Abstract
Low threshold voltage-gated T-type calcium channels have long been implicated in the electrical excitability and calcium signaling of cerebellar Purkinje neurons although the molecular composition, localization, and modulation of T-type channels within Purkinje cells have only recently been addressed. The specific functional roles that T-type channels play in local synaptic integration within Purkinje spines are also currently being unraveled. Overall, Purkinje neurons represent a powerful model system to explore the potential roles of postsynaptic T-type channels throughout the nervous system. In this review, we present an overview of T-type calcium channel biophysical, pharmacological, and physiological characteristics that provides a foundation for understanding T-type channels within Purkinje neurons. We also describe the biophysical properties of T-type channels in context of other voltage-gated calcium channel currents found within Purkinje cells. The data thus far suggest that one specific T-type isoform, Cav3.1, is highly expressed within Purkinje spines and both physically and functionally couples to mGluR1 and other effectors within putative signaling microdomains. Finally, we discuss how the selective potentiation of Cav3.1 channels via activation of mGluR1 by parallel fiber inputs affects local synaptic integration and how this interaction may relate to the overall excitability of Purkinje neuron dendrites.
Keywords: T-type, Calcium channel, Cav3.1, Purkinje, Parallel fiber, mGluR1
Introduction
The discovery of calcium spikes in Purkinje cell (PC) dendrites by Llinas and Sugimori [1] and their subsequent seminal studies initiated an era of unraveling the roles of dendritic voltage-dependent conductances in neurons. Since the characterization of high-voltage activated (HVA) P-type calcium channels in PCs [2, 3], and the finding that the selective pharmacological block of P-type channels inhibited calcium spikes in PC dendrites [3, 4], the study of calcium signaling in PC dendrites has largely been focused on the P-type calcium channel. However, the spatial resolution of calcium imaging techniques available in the 1980s could not adequately discriminate between secondary or tertiary dendrites and individual spines, while the temporal resolution did not allow for the fine description of calcium spikes. For almost 20 years now, robust low threshold calcium currents have been recorded from PCs, yet few physiological implications have been demonstrated from these studies. After a brief discussion of relevant T-type calcium channel properties, we will develop new hypotheses linking both T-type and P-type channels to functional dendritic integration of synaptic inputs in PC dendrites.
T-Type Channel Biophysics
T-type channels have many characteristic biophysical properties unique among voltage-gated calcium channels which enable them to serve specialized functions within the nervous system (see “Physiological Roles of T-Type Channels in the CNS: Promoting Bursting Modes”). These include fast activation and inactivation kinetics, a relatively hyperpolarized voltage-dependence of activation and inactivation, and slow deactivation kinetics [5]. The activation and inactivation kinetics of T-type channels are strongly voltage-dependent. After initial cloning, the recombinant T-type isoforms were biophysically well characterized, and these properties have been thoroughly reviewed [5]. In brief, comparison of the three rat Cav3 T-type isoforms revealed that Cav3.1 and Cav3.2 have properties similar to “typical” native T-type currents, while Cav3.3 possesses distinct biophysical properties. The Cav3.1 and Cav3.2 isoforms have fast activation and inactivation kinetics while Cav3.3 channel activation and inactivation kinetics are much slower [6]. A recent paper comparing T-type biophysical parameters at room temperature and at a physiological mammalian temperature (37°C) demonstrated that increasing the recording temperature dramatically alters many of these properties in a non-linear, isoform-specific manner [7]. In this regard, caution should be used when extrapolating specific T-type parameters measured at room temperature to models of physiological neuronal excitability. Alternative splicing creates additional functional diversity in T-type channel activity. For example, alternative splicing in the human Cav3.1 channel leads to multiple variants including those that shift the voltage dependence of inactivation in the hyperpolarizing direction and also increase inactivation kinetics [8]. Also, thalamic splice variants of the Cav3.2 channel are differentially affected by a missense mutation from the genetic absence epilepsy rats from Strasbourg model of absence epilepsy [9].
