inflammation and cellular differentiation and is an endogenous negative regulator of cardiac hypertrophy.
Objective
The purpose of this study was to test the hypothesis that vitamin D deficiency causes cardiomyocyte hypertrophy and increased proinflammatory profile in epicardial adipose tissue(EAT), and this correlates with decreased expression of SOCS3 in cardiomyocytes and EAT.
Methods
Eight female Yucatan miniswine were fed vitamin D-sufficient (900 IU/d) or vitamin D-deficient hypercholesterolemic diet. Lipid profile, metabolic panel, and serum 25(OH)D levels were regularly measured. After 12 months animals were euthanized and histological, immunohistochemical and qPCR studies were performed on myocardium and epicardial fat.
Results
Histological studies showed cardiac hypertrophy, as judged by cardiac myocyte cross sectional area, in vitamin D-deficient group. Immunohistochemical and qPCR analyses showed significantly decreased mRNA and protein expression of VDR and SOCS3 in cardiomyocytes of vitamin D-deficient animals. EAT from vitamin D-deficient group had significantly higher expression of TNF-α, IL-6, MCP-1, and decreased adiponectin in association with increased inflammatory cellular infiltrate. Interestingly, EAT from vitamin D-deficient group had significantly decreased expression of SOCS3.
Conclusion
These data suggest that vitamin D deficiency induces hypertrophy in cardiomyocytes which is associated with decreased expression of VDR and SOCS3. Vitamin D deficiency is also associated with increased inflammatory markers in EAT. Activity of VDR in the body is controlled through regulation of vitamin D metabolites. Therefore, restoration of VDR function by supplementation of VDR ligands in vitamin D- deficient population might be helpful in reducing inflammation and cardiovascular risk.
Keywords: Cardiac Hypertrophy, Coronary Artery Disease, Epicardial Fat, Inflammation, Vitamin D
Introduction
Vitamin D is a sectosteroid that functions through Vitamin D receptor (VDR), a transcription factor, which regulates various downstream signaling pathways and controls the transcription of many targets genes (Fleet, 2008; Nagpal et al., 2005). Prior to binding to VDR, vitamin D needs to be activated by a tightly regulated process of two enzymatic steps. Vitamin D3 is synthesized in the skin, metabolized in the liver to form 25(OH)D, and then further hydroxylated in the kidney to form the active hormone, 1,25- dihydroxyvitamin D3 (calcitriol)(Holick, 2007). The biological actions of calcitriol are mediated by VDR. Cardiovascular risk factors, including obesity, hypertension, hyperlipidemia, and diabetes are inversely associated with serum 25(OH)D levels (Martins et al., 2007). Low levels of 25(OH)D and 1, 25-dihydroxyvitamin D are associated with increased risk of incident congestive heart failure, myocardial infarction, and mortality due to cardiovascular disease (Dobnig et al., 2008; Giovannucci et al., 2008; Wang et al., 2008; Zittermann et al., 2003, 2006).
Epicardial adipose tissue (EAT) is a metabolically active organ that can substantially modulate the heart and coronary arteries through vasocrine or paracrine secretion of various bioactive molecules (Fain et al., 2010; Iacobellis et al., 2005; Kremen et al., 2006; Mazurek et al., 2003). The secretion of various epicardial inflammatory adipokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), adiponectin, monocyte chemoattractant protein-1 (MCP-1), contribute to inflammatory milieu and play a significant role in the development and progression of atherosclerosis (Iacobellis, 2009; Sacks and Fain, 2007). Subjects with coronary artery disease (CAD) have elevated inflammatory infiltrates in EAT (Mazurek et al., 2003).
Tyrosine kinase family -Jak activation and subsequent activation of STAT are key events in signal transduction of different cytokines and growth factors (Heinrich et al., 2003; Ihle, 2001). These proteins regulate different biological processes including inflammation, cellular differentiation, and the proliferation (Ehlting et al., 2007). Suppressor of cytokine signaling (SOCS)3, a physiologic inhibitory protein of JAK/STAT3, attenuates proinflammatory signaling by inhibiting STAT3 phosphorylation (Dominguez et al., 2010; Jo et al., 2005). SOCS3 also acts as an endogenous negative regulator of cardiac hypertrophy (Terrell et al., 2006; van Empel and De Windt, 2004; Yasukawa et al., 2001).
Recently, vitamin D deficiency has been identified as a potential risk factor for cardiovascular diseases (Michos and Melamed, 2008). However, most of these evidences are based on epidemiological and observational studies and underlying mechanism is still unclear. Here, we tested the hypothesis that vitamin D deficiency causes left ventricular hypertrophy and increase pro-inflammatory profile in EAT which is associated with decreased expression of SOCS3 in cardiomyocytes and EAT in atherosclerotic Yucatan miniswine.
Material and Methods
Experimental Animals
In this study, we used Yucatan miniswine, weighing 30-40 lbs, and purchased from Lonestar Laboratories (Sioux Center, IA). Although no model completely mimics the human pathology, the swine model represents a best choice for coronary artery disease because of similarities in the anatomy and physiology between human and porcine cardiovascular system. Also, the underlying disease process of atherosclerosis in swine develops in a similar manner to those in humans. The Institutional Animal Care and Use Committee of Creighton University approved all research protocols and the animals were housed in the Animal Resource Facility of Creighton University, Omaha, NE and cared for as per National Institute of Health standards.
