Abstract
Barrett’s Esophagus is considered to be a precursor to adenocarcinoma and the information on VDR expression in normal and Barrett’s esophagus is significantly lacking. In this study, we examined the expression of VDR in the lower esophagus and gastric cardia of normal and Barrett’s esophagus by immunofluorescence. Columnar mucosa but not squamous mucosa at the gastroesophageal junction showed positive immunofluorescence to VDR. Submucosal glands and ducts deep to the normal squamous mucosa stained positive for VDR and localized in the cytoplasm and perinuclear regions with no nuclear staining. Interestingly, the Barrett’s mucosa stained strongly positive for VDR. Glandular structures in the mucosal layer were far less abundant in the Barrett’s mucosa than in the normal gastric mucosa. As a result, fewer structures deep to the Barrett’s epithelial layer stained positive for VDR when compared to normal gastric mucosa. These findings suggest that in normal esophagus VDR expression is restricted to columnar epithelium and glandular structures. Furthermore, strong VDR expression in Barrett’s mucosa may indicate an increased sensitivity of this tissue to endogenous or therapeutic effects of Vitamin D.
Keywords: Barrett’s esophagus, Epithelium, Gastroesophageal junction, Vitamin D, Vitamin D receptor
INTRODUCTION
The vitamin D receptor (VDR), an intracellular transcriptional regulatory factor, was isolated from the human intestine in 1987 (1). In the rat intestine, VDR is involved in the regulation of a large number of genes involved in calcium homeostasis, intestinal absorption, intra and intercellular matrix modeling, immune responses, inflammatory processes, angiogenesis, and genes for proteases, enzymes and their inhibitors (2). In the mouse, VDR is expressed in some of its largest quantities throughout the digestive tract including the duodenum, jejunum, ileum, and colon (3, 4). The vitamin D receptor is of interest in these anatomical locations not only because of its role in calcium absorption and homeostasis, but also because of the possible anticancer role of vitamin D and its signaling pathways (5). Evidence for this role is supported by the association of malignancy in the human colon with the loss of VDR activity (6), and a correlation between a single nucleotide polymorphism in the VDR gene and the risk for colon cancer in human subjects (7).
Despite the rapid rise in the incidence and poor prognosis of adenocarcinoma of the esophagus (8, 9), the expression of VDR in the esophagus and stomach has received less attention than in the lower digestive tract. De Gottardi and colleagues detected expression of VDR mRNA in the esophagus using PCR (10), but the literature is otherwise scant. In addition, Barrett’s Esophagus, a histological change in the esophagus and considered to be a precursor to adenocarcinoma (11), is a third type of epithelium in addition to normal esophagus and gastric cardia. However, there is no information on the histological characterization of VDR expression. Barrett’s esophagus constitutes columnar epithelium, similar to the gastric cardia but with goblet cells, and existing in the lower esophagus, which normally is lined with stratified squamous epithelium (12). In this study, we provide the findings on the expression of VDR in the context of the histology of the lower esophagus and gastric cardia by immunofluorescence.
METHODS
Specimens
Resection specimens from patients undergoing treatment for esophageal adenocarcinoma at Creighton University Medical Center, Omaha, NE, between 2004-2009 were procured retrospectively. All patients received neoadjuvant therapy (chemoradiation prior to surgery) and subsequent surgical resection. From each resection specimen, areas of normal esophagus, Barrett’s esophagus, and normal gastric tissue were identified.
Immunofluorescence
After deparaffinization and rehydration, antigen retrieval was performed prior to immunostaining. Sections were incubated for 2 h in block/permeabilizing solutions containing PBS, 0.25% Triton X-100, and 5% (v/v) goat serum at room temperature. The slides were subsequently incubated with a primary antibody solution including mouse anti-VDR (SantaCruz Biotech, sc-13133) (1:200 in PBS) at 4 °C overnight. After washing with PBS four times for 5 min each, a secondary antibody (affinity purified goat anti-mouse cyanine 3 (cy3) antibody, 1:200 antibodies, PBS, 0.1% Triton X-100,1% goat serum) (Jackson ImmunoResearch, Westgrove, PA) was applied to the sections for 2 h in the dark. Negative controls were run in parallel with complete omission of primary antibody. Sections were washed with PBS four times for 5 min. Nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI). A single layer of nail polish was placed around the edge of slide to prevent escape of mounting media from the coverslip.
RESULTS
The normal squamous mucosa in the lower esophagus stained negative for VDR (Figure 1). Background fluorescence in these regions was similar in intensity to negative controls (Figure 2 D-F). In contrast, submucosal glands and ducts deep to the normal squamous mucosa stained positive for VDR (Figure 1 D-F). The transition between squamous and columnar mucosa at the gastroesophageal junction illustrates the difference in positive immunostaining between these two histologically mucosal subtypes (Figure 2 A-C).