T-Type Channel Pharmacology and Modulation
The study of T-type currents in native systems has been historically hindered by two major issues: (1) some HVA calcium channels in fact activate at relatively negative potentials (e.g., Cav1.3 L-type and Cav2.3 R-type) and contaminate what were previously thought to be pure low threshold T-type currents and (2) unlike the HVA calcium channel classes, no high affinity channel antagonists are commercially available that clearly distinguish T-type currents from HVA currents or that distinguish between individual T-type channel subtypes. These issues were compounded in early investigations of native T-type currents, where biophysical properties and sensitivities to pharmacological antagonists such as nickel (Ni2+) varied depending on the cell type. The cloning and characterization of the three main T-type isoforms has helped explain these divergent properties and has provided some clarification concerning the limitations and suitable uses of the pharmacological tools presently available.
While one of the earliest T-type current antagonists to be identified was Ni2+, the sensitivity to this agent is highly variable between different native systems. For example, Ni2+ inhibits T-type currents in chick skeletal muscle cells with an IC50 of 21 μM [10], while it is a much less effective T-type current inhibitor (IC50 = 110 μM) in cerebellar PCs [11]. Molecular identification of the three Cav3 channels revealed that Cav3.2 is the only T-type isoform highly sensitive to Ni2+, with an IC50 of 12 μM compared to IC50 values of 250 and 216 μM for Cav3.1 and Cav3.3, respectively [12]. Furthermore, Ni2+ blocks Cav1.2 L-type and Cav2.3 R-type channels with a higher potency than either of the Cav3.1 or Cav3.3 T-type isoforms [12, 13]. Therefore, low concentrations (e.g., ~50 μM) of Ni2+ can only be used to selectively block Cav3.2-mediated T-type currents (with minimal blockade of Cav3.1- and Cav3.3-mediated T-type currents) but notably cannot reliably distinguish between Cav3.2- and Cav2.3-mediated R-type currents at this concentration.
There are a number of clinically used agents that also nonspecifically target T-type channels. Mibefradil (now withdrawn from the market) is a prime example of an agent with good efficacy in blocking T-type currents. For research purposes, mibefradil was found to selectively inhibit T-type currents (IC50 ranging from 14 nM to 1 μM) over HVA currents in some native systems, with state-dependent block causing greater inhibition of T-type currents at more depolarized potentials [14]. However, other studies showed that mibefradil can potently block R-type currents in NG108-15 cells [15] and can also block N-type, L-type, and P-type calcium currents at concentrations of ~1 μM in spinal motor neurons [16]. Due to the nonspecificity of mibefradil action, this compound is now deemed to be a relatively nonspecific T-type antagonist. In recent developments, pharmaceutical companies are beginning to identify small organic molecules that are proving highly efficacious (nanomolar affinity) in blocking T-type channels [17]. One such compound, TTA-P2, potently (IC50 = 20 nM) blocks T-type currents in thalamocortical and reticular thalamic neurons without altering HVA calcium currents, sodium currents, or glutamatergic and GABAergic synaptic currents [17].
T-type channels were originally thought to be largely resistant to modulation by intracellular signaling pathways. Subsequent studies on T-type modulation in native systems revealed a number of discrepancies, with individual neurotransmitter types being reported to inhibit, stimulate, or have no effect on T-type currents depending upon the tissue and cell type being examined (reviewed in [18]). After a lull following their initial cloning and characterizations, the study of recombinant T-type channel modulation is emerging as an essential tool aimed at shedding further light on low threshold current regulation within native systems. Of the three T-type isoforms, Cav3.2 is the prototypical modulated T-type isoform as it appears to be specifically targeted by Gβγ [19, 20], CAMKII [21], and redox agents [22]. More recent studies are also now identifying signaling pathways that act on the Cav3.1 and Cav3.3 isoforms [23–26]. As discussed in subsequent sections, we have identified a differential modulation of T-type isoforms that results in the potentiation of Cav3.1-mediated T-type currents within PCs [27].