Hypercholesterolemic Vitamin D- Deficient Swine Model
Yucatan miniswine were randomly divided into 2 groups of 4 animals in each group. Group 1 was fed with 1-1.5 lb/swine/day of a vitamin D-sufficient high cholesterol diet (Harlan, USA) with the following major ingredients: 37.2.% corn (8.5% protein), 23.5% soybean meal (44% protein), 20% chocolate mix, 5% alfalfa, 4% cholesterol, 4% peanut oil, 1.5% sodium cholate, and 1% lard. Animals in group 2 received vitamin D-deficient high cholesterol swine diet (Harlan, USA) with the following major ingredients: 19% casein “vitamin free”, 23.5% sucrose, 23.9% corn starch, 13% maltodextrin, 4% soybean oil, 4% cholesterol, and 10% cellulose. Venous blood (10 ml) from the ear vein was drawn every 8 weeks to examine complete blood count (CBC), complete metabolic profile, complete lipid profile, and serum 25(OH)D levels. Animals were housed in the Creighton animal facility under controlled conditions, 12:12-h light-dark cycle at 20–24°C, without exposure to sunlight and fed a controlled diet to avoid any variation in the 25(OH)D levels due to season or diet. After 48 weeks, animals were euthanized by administering high dose of barbiturates (Beuthanasia-D 1.0ml/10 lb i.v.).
Tissue Harvest and Processing
Immediately after euthanasia swine hearts were removed and placed in a medium (RPMI with 10% FBS, 500U/ml penicillin, 500μg/ml streptomycin). Epicardial adipose tissue (EAT) surrounding the coronary vessel extending from 10 mm up to 20 mm on each side of the vessel wall was removed from the heart surface. Three tissue sections (1 × 1 cm) from left ventricle (LV) free wall were also collected. Small portion of EAT and LV tissue were placed in an eppendorf tube sitting on dry ice and the remaining tissues were fixed in 4% formalin. Formalin-fixed tissues were embedded in paraffin and thin sections (5 μm) were obtained using microtome (Leica, Germany) for histomorphometric and immunohistochemical evaluation.
mRNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
Approximately 500 mg of frozen tissue was used to isolate total RNA using the Trizol reagent (Sigma, St. Louis, MO). The yield of RNA was quantified by Nanodrop (GE Healthcare, USA) and subjected to reverse transcription using the Improm-II reverse transcription kit (Promega, Madison, WI). Then, c DNA was subjected to qPCR for VDR gene using SYBR Green PCR kit (Promega, Madison, WI). Quantification was done by normalization against GAPDH. Following primer sequences were used: VDR: forward, 5′- AATGGCGGCCAGCACTTCCC; reverse,5′- CTGGCAGTGGCGTCGGTTGT; SOCS3: forward, 5′- CTTCAGCTCCAAGACTCGAGT; reverse, 5′- AACACCAGGGGGATCTTCCT; GAPDH: forward, 5′-ACACTCACTCTTCTACCTTTG; reverse, 5′- CAAATTCATTGTCGTACCAG.
Histology and Measurements of Cardiomyocyte Area
For histomorphometric examination paraffin-embedded samples were cut (5 μm), deparaffinized, rehydrated and stained with hematoxylin and eosin (H&E) and trichrome stain. The size of the cardiomyocyte was assessed on H&E-stained sections using NIH Image J software (http://rsb.info.nih.gov/ij/). Randomly chosen cardiomyocytes (50-100) from each heart were used to measure cross-sectional area of the cardiomyocytes.
Immunohistochemistry
For immunohistochemical studies samples were treated by steam heating for antigen retrieval (20-30 min) using DAKO antigen retrieval solution (DAKO, Carpenteria, CA). Slides were washed twice 5 min each using phosphate buffered saline (PBS). Endogenous peroxidase was inhibited by immersing the slides in a 3% hydrogen peroxide solution for 20 min. Slides were then washed twice for 5 min in PBS. After pre-incubation of the tissue sections with 10% serum for 1 h to avoid non-specific binding (VECTASTAIN Elite ABC system, Vector Laboratories, Burlingame, CA), the sections were incubated with primary antibodies; rabbit anti- SOCS-3 (Ab16030; Abcam), mouse anti-TNF-α(Ab1793; Abcam), rabbit anti-MCP-1 (Ab7202; Abcam), rabbit anti-IL-6 (Ab6672-Abcam), mouse anti-adiponectin (Ab22554; Abcam) overnight at 4°C. Antibodies were diluted (1:200) with PBS. Slides were washed twice with PBS and consecutively incubated with biotinylated secondary antibody for 1 hr. Slides were washed twice with PBS and incubated with ABC solution (VECTASTAIN Elite ABC system, Vector Laboratories, Burlingame, CA) for 30 min. Slides were washed twice again with PBS and finally incubated with diaminobenzidine (Vector Laboratories, Burlingame, CA) for 1 min. Immediately after staining, slides were washed with distilled water for 5 min and counterstained with hematoxylin for 7 sec. Slides were rinsed for 5 min with distilled water and dehydrated for 3–5 min each with 70–100% isopropanol. Finally, samples were immersed in xylene for 5 min each and mounted by using permount (Fisher Scientific, Pittsburg, PA). Sections incubated without the primary antibody served as negative controls. Slides were examined using an inverted microscope (Olympus).
Immunofluorohistochemistry
After deparaffinization and rehydration, antigen retrieval was performed prior to immunostaining. Sections were incubated for 2 hr in block/permeabilizing solutions containing PBS, 0.25 % Triton X-100, and 5% (v/v) goat serum at room temperature. The slides were subsequently incubated with primary antibodies including mouse anti-VDR (SantaCruz Biotech; sc-1008), rabbit anti- SOCS-3 (Abcam; Ab16030), (1:100 antibodies) at 4°C overnight. After washing with PBS times for 5 minutes each, a secondary antibody (affinity purified goat anti-mouse and goat anti-rabbit cyanine 3 (cy3) antibody, 1:200) (Jackson ImmunoResearch, Westgrove, PA) was applied to the sections for 1 hr in dark. Negative controls were run in parallel with normal host IgG including chromPure mouse IgG, and ChromPure rabbit IgG or complete omission of primary antibody. Sections were washed with PBS three times for 5 min. Nuclei were counterstained with 4′,6- diamidino-2-phenylindole (DAPI). A single layer of nail polish was placed around the edge of slide to prevent escape of mounting media from the coverslip. Pictures were taken within 1 hr of mounting using Olympus DP71 camera. Total fluorescence measurements were performed with NIH ImageJ software.
