Abstract
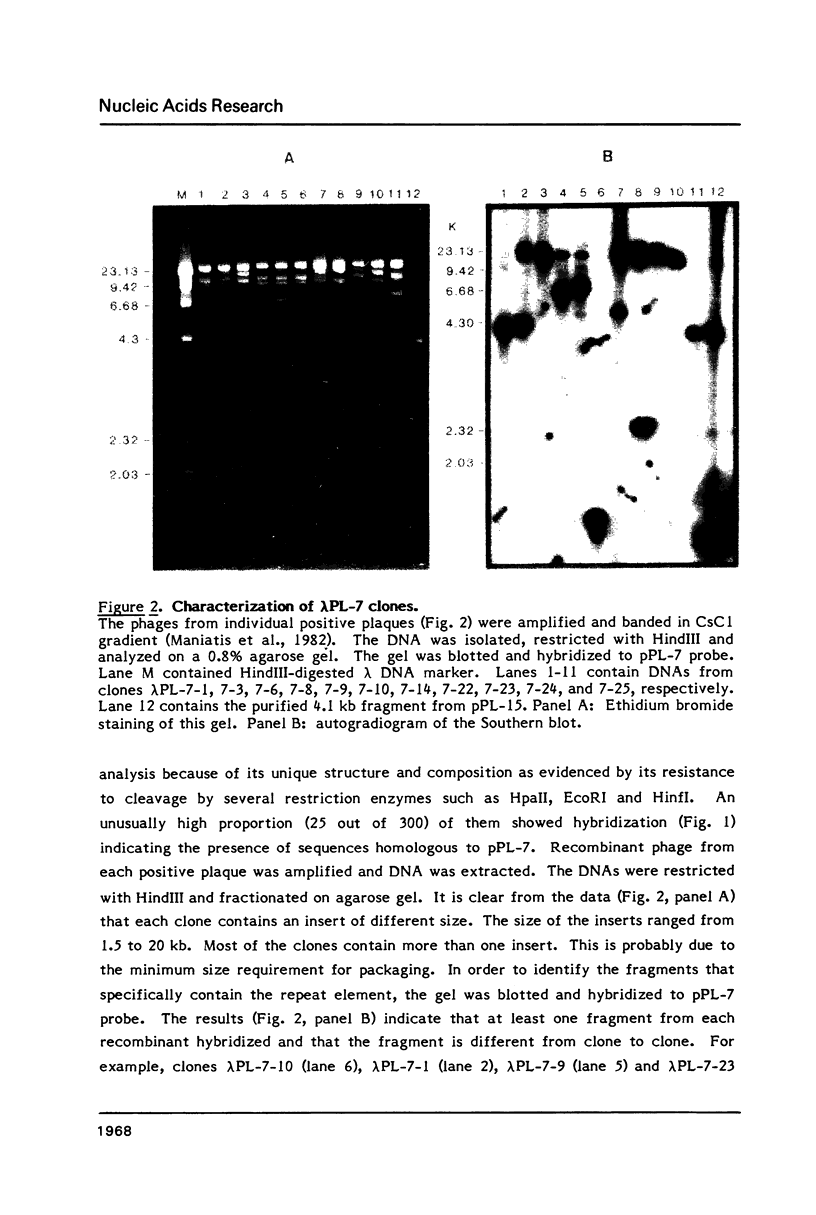
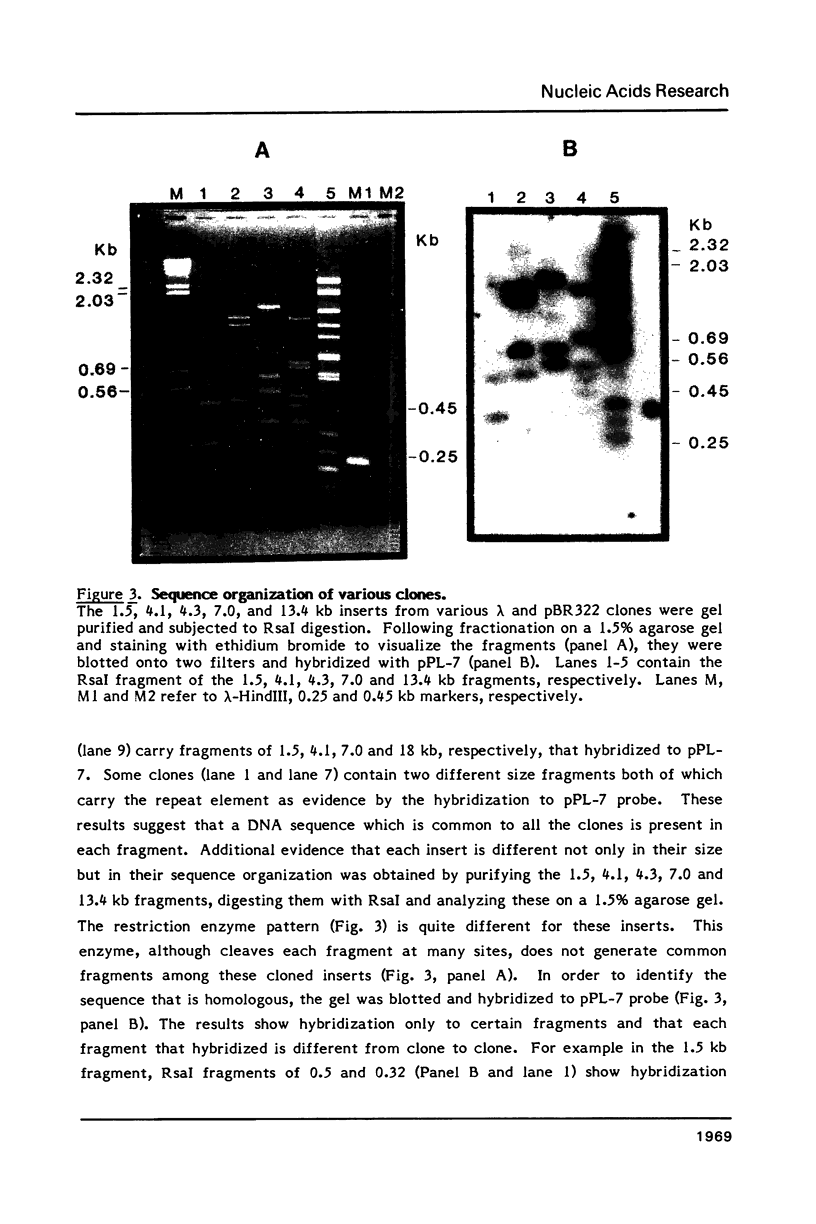
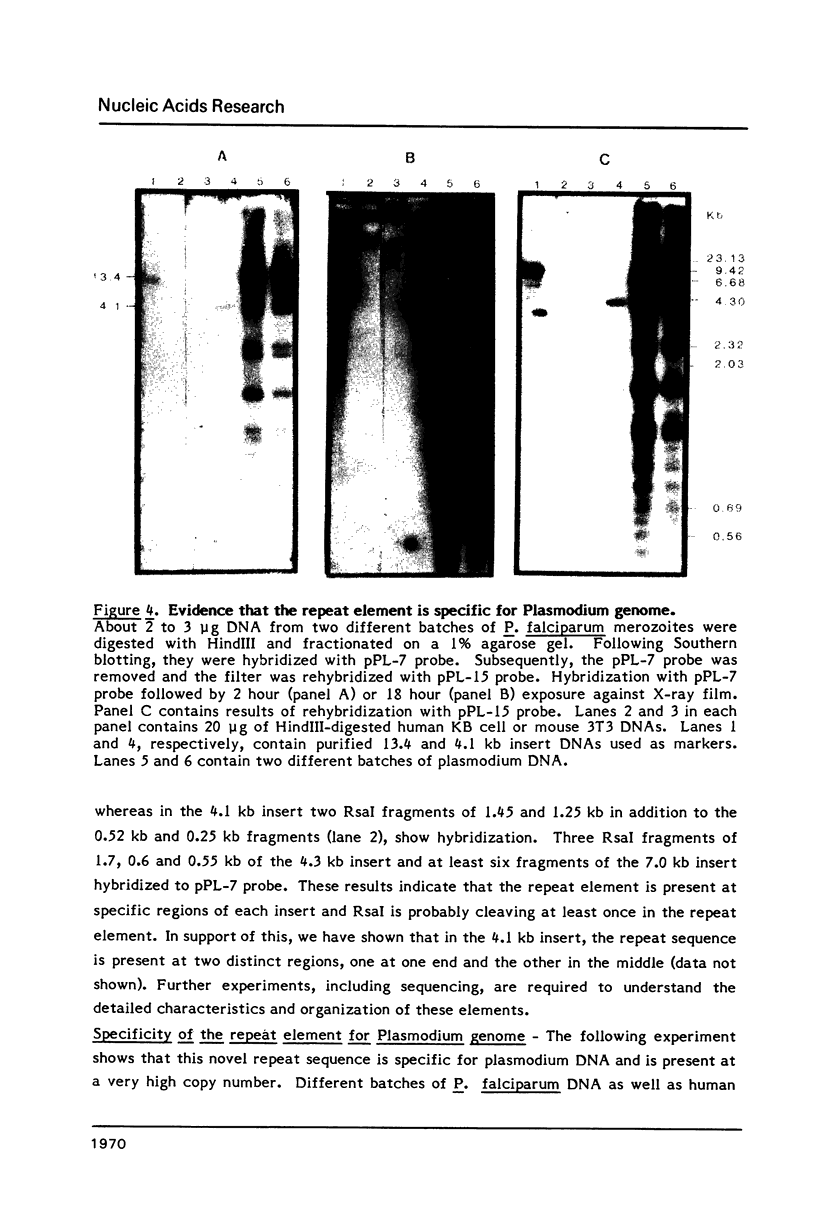
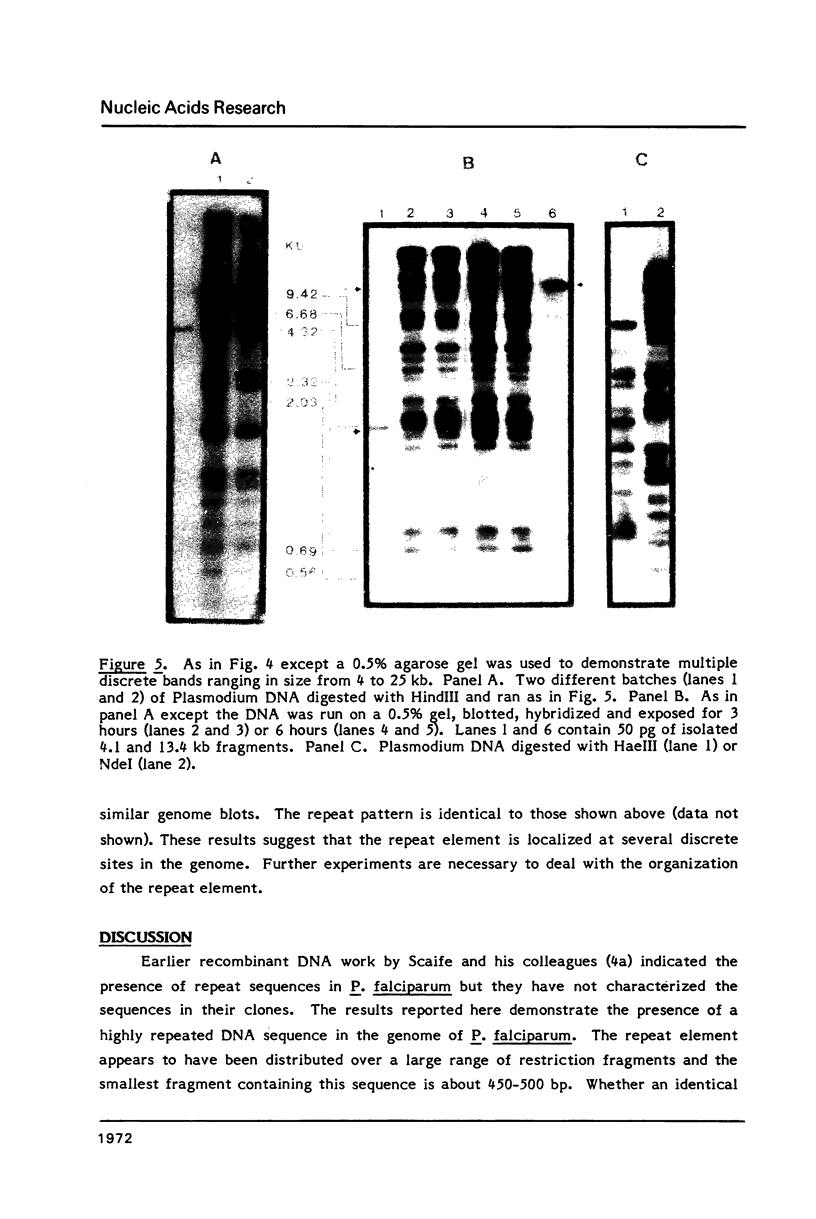
Plasmodium falciparum DNA, isolated from the merozoite stage, was cleaved with HindIII and cloned in pBR322 and lambda L47.1 vectors. Plasmid clones containing 13.4, 7.0, 4.3, 4.1 and 1.5 kb inserts were characterized in some detail. The inserts contain several repeating units of smaller size. Nucleic acid hybridization studies showed that the repeat element is present in the Plasmodium DNA at a very high copy number and appears to be distributed widely throughout the genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Brown G. V., Edwards A. Characterization of an S antigen synthesized by several isolates of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6652–6656. doi: 10.1073/pnas.80.21.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birago C., Bucci A., Dore E., Frontali C., Zenobi P. Mosquito infectivity is directly related to the proportion of repetitive DNA in Plasmodium berghei. Mol Biochem Parasitol. 1982 Jul;6(1):1–12. doi: 10.1016/0166-6851(82)90048-2. [DOI] [PubMed] [Google Scholar]