Physiological Roles of T-Type Channels in the CNS: Promoting Bursting Modes
The low threshold activation of T-type currents enables them to open in response to relatively small membrane depolarizations and to generate “window currents” whereby a fraction of channels are tonically open at resting membrane potentials. Both of these properties are highly dependent on the resting membrane potential, as T-type channels become completely inactivated at more depolarized potentials and are subsequently not available to open without a de-inactivating hyperpolarization [28]. In thalamocortical relay cells, the T-type window current contributes directly to the membrane potential [17] and has been suggested to be essential for the slow sleep oscillations of thalamic neurons [29].
The above properties enable T-type currents to be “first responders” to changes in membrane potential, which potentially impacts both calcium-mediated signaling pathways as well as electrical firing patterns. For example, T-type calcium spikes can have profound effects on global neuronal excitability. Low-threshold calcium spikes were first identified from brain slices of the inferior olive, where removal of T-type inactivation with hyperpolarization initiated a spontaneous “rebound-burst” spike [30]. T-type channels have now been shown to underlie regenerative low-threshold spikes and burst firing in neurons throughout the central nervous system (CNS), including in the thalamus, inferior olive, cerebellum, hippocampus, cortex, and neocortex (reviewed in [31]). In some neurons, low threshold spikes and burst firing can alter neuronal oscillations, causing cells to switch from tonic firing to a phasic mode with regular intervals of high frequency bursts of spikes [32, 33]. Within the thalamus, T-type-mediated changes in rhythmic oscillations directly underlie physiological sleep–wake gating and pathophysiological epileptic absence seizure activity [34, 35].
Recent immunostaining experiments have revealed a differential subcellular localization of the three T-type isoforms in the spines, dendrites, and soma of neurons throughout the CNS [36, 37]. The predominant expression of T-type currents in neuronal dendrites also implicates their potential involvement in signal integration at synaptic inputs. In both pyramidal cortical and hippocampal CA1 neurons, subthreshold excitatory postsynaptic potentials (EPSPs) can activate T-type currents and generate a localized increase in dendritic calcium levels [38, 39]. This T-type activity may act to boost dendritic depolarizations and, therefore, increase excitability, or conversely, may activate calcium-activated potassium currents to cause membrane hyperpolarizations [40]. T-type channel inputs have also been linked to synaptic plasticity [41, 42], although the use of imperfect pharmacological tools make these postulations preliminary. More thorough investigations involving high-resolution two-photon calcium imaging, Cav3 knock-out (KO) mice, Cav3 RNAi-mediated knock-down, and/or more specific T-type antagonists are required to help elucidate the exact physiological roles of dendritic T-type currents in both plasticity and excitability.
Expression and Localization of Voltage-Gated Calcium Channels in Purkinje Neurons
Voltage-Gated Calcium Channel Distribution and Functional Expression
Of the various calcium channel classes, P-type channels (Cav2.1) display the highest functional expression within PCs, forming whole-cell currents on the order of several nanoamps [2, 3, 43]. Cav2.1 channel proteins are expressed throughout the PC dendrites, soma, and even axons, but are most highly expressed within dendritic spines that are postsynaptic to parallel fiber (PF) inputs [44, 45]. There is also both immunohistochemical [46] and electrophysiological [47] evidence for the expression of functional L-type channels (Cav1.2, Cav1.3) in the soma of PCs, but the relative expression of L-type channels appears to decrease during PC maturation [43], potentially due to the increased expression of other calcium channel classes within developing PC dendrites. There is little evidence for the expression of ω-conotoxin-sensitive N-type currents (Cav2.2) in PCs [48] and while the R-type channel isoform, Cav2.3, has been shown to be expressed at the protein level in PCs [49, 50] there has yet to be evidence of functional expression.