Statistical analysis
Data was analyzed using GraphPad Prism 5.0 biochemical statistical package (GraphPad Software, Inc., San Diego, CA). Values of all measurements were expressed as mean ± SEM. Statistical analysis was performed using two-tailed unpaired student t-test or one-way ANOVA with Bonferroni's multiple comparison test to analyze statistically significant differences between groups. Differences at p<0.05 were considered significant. The semi-quantitative scoring of immunostaining was performed as follows: 0- undetectable, 1+- weekly positive, 2+- moderately positive, 3+- strongly positive
Results
Vitamin D and Lipid Profile
Vitamin D-deficient high cholesterol diet produced significant vitamin D deficiency in swine. At the time of euthanasia, serum levels of 25(OH)D, the major circulating form of vitamin D, were significantly (p <0.01) decreased in swine on vitamin D-deficient diet (16.67 ± 1.20 ng/ml) compared to swine on vitamin D-sufficient high cholesterol diet (24.33±2.33 ng/ml) (Fig. 1A). There was no difference in total serum cholesterol, serum high density lipoprotein (HDL) and serum low density lipoprotein (LDL) levels between the two groups. Mean serum cholesterol, LDL and HDL levels at the time of euthanasia were 417±27, 389±15 and 99.5± 13.8 in vitamin D-deficient hypercholesterolemic group versus 402±30, 402.3±25.9 and 96±7 in the vitamin D-sufficient hypercholesterolemic group, respectively (Fig. 1B-D).
Figure 1.
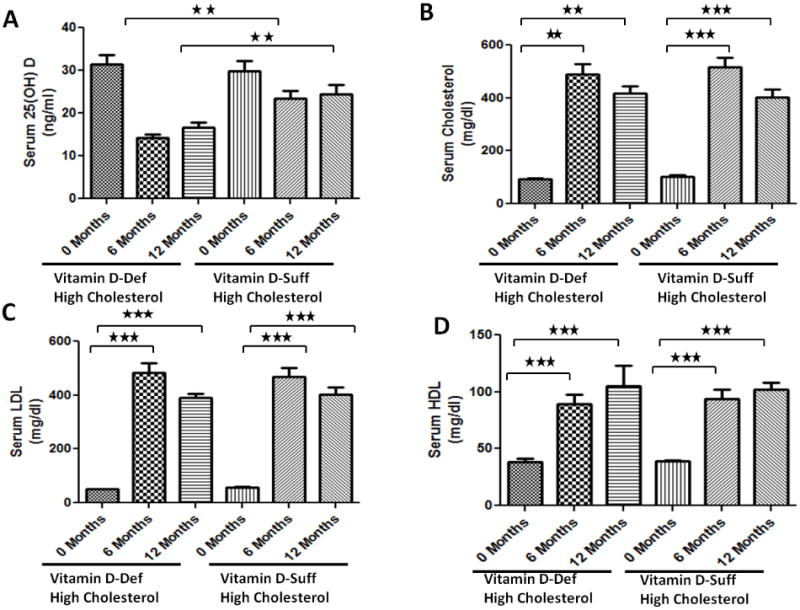
The effects of vitamin D-deficient high cholesterol and vitamin D-sufficient high cholesterol diet on (A) serum 25-hydroxy vitamin D, (B) total cholesterol, (C) low density lipoprotein (LDL), and (D) high density lipoprotein (HDL) levels of the female Yucatan miniswine following 12 months of administration of the diet. Data are shown as mean ± SEM (N=8); *P <0.05, * *P< 0.01, * * *P< 0.001.
Vitamin D deficiency is associated with cardiomyocyte hypertrophy in hypercholesterolemic swine
Morphometric analyses of Masson's trichrome (Fig.2 A,B) and H&E (Fig.2C,D)- stained sections demonstrated that cross-sectional area of ventricular cardiomyocytes from vitamin D-deficient hypercholesterolemic swine was significantly increased compared to those in vitamin D-sufficient hypercholesterolemic swine (12552 ± 783 μm2 and 7817± 402 μm2, respectively; P< 0.001) (Fig.2E). Masson trichrome staining revealed no difference in ventricular collagen deposition between the groups.
Figure 2.
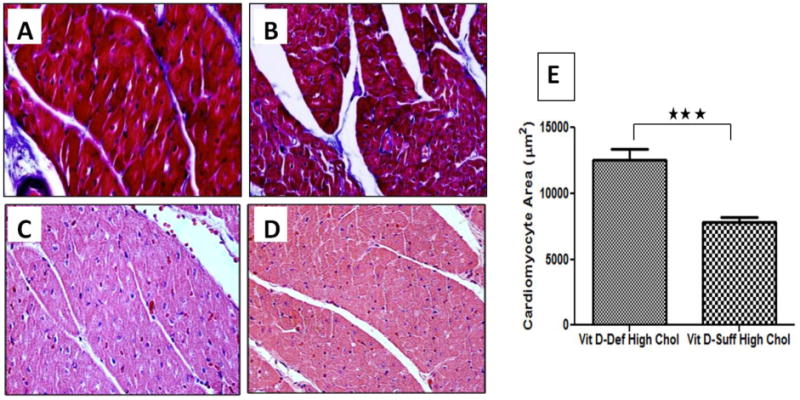
Histological analysis of left ventricular myocytes from vitamin D-deficient hypercholesterolemic and vitamin D-sufficient hypercholesterolemic swine. Paraffin embedded thin sections were cut, deparaffinized and histological analysis was performed using H&E, and Masson's trichrome stains. (A, B): Masson's trichrome staining of vitamin D-deficient hypercholesterolemic (A) and vitamin D-sufficient hypercholesterolemic swine (B); (C, D): H&E staining of vitamin D-deficient hypercholesterolemic (C) and vitamin D-sufficient hypercholesterolemic swine (D). Note the larger size of cardiomyocytes in vitamin D-deficient hypercholesterolemic group; E: Mean cross-sectional area of cardiomyocytes (±SEM) in vitamin D-deficient hypercholesterolemic and vitamin D-sufficient hypercholesterolemic swine was measured. Two-tailed unpaired Student t tests were performed to determine statistical relevance; significant P value is shown; *P <0.05, * *P< 0.01, * * *P< 0.001. Magnification 600×
VDR mRNA and protein expression are decreased in cardiomyocytes of vitamin D-deficient swine
VDR mRNA expression in the cardiomyocytes of vitamin D-deficient hypercholesterolemic swine was significantly lower (∼3 folds) than that in vitamin D- sufficient hypercholesterolemic swine (P<0.05; Fig.3A). Furthermore, VDR protein expression in the left ventricular cardiomyocytes was significantly reduced in vitamin D- deficient hypercholesterolemic swine compared to vitamin D-sufficient hypercholesterolemic swine (Fig. 3B and 3C). VDR protein expression was quantified based on immunofluorescence intensity and the data are expressed as mean ± SEM in arbitrary units (AU). Vitamin D deficient hypercholesterolemic group showed significantly decreased (P< 0.001) VDR protein expression (51,591 ±1,841) compared to hypercholesterolemic group (159,222 ± 3,454 (Fig. 3D).