- Campbell D. A., Thornton D. A., Boothroyd J. C. Apparent discontinuous transcription of Trypanosoma brucei variant surface antigen genes. 1984 Sep 27-Oct 3Nature. 311(5984):350–355. doi: 10.1038/311350a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Dorfman D. M., Donelson J. E. Characterization of the 1.35 kilobase DNA repeat unit containing the conserved 35 nucleotides at the 5'-termini of variable surface glycoprotein mRNAs in Trypanosoma brucei. Nucleic Acids Res. 1984 Jun 25;12(12):4907–4920. doi: 10.1093/nar/12.12.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard W., Meitinger T., Höchtl J., Zachau H. G. A new family of interspersed repetitive DNA sequences in the mouse genome. J Mol Biol. 1982 May 25;157(3):453–471. doi: 10.1016/0022-2836(82)90471-5. [DOI] [PubMed] [Google Scholar]
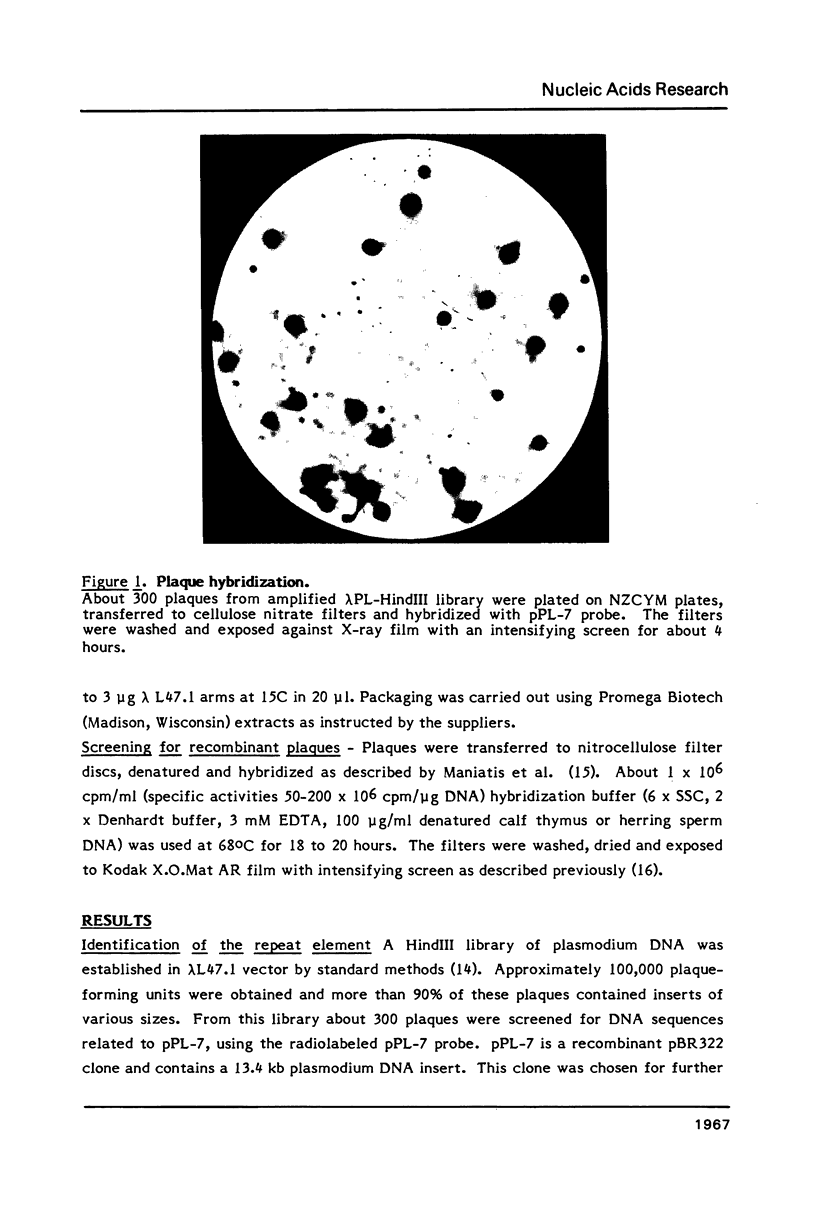
- Goman M., Langsley G., Hyde J. E., Yankovsky N. K., Zolg J. W., Scaife J. G. The establishment of genomic DNA libraries for the human malaria parasite Plasmodium falciparum and identification of individual clones by hybridisation. Mol Biochem Parasitol. 1982 Jun;5(6):391–400. doi: 10.1016/0166-6851(82)90012-3. [DOI] [PubMed] [Google Scholar]