T-type calcium channel activity was first identified in PCs using sharp electrode intracellular recordings in adult rat cerebellar slices. These recordings on mature PCs revealed a prominent inward-rectifying hyperpolarization-activated current (Ih) as well as a putative low threshold calcium conductance that was de-inactivated by hyperpolarization [51]. One single channel recording study failed to identify low threshold calcium currents in acute cerebellar slices from adult guinea pigs and so the presence of T-type currents in mammalian PCs was initially somewhat controversial [3]. T-type currents were however subsequently identified in PCs of both juvenile and adult rats and mice through recordings on acutely dissociated and primary cultured PCs, PCs from slice cultures and PCs from acute brain slices [11, 52–58]. Purkinje neuron T-type currents possess all of the hallmark T-type current biophysical properties, including low threshold activation ranging between −60 and −40 mV, small single channel conductance between 7 and 9 pS, a relatively hyperpolarized voltage dependence of inactivation, fast activation and inactivation kinetics, and relatively slow deactivation kinetics [11, 52–54, 57]. Cell-attached recordings on PCs from newborn rat slice cultures demonstrated that T-type currents are distributed more densely on dendritic membranes compared to somatic membranes [54]. This finding is consistent with the observation that in a cell culture model of PC development T-type currents are only present in PCs that have developed a dendritic structure [58]. We furthered these investigations by using a combination of two photon calcium imaging and voltage-clamp recordings on PCs from juvenile rat acute cerebellar slices to show that T-type calcium currents are present in both the spines and dendrites of PCs [57]. The T-type currents have a large peak amplitude (approx. −2 nA at postnatal day 10) that increases with developmental age, which indicates that T-type currents are functionally expressed in adult rodent PCs [27, 57] (Fig. 1a). Several studies have now reached the consensus that P-type and T-type currents comprise the vast majority (up to 95%) of calcium channel currents in mature cerebellar PCs [3, 56, 57, 59]. The specific Cav3 isoform(s) that compose the native T-type currents within PCs is discussed in “The Cav3.1 Isoform is the Major Functional T-Type Calcium Channel Expressed in Rodent Purkinje Cells.”
Fig. 1.
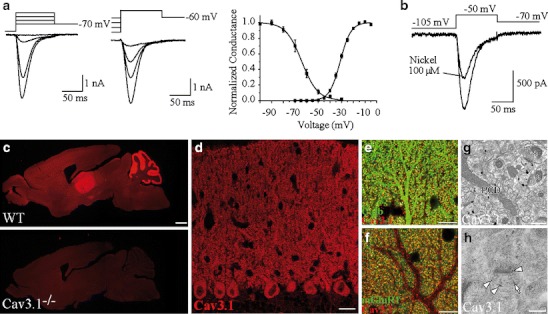
Biophysical properties of T-type currents in juvenile rodents and Cav3.1 expression in adult rodents. a T-type current activation and inactivation in juvenile rats. Left panel depolarizing steps to −65, −55, −45, and −40 mV were applied from −105 mV. Middle panel depolarizing steps to −40 mV were preceded by conditioning steps to −80, −75, −70, and −60 mV. Right panel normalized conductance curves for activation and steady-state inactivation fitted with Boltzmann equations. b Effect of nickel application (100 μM) on T-type current (mean inhibition was 35 ± 15%, n = 4). c–h Immunofluorescence showing predominant distribution of Cav3.1 in the dendritic spines of cerebellar Purkinje cells. c Specificity of Cav3.1 antibody in mouse brain. Note intense labeling in the cerebellum (Cb) and thalamus (Th) of WT but not Ca v 3.1 −/−. Scale bar 1 mm. d Immunofluorescence for Cav3.1 in the cerebellar cortex, scale bar 20 μm. e, f Double immunofluorescence for Cav3.1 (red) and calbindin (green) in (e) and mGluR1 (green) in (f), scale bar 10 μm. Note the colocalization in spines. g, h Pre-embedding silver-enhanced immunogold in (g) and postembedding immunogold in (h) showing Cav3.1 perisynaptic localization. Scale bar 500 nm in (g) and 200 nm in (h). Panel a from Isope and Murphy [57] and panels c–h from Hildebrand et al. [27] with permission