Figure 3.
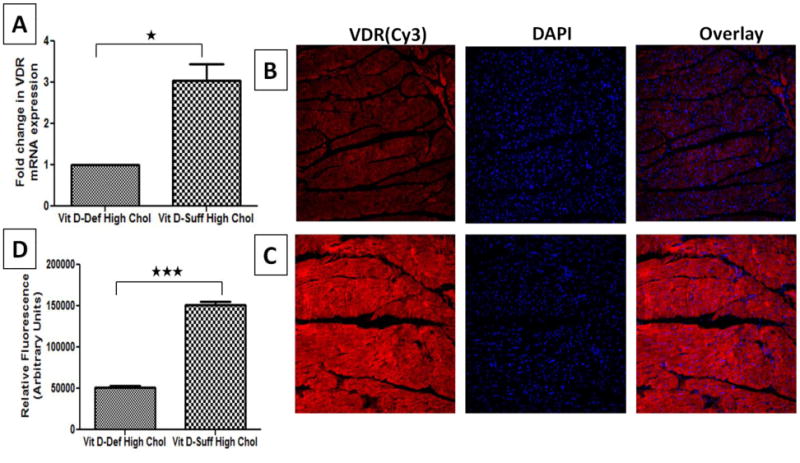
Expression of VDR in cardiomyocytes of hypercholesterolemic swine following vitamin D deficiency. A: Total RNA was isolated from cardiac tissue and expression of VDR was detected by quantitative PCR. The fold change in VDR expression between samples was calculated by Fold change= 2 –ΔΔCt method. The results were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B, C): Immunofluorescence of cardiac VDR expression in vitamin D-deficient hypercholesterolemic (B) and vitamin D-sufficient hypercholesterolemic (C) swine. Sections were stained using mouse anti- VDR antibody and goat anti-mouse cy3 as secondary antibody. DAPI was used to stain the nuclei. DAPI overlay is shown with mouse anti- VDR and goat anti-mouse cy3 as secondary antibody; D: Mean fluorescent intensity of staining obtained with anti-VDR antibody was measured in arbitrary units using NIH Image J software. Two-tailed unpaired student t tests were performed to determine statistical relevance; significant P values are shown. Data are shown as mean ± SEM (N=8); *P <0.05, * *P< 0.01, * * *P< 0.001. Magnifications 200×.
SOSC3 mRNA and protein expression are decreased in cardiomyocytes of vitamin D-deficient swine
SOCS3 mRNA expression was significantly lower in the cardiomyocytes of vitamin D-deficient hypercholesterolemic swine and was approximately 4-fold lower than in the cardiomyocytes of vitamin D-sufficient hypercholesterolemic swine (P<0.05; Fig. 4A). SOCS3 protein expression in the left ventricular cardiomyocytes was significantly reduced in vitamin D-deficient hypercholesterolemic swine compared to vitamin D- sufficient hypercholesterolemic swine (Fig. 4C, D).
Figure 4.
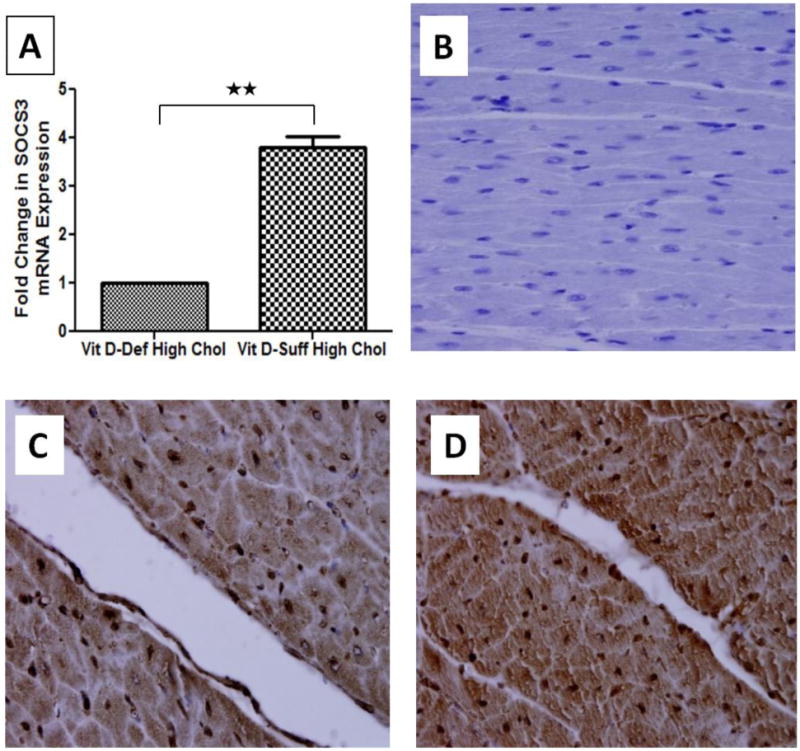
Expression of SOCS3 in cardiomyocytes of hypercholesterolemic swine following vitamin D deficiency. A: Total RNA was isolated from cardiac tissue and expression of SOCS3 was detected by quantitative PCR. The fold change in SOCS3 expression between samples was calculated by Fold change= 2 –ΔΔCt method. The results were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data shown are mean ± SEM (N=8). *P <0.05, * *P< 0.01, * * *P< 0.001. (C, D): Immunohistochemical expression of cardiac SOCS3 in vitamin D-deficient hypercholesterolemic (C) and vitamin D-sufficient hypercholesterolemic (D) swine. Sections were stained with DAB as chromogen and counterstained using hematoxylin. Decreased SOCS3 expression was observed in vitamin D-deficient hypercholesterolemic group. Negative control is shown (B). Magnification 600×
Vitamin D deficiency is associated with increased inflammatory infiltrates and decreased SOCS3 expression in epicardial adipose tissue
The EAT samples were examined by H&E staining and immunohistochemistry. Compared to the vitamin D-sufficient high cholesterol diet group, the EAT from the vitamin D-deficient high cholesterol group showed higher dense inflammatory cell infiltrate (Fig. 5A, B). SOCS3 protein expression was significantly decreased in EAT of vitamin D-deficient hypercholesterolemic swine compared to vitamin D-sufficient hypercholesterolemic swine (Figure 5C, D).