- Green T. J., Morhardt M., Brackett R. G., Jacobs R. L. Serum inhibition of merozoite dispersal from Plasmodium falciparum schizonts: indicator of immune status. Infect Immun. 1981 Mar;31(3):1203–1208. doi: 10.1128/iai.31.3.1203-1208.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Rao P. Y., Mitsialis S. A., Katz R. Modification of avian sarcoma proviral DNA sequences in nonpermissive XC cells but not in permissive chicken cells. J Virol. 1980 May;34(2):569–572. doi: 10.1128/jvi.34.2.569-572.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Weiner A. J. Effect of dibutyryl cyclic AMP on intracellular levels of avian sarcoma virus specific RNA. Nature. 1978 Jul 20;274(5668):274–276. doi: 10.1038/274274a0. [DOI] [PubMed] [Google Scholar]
- Haynes S. R., Jelinek W. R. Low molecular weight RNAs transcribed in vitro by RNA polymerase III from Alu-type dispersed repeats in Chinese hamster DNA are also found in vivo. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6130–6134. doi: 10.1073/pnas.78.10.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Toomey T. P., Leinwand L., Jelinek W. R. The Chinese hamster Alu-equivalent sequence: a conserved highly repetitious, interspersed deoxyribonucleic acid sequence in mammals has a structure suggestive of a transposable element. Mol Cell Biol. 1981 Jul;1(7):573–583. doi: 10.1128/mcb.1.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller D., Jackson M., Leinwand L. Organization and expression of non-Alu family interspersed repetitive DNA sequences in the mouse genome. J Mol Biol. 1984 Mar 15;173(4):419–436. doi: 10.1016/0022-2836(84)90389-9. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Zolg J. W., Scaife J. G. Isolation and characterisation of ribosomal RNA from the human malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 1981 Dec 31;4(5-6):283–290. doi: 10.1016/0166-6851(81)90061-x. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Cowman A. F., Saint R. B., Brown G. V., Anders R. F. Expression of Plasmodium falciparum blood-stage antigens in Escherichia coli: detection with antibodies from immune humans. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3787–3791. doi: 10.1073/pnas.80.12.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Mitsialis S. A., Young J. F., Palese P., Guntaka R. V. An avian tumor virus promoter directs expression of plasmid genes in Escherichia coli. Gene. 1981 Dec;16(1-3):217–225. doi: 10.1016/0378-1119(81)90078-0. [DOI] [PubMed] [Google Scholar]
- Ozaki L. S., Svec P., Nussenzweig R. S., Nussenzweig V., Godson G. N. Structure of the plasmodium knowlesi gene coding for the circumsporozoite protein. Cell. 1983 Oct;34(3):815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Parsons M., Nelson R. G., Watkins K. P., Agabian N. Trypanosome mRNAs share a common 5' spliced leader sequence. Cell. 1984 Aug;38(1):309–316. doi: 10.1016/0092-8674(84)90552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack Y., Katzen A. L., Spira D. T., Golenser J. The genome of Plasmodium falciparum. I: DNA base composition. Nucleic Acids Res. 1982 Jan 22;10(2):539–546. doi: 10.1093/nar/10.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Shafit-Zagardo B., Maio J. J., Brown F. L. KpnI families of long, interspersed repetitive DNAs in human and other primate genomes. Nucleic Acids Res. 1982 May 25;10(10):3175–3193. doi: 10.1093/nar/10.10.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]