There are several technical limitations and caveats to recording calcium currents in PCs that are not always explicitly addressed in studies but that should be highlighted. The high expression of calcium channels in the extensive dendritic arbor of PCs means that space clamp issues arise during whole-cell recordings from intact PCs in cerebellar slices. Thus, whole-cell calcium currents can only be adequately clamped up to approximately postnatal day 14 or even younger (especially for large P-type currents) in PCs from slices ([57] and our unpublished observations), which inversely correlates with dendritic development during PC maturation [60, 61]. In fact, in more mature PCs, the space clamp can become so poor that dendrites are able to fire calcium spikes [56]. Cell-attached recordings can be performed on the soma and dendrites of PCs although this technique is technically challenging, restricts quantitative investigations, and is affected by heterogeneity in channel distribution [43]. Space clamp issues are minimized in recordings on acutely dissociated PCs, but these neurons lack the dendritic tree where many calcium channels predominate and the protease treatments used during dissociation can significantly alter calcium channel expression compared to recordings on equivalent PCs from slices (our unpublished observations and [62]). Similarly, cultures of isolated PCs or cerebellar slices are taken from newborn or juvenile rats and subsequent dendritic growth and channel expression is determined by in vitro culture conditions [63]. A combination of pharmacological tools, genetically engineered mice strains, and two-photon calcium imaging has thus far been the only configuration to date that allows adequate study of calcium channel activity in mature animals [27].
The Cav3.1 Isoform is the Major Functional T-Type Calcium Channel Expressed in Rodent Purkinje Cells
The properties of T-type currents recorded from PCs include low sensitivity to Ni2+, fast inactivation kinetics, and slow deactivation kinetics, which are more characteristic of Cav3.1 currents than either Cav3.2 or Cav3.3 currents [11, 57] (Fig. 1a, b). In support, the overall consensus from in situ hybridization and immunohistochemical experiments is that T-type channels are robustly expressed in the soma and dendrites of PCs, with predominant expression of Cav3.1 and the potential expression of Cav3.3 in a subset of cells [27, 36, 64–67].
We have further provided several lines of evidence which demonstrate that the Cav3.1 T-type isoform conducts the majority of functional T-type currents within PCs of both juvenile and adult rodents [27]. Recordings on both juvenile and mature PCs from transgenic Cav3.1 KO mice show a near complete elimination of T-type currents and calcium transients compared to wild-type (WT) mice. Furthermore, immunofluorescence with a Cav3.1-specific antibody reveals a robust expression of Cav3.1 in PCs that rivals the thalamus for the highest overall Cav3.1 expression in the brain (Fig. 1c). At a subcellular level, confocal and electron microscopy demonstrate that Cav3.1 is predominantly localized to dendritic spines at PF synapses in both juvenile and adult mice (Fig. 1d–h).
Native Cav3.1 T-Type Channels are Potentiated by mGluR1
Both the synaptic and pharmacological activation of mGluR1 results in a robust and reversible potentiation of Cav3.1-mediated T-type transients within PCs. This potentiation involves both an increase in maximal currents and a slight hyperpolarizing shift in the voltage-dependence of T-type channel activation, enabling T-type channels to more potently respond to small depolarizations near the PC resting membrane potential [27]. Interestingly, the potentiation of T-type currents by mGluR1 occurs through the same non-canonical G-protein mediated pathway that couples mGluR1 to the TRPC-mediated slow excitatory postsynaptic current (sEPSC) within PCs [68, 69]. Extensive pharmacological investigations revealed that both the activation of the sEPSC and the potentiation of T-type currents by mGluR1 involve an intracellular pathway that is independent of phospholipase C and other classical downstream mGluR1 effectors but that is dependent upon tyrosine phosphorylation/dephosphorylation [27, 70]. Both sEPSC activation and T-type potentiation are blocked by tyrosine phosphatase antagonists such as orthovanadate and bpV(phen) and are conversely potentiated by PP1, an inhibitor of Src-family tyrosine kinases (Fig. 2a). The augmentation of T-type currents by mGluR1 also involves intracellular calcium signaling but does not require the activation of the sEPSC channels themselves [27]. As sEPSCs are activated by PF stimulation within PC dendritic spines [71], the above observations have led us to hypothesize that Cav3.1 T-type channels are physically coupled with mGluR1 and various other signaling elements within postsynaptic PC spines (Fig. 2b).