Figure 5.
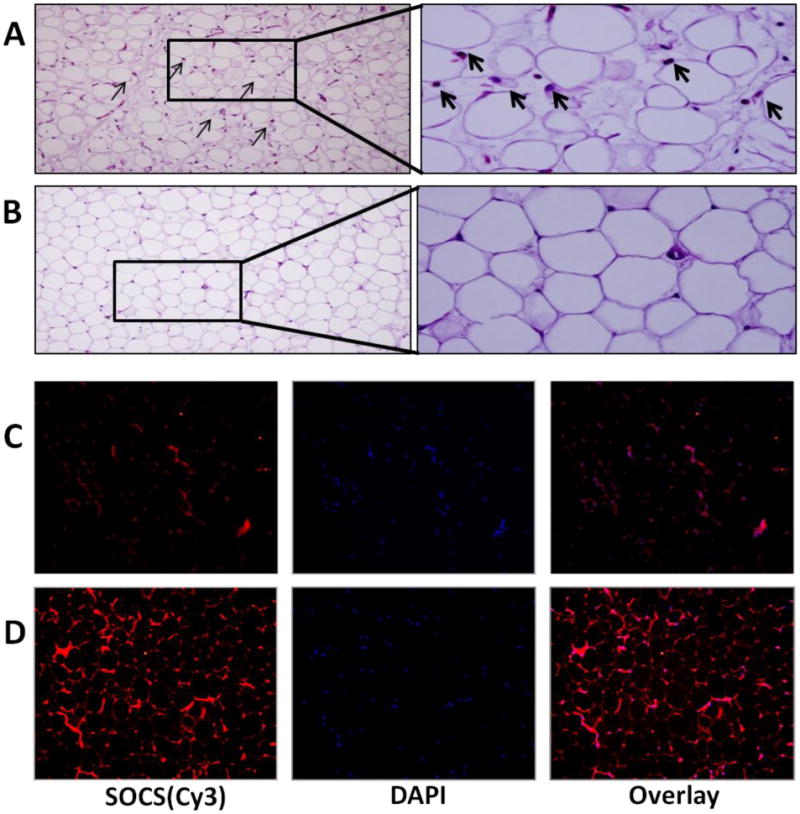
Vitamin D- deficiency mediated increased inflammatory infiltrates and decreased SOCS3 expression in EAT of hypercholesterolemic swine. (A, B): H&E staining of paraffin sections of EAT from both vitamin D-deficient hypercholesterolemic (A) and vitamin D-sufficient hypercholesterolemic swine (B). Arrows indicate inflammatory cells. EAT of vitamin D-deficient hypercholesterolemic swine showed significantly increased inflammatory cellular infiltrate. Magnification (200-600×); (C, D): Immunofluorescence of SOCS3 expression in vitamin D-deficient hypercholesterolemic (C) and vitamin D-sufficient hypercholesterolemic (D) swine EAT. Sections were stained using rabbit anti- SOCS3 antibody and goat anti-rabbit cy3 as secondary antibody. DAPI was used to stain the nuclei. DAPI overlay is shown with rabbit anti-SOCS3 and goat anti-rabbit cy3 as secondary antibody. Decreased expression of SOCS3 was observed in vitamin D-deficient hypercholesterolemic group. Magnification (200×)
Vitamin D deficiency increases adipokine protein expression in epicardial adipose tissue
Expression of TNF-α, MCP-1, IL-6, and adiponectin was evaluated by immunohistochemistry. There was a marked increase in TNF-α (Figure 6A, B), MCP-1 (Figure 6C, D), and IL-6 (Figure 7A, B) expression in the EAT of vitamin D-deficient hypercholesterolemic animals compared to that in the vitamin D-sufficient high cholesterol diet group. However, adiponectin (Figure 7C, D) expression was significantly greater in vitamin D-sufficient high cholesterol diet group compared to vitamin D- deficient hypercholesterolemic swine.
Figure 6.