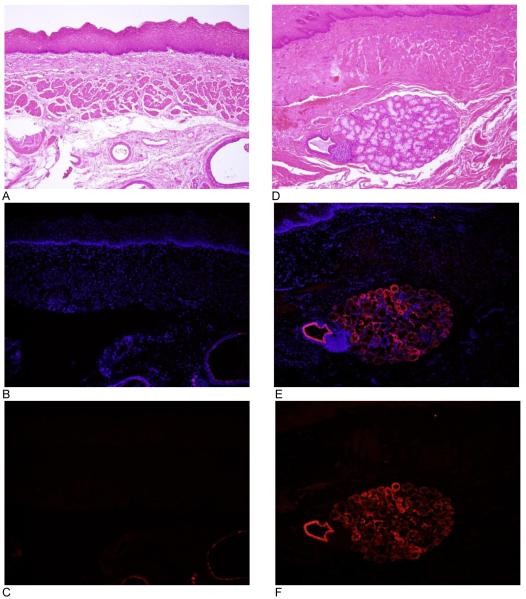
Figure 1.
VDR expression in the human esophageal tissue. Each image is a representative photograph from 5 subjects from which tissue was acquired. Images A-C are from normal esophagus, displaying absence of staining in the squamous mucosa of this tissue. Images D-F are also from normal esophagus, highlighting VDR staining of a submucosal gland and duct. Images A and D are hematoxylin and eosin. Images B and E are an overlay of VDR immunofluorescence and DAPI (nuclear counterstain). Images C and F are VDR immunofluorescence without DAPI. All images are taken at 10x objective.
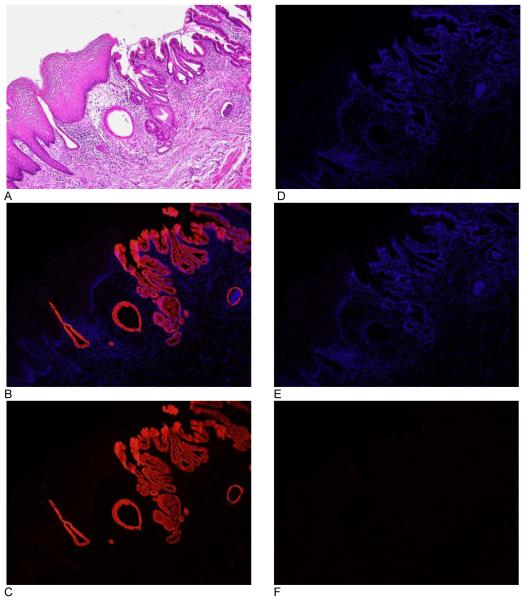
Figure 2.
VDR expression in the human gastroesophageal junction. Images A-C are from gastroesophageal junction illustrating the difference in staining between the proximal squamous mucosa and distal columnar mucosa. Images D-F are from a negative control. Image A shows staining with hematoxylin and eosin. Images B and E are an overlay of VDR immunofluorescence and DAPI (nuclear counterstain). Images C and F are VDR immunofluorescence without DAPI. Image D is DAPI. All images are taken at 10x objective.
Normal gastric mucosa and the underlying gastric and fundic glands also showed positive immunofluorescence (Figure 3 A-C). Immunofluorescence for VDR appeared to be localized in the cytoplasm and perinuclear regions, with nuclear staining absent (Figure 3 D-F).
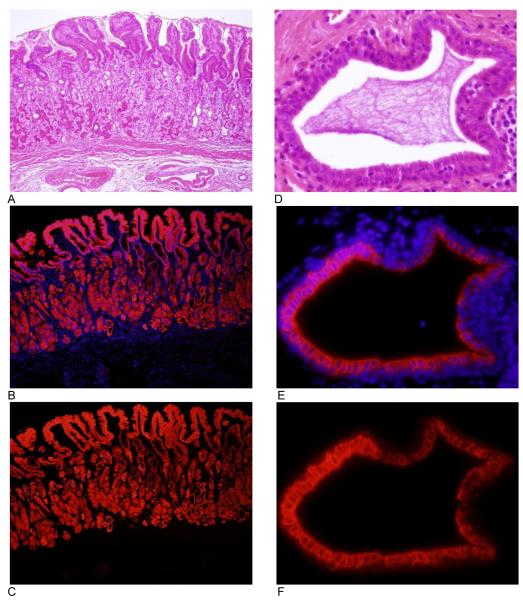
Figure 3.