Fig. 2.
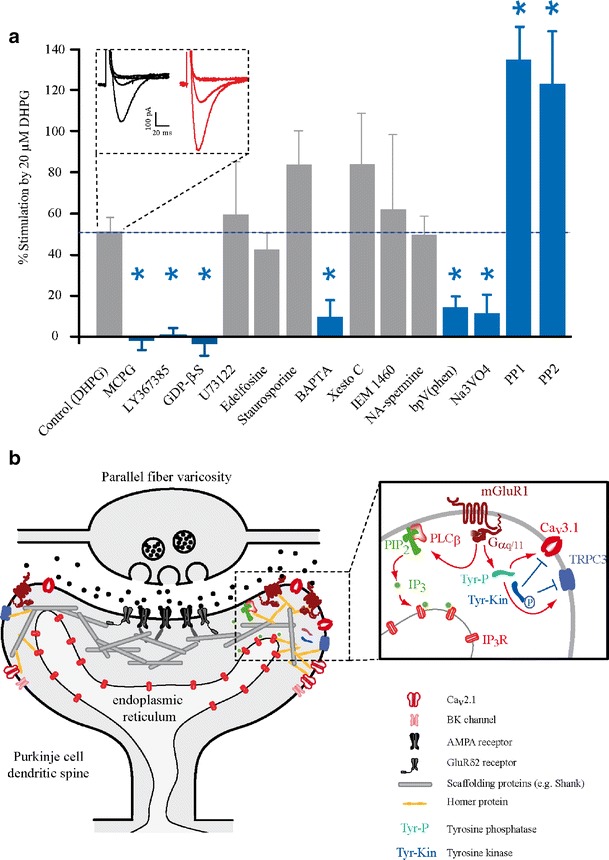
The spine machinery: intracellular pathway involved in the functional coupling between Cav3.1 and mGluR1. a Bar graph showing T-type channel potentiation values during various pharmacological treatments compared with the control (DHPG) potentiation value (indicated by dashed line). Blocking mGluR1 receptors with 500 μM MCPG (n = 6) or 100 μM LY367385 (n = 8), inhibiting G-protein signaling with 2 mM GDP-β-S (n = 5), buffering intracellular Ca2+ through inclusion of 20 mM BAPTA (n = 6) in the pipette, and blocking tyrosine phosphatases with 100 μM bpV(phen) (n = 6) or 1 mM Na3VO4 (n = 5) all significantly (blue bars; p < 0.02) reduced the DHPG-mediated increase. Conversely, blocking Src-family tyrosine kinases with inclusion of 10 μM PP1 (n = 5) or 10 μM PP2 (n = 6) in the pipette significantly (p < 0.02) augmented the DHPG-induced increase. Blocking phospholipase C with 1 μM U73122 (n = 6) or 10 μM edelfosine (n = 7), serine/threonine kinases (such as protein kinase C) with 1 to 2.5 μM staurosporine (n = 8), IP3Rs with 1 μM xestospongin C (n = 6), and sEPSC currents with 250 μM IEM 1460 (n = 6) or 100 μM NA-spermine (n = 5) all caused no significant (p > 0.05) change in the level of DHPG-mediated increase in T-type currents. Inset Representative voltage-clamped current traces from rat PCs during depolarizations to potentials ranging from –60 to –30 mV before (left; black) and after (right; red) mGluR1 was activated with 30 μM DHPG. From Hildebrand et al. [27] with permission. b Synaptic scaffolding of the parallel fiber to Purkinje cell synapse. Glutamate AMPA and GluRδ2 receptors are embedded in a subsynaptic ultrastructure composed notably by Shank and Homer proteins. Homer proteins also tether various calcium signaling receptors and channels to scaffolding proteins, creating a perisynaptic molecular microdomain ideally suited for calcium signal integration within Purkinje cell spines. Inset mGluR1 receptor activation leads either to a release of calcium from internal stores via the PLCβ/IP3 pathway or to activation of Cav3.1 and TRPC3 via tyrosine phosphatases
Cav3.1 Channels are Embedded in a Scaffolding and Calcium Signaling Complex within Dendritic Spines
Unraveling the fine microstructure and protein scaffolding organization within PC dendritic spines is yielding exciting new results towards understanding local synaptic integration. Biochemical assays have demonstrated physical interactions between several calcium signaling pathways and the structural organization of the spine [72, 73] (Fig. 2b). The scaffolding of postsynaptic densities (PSDs) in PC spines includes tight interactions between PDZ domain proteins such as PSD-93 and Shank [72, 74, 75]. AMPA receptors (containing GluR2) that mediate fast synaptic transmission are linked to this structure via GRIP and PICK1 proteins [76, 77]. Purkinje cell spines that receive PF inputs contain a specific PSD that lacks NMDA receptors but includes the GluRδ2 receptor, a