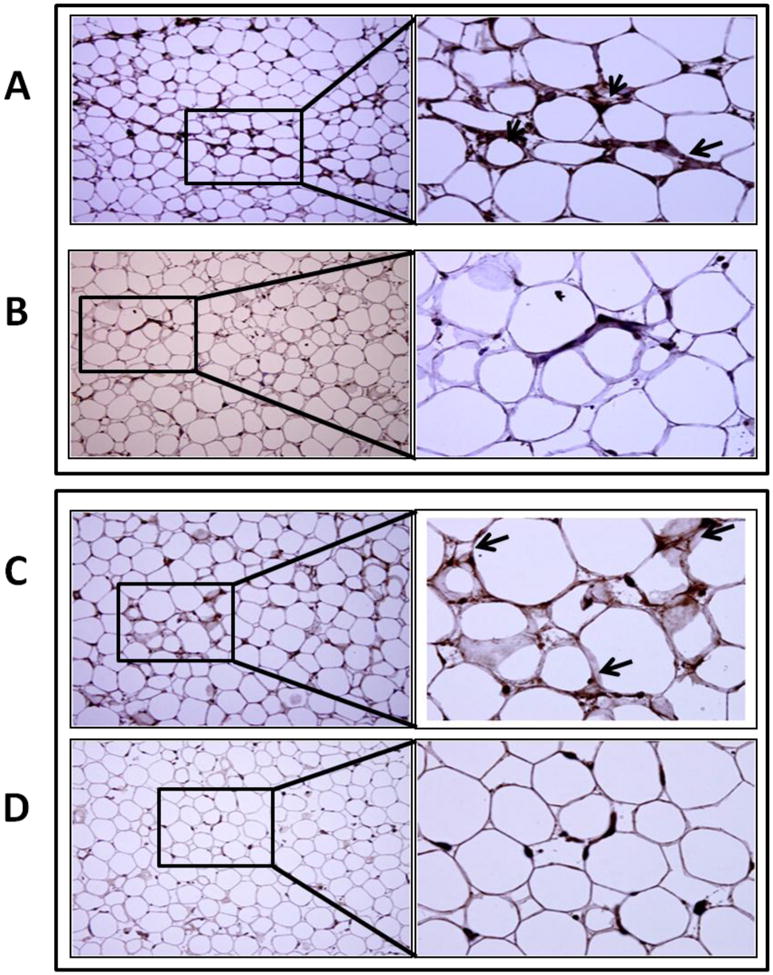
Immunohistochemical detection of adipokines in EAT. Sections were stained with DAB as chromogen and counterstained using hematoxylin. (A, B): Expression of TNF-α in EAT of vitamin D-deficient hypercholesterolemic (A) and vitamin D-sufficient hypercholesterolemic swine (B). Increased TNF-α expression (arrows) was observed in vitamin D-deficient hypercholesterolemic group. (C, D): Expression of MCP-1 in EAT of vitamin D-deficient hypercholesterolemic (C) and vitamin D-sufficient hypercholesterolemic swine (D). Increased MCP-1 expression (arrows) was observed in vitamin D-deficient hypercholesterolemic group. Magnification (200 ×- 600×).
Figure 7.
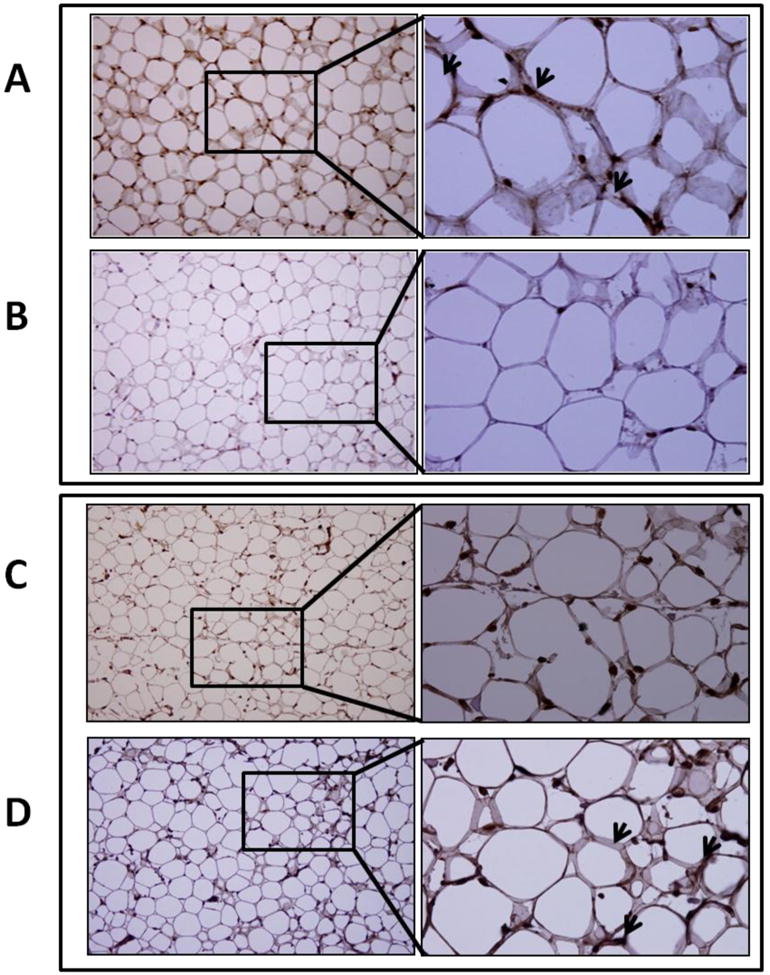
Immunohistochemical detection of adipokines in EAT. Sections were stained with DAB as chromogen and counterstained using hematoxylin. (A, B): Expression of IL-6 in EAT of vitamin D-deficient hypercholesterolemic (A) and vitamin D-sufficient hypercholesterolemic swine (B). Increased IL-6 expression (arrows) was observed in vitamin D-deficient hypercholesterolemic group. (C, D): Expression of adiponectin in EAT of vitamin D-deficient hypercholesterolemic (C) and vitamin D-sufficient hypercholesterolemic swine (D). Adiponectin expression (arrows) was higher in vitamin D-sufficient hypercholesterolemic group. Magnification (200 ×- 600×).