VDR expression in normal gastric cardia.. Each image is a representative photograph from the 5 subjects from which tissue was acquired. Images A-C are from normal gastric cardia at 10x objective. Images D-F are images of the mucus gland duct pictured in Figure 1 D-F at 60x objective. Images A and D are hematoxylin and eosin. Images B and E are an overlay of VDR immunofluorescence and DAPI (nuclear counterstain). Images C and F are VDR immunofluorescence without DAPI.
The Barrett’s mucosa was the third histological subtype of mucosa that was examined. The Barrett’s mucosa also stained positive for VDR (Figure 4). Glandular structures in the mucosal layer were far less abundant in the Barrett’s mucosa than in the normal gastric mucosa. As a result, fewer structures deep to the Barrett’s epithelial layer stained positive for VDR when compared to normal gastric mucosa. An island of Barrett’s metaplasia interpolated in the normal squamous epithelium of the lower esophagus is displayed in Figure 4 D-F.
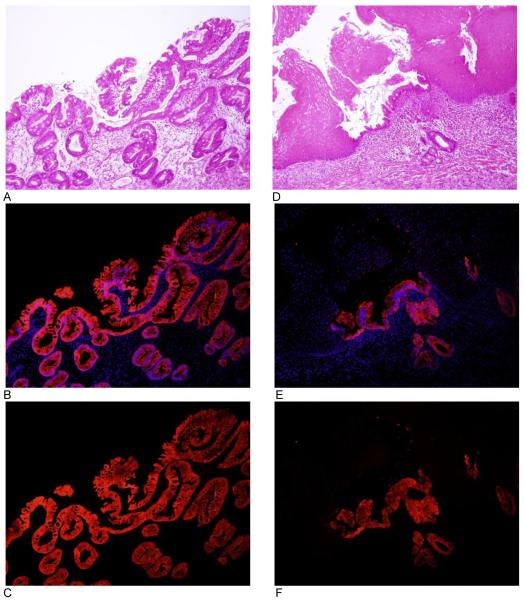
Figure 4.
VDR expression in Barrett’s esophagus. Each figure is a representative photograph from the 4 subjects with Barrett’s esophagus from which tissue was acquired. Images A-C are an area of complete columnar metaplasia with goblet cells. Images D-F is a region of metaplasia interpolated between normal squamous mucosa. Images A and D are hematoxylin and eosin. Images B and E are an overlay of VDR immunofluorescence and DAPI (nuclear counterstain). Images C and F are VDR immunofluorescence without DAPI. All images are taken at 10x objective.
DISCUSSION
The most apparent finding was the restriction of immunofluorescent staining to columnar epithelium and glandular structures; the squamous epithelium of the normal esophagus universally stained negative for VDR. Staining of the columnar mucosa and glandular structures is consistent with findings in other tissues that share architectural similarities with the mucosa of the esophagus and gastric cardia. Barrett’s mucosa showed immunopositivity for VDR unlike normal squamous epithelium of esophagus. Upregulation of VDR expression in Barrett’s mucosa may indicate an increased sensitivity of this tissue to endogenous or therapeutic effects of Vitamin D.
Columnar and glandular epithelium displays the strongest immunohistochemical staining in the murine placenta (13), and the glandular epithelium of the tubuloalveolar glands of neoplastic prostate glands also displays immunohistochemical staining for VDR (14). In the latter, there was considerable variability in the intensity of staining. This is consistent with the variability in the immunofluorescent staining of esophageal adenocarcinoma (15) and may be associated with the neoplastic process.
The absence of staining in the squamous mucosa of the esophagus is in contrast to what is seen in the squamous epithelium of the epidermis and cervix where nuclei stain positive throughout the thickness of the epithelial layer and strongest in the basal cell layer (16, 17). Cytoplasmic staining is also observed but to a lesser degree (18). However, the epidermal mucosa is composed of keratinized epithelium whereas the esophageal mucosa is non-keratinized epithelium, and immunohistochemistry was utilized as an assay as compared to immunofluorescence. These are two differences that may partially explain the discrepancies observed in studies of the VDR expression in the epidermis with this study.
Certain nuclear receptors like VDR shuttle between the cytoplasm and the nucleus even in the absence of their ligands (19), and in the unliganded state VDR distributes evenly between the cytoplasm and nucleus (20). Upon ligand binding, the steady state shuttling between cytoplasm and nucleus favors nuclear localization (19). Despite this, the immunofluorescent staining used here appeared to show mainly cytoplasmic staining with relative sparing of the nuclei. This is also in contrast to VDR immunohistochemistry of the epidermis, which showed mainly nuclear staining (16-18).