Discussion
Vitamin D deficiency is associated with the etiology and pathogenesis of heart disease, including congestive heart failure (Park et al., 1999; Wu-Wong, 2011; Zittermann et al., 2003). Evidence from recent studies in animal models suggest that vitamin D or vitamin D receptor deficiency promotes cardiac hypertrophy, which might be the one of the mechanisms for increased cardiovascular risk (Simpson et al., 2007; Xiang et al., 2005). Maternal vitamin D deficiency leads to cardiac hypertrophy in rat offspring (Gezmish et al., 2010). However, all these studies have been done in small animal models including mice or rat and their significance to human is not clear.
In our study, we for the first time report the effect of vitamin D deficiency on cardiomyocytes hypertrophy and EAT inflammation in a hypercholesterolemic swine model, which is similar to human disease. Results from our studies confirm that vitamin D deficiency is associated with cardiomyocyte hypertrophy in hypercholesterolemic Yucatan miniswine. Heart is believed to be the target organ of vitamin D because VDR is expressed in cardiomyocytes (Fraga et al., 2002). Results from in vitro studies have also suggested that vitamin D inhibits cardiomyocyte proliferation and hypertrophy (Chen et al., 1998; Wu et al., 1996). Most of these biological actions of vitamin D are exerted through the nuclear VDR-mediated control of target genes (Kato, 2000). We observed significantly decreased expression of VDR in ventricular cardiomyocytes of vitamin D-deficient swine. These finding are in accordance with the previous studies which suggest vitamin D deficiency is associated with decreased VDR expression in different tissues (Healy et al., 2003; Zineb et al., 1998).
Negative feedback control of JAK-STAT by SOCS3 provides a critical key molecular switch for a negative feedback circuit for the hypertrophy of cardiomyocytes (Podewski et al., 2003; Yasukawa et al., 2001). Therefore, we examined the expression of SOCS3 in left ventricular cardiomyocytes. Interestingly, there was significantly decreased expression of SOCS3 in left ventricular cardiomyocytes of vitamin D-deficient swine compared to the vitamin D-sufficient group. These observations suggest that the mechanism underlying cardiomyocyte hypertrophy in vitamin D-deficient swine, at least in part, might be the consequence of decreased SOCS3 expression in the cardiomyocytes. However, potentially causal effect in the pathogenesis of coronary artery disease and the underlying cellular and molecular mechanisms on relationship between vitamin D and SOCS3 warrant further investigation.
EAT is a key source of both pro- (TNF-α, IL-6) and anti- (adiponectin) inflammatory adipokines and chemokines, including monocyte chemoattractant factor (MCP)-1 (Baker et al., 2006; Mazurek et al., 2003). The disorders of endocrine factors secreted by EAT can induce inflammation that contributes to the occurrence of cardiovascular disease including coronary artery disease (Zhou et al., 2011). Recent studies have demonstrated that vitamin D, particularly its metabolite 25(OH)D, has immunomodulatory effects and it decreases the risk of many chronic illnesses, including cancers, infectious diseases, autoimmune diseases, and cardiovascular disease (Holick, 2007). The data in our study demonstrate that there was significantly increased expression of IL-6, TNF-α, and MCP-1 in the EAT of vitamin D-deficient hypercholesterolemic swine compared to vitamin D-sufficient hypercholesterolemic group.
A low-circulating or tissue level of adiponectin is associated with various cardiovascular diseases, including CAD (Antoniades et al., 2009; Zhou et al., 2011). We observed significantly decreased expression of adiponectin in the EAT of vitamin D- deficient swine. These findings imply vitamin D deficiency as a contributory factor in the activation of inflammatory adipokines in EAT, suggesting the immunomodulatory role of 25(OH)D in the pathogenesis of CAD. The production of inflammatory mediators depends on tightly regulated intracellular signaling. SOCS3 attenuates pro-inflammatory signaling mediated by the signal transducer and activator of transcription (STAT) family proteins (Jo et al., 2005). Recent studies have demonstrated that SOCS3 play a negative regulatory role in inflammatory arthritis (Shouda et al., 2001) and intestinal inflammation (Suzuki et al., 2001). Moreover, it has been shown that reduced SOCS3 expression is associated with increased leukocytic infiltration (Ortiz-Munoz et al., 2009; Tomita et al., 2011). In our study, significantly decreased SOCS3 expression and increased leukocytic infiltration was observed in the EAT of vitamin D-deficient hypercholesterolemic swine. Thus, our current data support the possibility that vitamin D deficiency may contribute to reduced SOCS3 expression in EAT leading to shift in adipokines profile and increased inflammatory cellular infiltration. However, additional studies on the association between vitamin D status and SOCS3 are needed for a better understanding of the regulatory mechanism.
Conclusion
Vitamin D deficiency results in the hypertrophy of left ventricular cardiomyocyes and increased inflammatory cellular infiltrate and increased pro-inflammatory adipokine expression in EAT. These effects are associated with decreased SOCS3 expression in both cardiomyocytes and EAT. Taken together, data from this study suggest that vitamin D regulates cardiovascular functions by various mechanisms involving both direct effects on the cardiomyocytes hypertrophy and inflammatory profile in EAT. This could be a potential contributing factor for increased atherogenesis and altered cardiac function in vitamin D-deficient population. Combined with the epidemiologic data demonstrating a protective effect of vitamin D on cardiovascular health, this study supports the notion that vitamin D sufficiency might play a vital role in either preventing or decreasing the burden of cardiovascular diseases.
Acknowledgments
This work was supported by NIH grants R01HL104516 and R01HL116042 to DKA.
Abbreviations
- EAT
Epicardial Adipose Tissue
- SOCS3
Suppressor of Cytokine Signaling 3
- VDR
Vitamin D Receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoniades C, Antonopoulos AS, Tousoulis D, Stefanadis C. Adiponectin: from obesity to cardiovascular disease. Obes Rev. 2009;10:269–279. doi: 10.1111/j.1467-789X.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wu J, Hsieh JC, Whitfield GK, Jurutka PW, Haussler MR, Gardner DG. Suppression of ANP gene transcription by liganded vitamin D receptor: involvement of specific receptor domains. Hypertension. 1998;31:1338–1342. doi: 10.1161/01.hyp.31.6.1338. [DOI] [PubMed] [Google Scholar]
- Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M. SOCS3- mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci. 2010;30:5754–5766. doi: 10.1523/JNEUROSCI.5007-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlting C, Lai WS, Schaper F, Brenndorfer ED, Matthes RJ, Heinrich PC, Ludwig S, Blackshear PJ, Gaestel M, Haussinger D, Bode JG. Regulation of suppressor of cytokine signaling 3 (SOCS3) mRNA stability by TNF-alpha involves activation of the MKK6/p38MAPK/MK2 cascade. J Immunol. 2007;178:2813–2826. doi: 10.4049/jimmunol.178.5.2813. [DOI] [PubMed] [Google Scholar]
- Fain JN, Sacks HS, Bahouth SW, Tichansky DS, Madan AK, Cheema PS. Human epicardial adipokine messenger RNAs: comparisons of their expression in substernal, subcutaneous, and omental fat. Metabolism. 2010;59:1379–1386. doi: 10.1016/j.metabol.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Mol Aspects Med. 2008;29:388–396. doi: 10.1016/j.mam.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga C, Blanco M, Vigo E, Segura C, Garcia-Caballero T, Perez-Fernandez R. Ontogenesis of the vitamin D receptor in rat heart. Histochem Cell Biol. 2002;117:547–550. doi: 10.1007/s00418-002-0413-3. [DOI] [PubMed] [Google Scholar]
- Gezmish O, Tare M, Parkington HC, Morley R, Porrello ER, Bubb KJ, Black MJ. Maternal vitamin D deficiency leads to cardiac hypertrophy in rat offspring. Reprod Sci. 2010;17:168–176. doi: 10.1177/1933719109349536. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy KD, Zella JB, Prahl JM, DeLuca HF. Regulation of the murine renal vitamin D receptor by 1,25-dihydroxyvitamin D3 and calcium. Proc Natl Acad Sci U S A. 2003;100:9733–9737. doi: 10.1073/pnas.1633774100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 2009;17:625. doi: 10.1038/oby.2008.575. author reply 626-627. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13:211–217. doi: 10.1016/s0955-0674(00)00199-x. [DOI] [PubMed] [Google Scholar]
- Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med. 2005;11:892–898. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- Kato S. The function of vitamin D receptor in vitamin D action. J Biochem. 2000;127:717–722. doi: 10.1093/oxfordjournals.jbchem.a022662. [DOI] [PubMed] [Google Scholar]
- Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, Haluzikova D, Bosanska L, Vokurka M, Svacina S, Haluzik M. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91:4620–4627. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008;11:7–12. doi: 10.1097/MCO.0b013e3282f2f4dd. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- Ortiz-Munoz G, Martin-Ventura JL, Hernandez-Vargas P, Mallavia B, Lopez-Parra V, Lopez-Franco O, Munoz-Garcia B, Fernandez-Vizarra P, Ortega L, Egido J, Gomez-Guerrero C. Suppressors of cytokine signaling modulate JAK/STAT-mediated cell responses during atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:525–531. doi: 10.1161/ATVBAHA.108.173781. [DOI] [PubMed] [Google Scholar]
- Park CW, Oh YS, Shin YS, Kim CM, Kim YS, Kim SY, Choi EJ, Chang YS, Bang BK. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33:73–81. doi: 10.1016/s0272-6386(99)70260-x. [DOI] [PubMed] [Google Scholar]
- Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H. Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation. 2003;107:798–802. doi: 10.1161/01.cir.0000057545.82749.ff. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Shouda T, Yoshida T, Hanada T, Wakioka T, Oishi M, Miyoshi K, Komiya S, Kosai K, Hanakawa Y, Hashimoto K, Nagata K, Yoshimura A. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest. 2001;108:1781–1788. doi: 10.1172/JCI13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–524. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, Akira S, Matsumoto S, Toyonaga A, Sata M, Yoshimura A. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell AM, Crisostomo PR, Wairiuko GM, Wang M, Morrell ED, Meldrum DR. Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock. 2006;26:226–234. doi: 10.1097/01.shk.0000226341.32786.b9. [DOI] [PubMed] [Google Scholar]
- Tomita S, Ishibashi K, Hashimoto K, Sugino T, Yanagida T, Kushida N, Shishido K, Aikawa K, Sato Y, Suzutani T, Yamaguchi O. Suppression of SOCS3 increases susceptibility of renal cell carcinoma to interferon-alpha. Cancer Sci. 2011;102:57–63. doi: 10.1111/j.1349-7006.2010.01751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Empel VP, De Windt LJ. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc Res. 2004;63:487–499. doi: 10.1016/j.cardiores.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Wong JR. Vitamin D therapy in cardiac hypertrophy and heart failure. Curr Pharm Des. 2011;17:1794–1807. doi: 10.2174/138161211796391038. [DOI] [PubMed] [Google Scholar]
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97:1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Hoshijima M, Gu Y, Nakamura T, Pradervand S, Hanada T, Hanakawa Y, Yoshimura A, Ross J, Jr, Chien KR. Suppressor of cytokine signaling-3 is a biomechanical stress-inducible gene that suppresses gp130-mediated cardiac myocyte hypertrophy and survival pathways. J Clin Invest. 2001;108:1459–1467. doi: 10.1172/JCI13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, Dong N, Chen X. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol. 2011;10:2. doi: 10.1186/1475-2840-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zineb R, Zhor B, Odile W, Marthe RR. Distinct, tissue-specific regulation of vitamin D receptor in the intestine, kidney, and skin by dietary calcium and vitamin D. Endocrinology. 1998;139:1844–1852. doi: 10.1210/endo.139.4.5903. [DOI] [PubMed] [Google Scholar]
- Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]