The results presented here are the first that describe the histological distribution of VDR in the esophagus. The intensity of immunohistochemical staining has been correlated with neoplastic disease in both prostate (14) and basal cell carcinoma (17), and VDR gene single nucleotide polymorphisms (7) and loss of VDR activity (6) are both associated with malignancy. If the trend of examining the role of VDR in the process of neoplasia continues up the digestive tract to the stomach and esophagus, understanding the normal histology of these tissues will be of important value.
Highlights.
Esophageal squamous mucosa is negative to VDR expression
VDR expression in normal esophagus is restricted to columnar mucosa and glandular structures
Barrett’s mucosa stained strongly positive for VDR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, et al. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988;85(10):3294–8. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kutuzova GD, Deluca HF. Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys. 2004;432(2):152–66. doi: 10.1016/j.abb.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–99. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangelsdorf D. VDR : Anatomical Q-PCR Expression Data. Nuclear Receptor Singaling Atlas; 2005. [Google Scholar]
- 5.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 6.Meggouh F, Lointier P, Pezet D, Saez S. Evidence of 1,25- dihydroxyvitamin D3-receptors in human digestive mucosa and carcinoma tissue biopsies taken at different levels of the digestive tract, in 152 patients. J Steroid Biochem. 1990;36(1-2):143–7. doi: 10.1016/0022-4731(90)90124-b. [DOI] [PubMed] [Google Scholar]
- 7.Mahmoudi T, Arkani M, Karimi K, Safaei A, Rostami F, Arbabi E, et al. The -4817 G>A (rs2238136) variant of the vitamin D receptor gene: a probable risk factor for colorectal cancer. Mol Biol Rep. 2011 doi: 10.1007/s11033-011-1325-x. [DOI] [PubMed] [Google Scholar]
- 8.Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer. 2002;99(6):860–8. doi: 10.1002/ijc.10427. [DOI] [PubMed] [Google Scholar]
- 9.Institute NC . Surveillance Epidemiology and End Results: Cancer Statistics Review, 1975-2008. National Cancer Institute; 2011. [Google Scholar]
- 10.De Gottardi A, Dumonceau JM, Bruttin F, Vonlaufen A, Morard I, Spahr L, et al. Expression of the bile acid receptor FXR in Barrett’s esophagus and enhancement of apoptosis by guggulsterone in vitro. Mol Cancer. 2006;5:48. doi: 10.1186/1476-4598-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103(3):788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 12.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ, American Gastroenterological A American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):e18–52. doi: 10.1053/j.gastro.2011.01.031. quiz e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahbazi M, Jeddi-Tehrani M, Zareie M, Salek-Moghaddam A, Akhondi MM, Bahmanpoor M, et al. Expression profiling of vitamin D receptor in placenta, decidua and ovary of pregnant mice. Placenta. 2011;32(9):657–64. doi: 10.1016/j.placenta.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickson WK, Flavin R, Kasperzyk JL, Fiorentino M, Fang F, Lis R, et al. Vitamin D receptor protein expression in tumor tissue and prostate cancer progression. J Clin Oncol. 2011;29(17):2378–85. doi: 10.1200/JCO.2010.30.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trowbridge R, Sharma P, Hunter WJ, Agrawal DK. Vitamin D receptor expression and neoadjuvant therapy in esophageal adenocarcinoma. Exp. Mol. Pathol. 2012 doi: 10.1016/j.yexmp.2012.04.018. doi:10.1016/j.yexmp.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milde P, Hauser U, Simon T, Mall G, Ernst V, Haussler MR, et al. Expression of 1,25-dihydroxyvitamin D3 receptors in normal and psoriatic skin. J Invest Dermatol. 1991;97(2):230–9. doi: 10.1111/1523-1747.ep12480255. [DOI] [PubMed] [Google Scholar]
- 17.Reichrath J, Kamradt J, Zhu XH, Kong XF, Tilgen W, Holick MF. Analysis of 1,25-dihydroxyvitamin D(3) receptors (VDR) in basal cell carcinomas. Am J Pathol. 1999;155(2):583–9. doi: 10.1016/s0002-9440(10)65153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum Pathol. 2011;42(5):618–31. doi: 10.1016/j.humpath.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prufer K, Barsony J. Retinoid X receptor dominates the nuclear import and export of the unliganded vitamin D receptor. Mol Endocrinol. 2002;16(8):1738–51. doi: 10.1210/me.2001-0345. [DOI] [PubMed] [Google Scholar]
- 20.Prufer K, Racz A, Lin GC, Barsony J. Dimerization with retinoid X receptors promotes nuclear localization and subnuclear targeting of vitamin D receptors. J Biol Chem. 2000;275(52):41114–23. doi: 10.1074/jbc.M003791200. [DOI] [PubMed] [Google Scholar]