GluR subunit with thus far no assigned ionotropic activity [78]. After 3 weeks of age, GluRδ2 is specifically expressed at the PF-PC synapse and is essential for both stabilization and plasticity (long-term depression; LTD) of this synapse via an interaction between its C terminus and PSD scaffolding proteins such as Shank [79, 80] [72]. Homer 3 is another highly expressed and critical scaffolding protein that forms a lattice around the PSD of PF-PC synapses [81–83]. Homer also binds calcium signaling proteins; indeed, homer co-immunoprecipitates with GluRδ2, Shank, mGluR1a, PLCβ4 and IP3 receptors (IP3Rs) [72, 84]. This architecture surrounds the PSD like a belt and links it to the endoplasmic reticulum via IP3Rs, suggesting that proteins of the mGluR1/PLC/IP3 pathway, involved in the induction of LTD (for review, see [85]), are tethered to each other by homer proteins. Electron microscopy studies have shown that mGluR1 and PLCβ4 receptors are highly expressed at the perisynaptic site of the PF synapse [73, 86]. Upon glutamate binding to mGluR1, the coupled Gαq/11 activates two independent intracellular pathways: one leads to calcium release from internal stores through IP3Rs [87–89], while a second pathway activates a slow EPSP [90] mediated by a nonselective cation channel that is calcium permeable and recently identified as the TRPC3 channel [68] (Fig. 2b). Like IP3-mediated calcium release, this sEPSC has a delayed onset in the range of hundreds of milliseconds [90]. PTPMEG tyrosine phosphatase is highly expressed in PC spines and binds to GluRδ2 receptors in the PSD structure and thus, could be directly involved in the tyrosine-phosphatase dependent activation of sEPSCs (see “Native Cav3.1 T-Type Channels are Potentiated by mGluR1”). Interestingly, PTPMEG KO mice and TRPC3 KO mice exhibit severe impairment in rotarod tests and walking coordination, respectively [68, 91].
Although membrane depolarization is thought to spread throughout the spines and dendrites of PCs, suggesting that voltage-dependent channels do not need to be part of a microdomain to be activated, recent studies have demonstrated that calcium channels are also part of this scaffolding belt surrounding the PSD in PF spines and that they can be modulated by physical interactions. Functional and physical interactions between T-type and P-type calcium channels and scaffolding architecture might impact calcium signaling within PC spines and dendrites. Electron microscopy and biochemical assays have demonstrated a physical link between mGluR1s and Cav2.1 (P-type) channels in spines via their carboxyl terminal intracellular domains, leading to a decrease in calcium conductance [44, 92]. Conversely, a coactivation of mGluR1 and Cav2.1 channels can also induce large calcium increases within PCs [92]. Furthermore, functional and anatomical studies have demonstrated that a combination of P-type calcium channel activity and mGluR1 activity can activate BK potassium currents in PC spines, indicating that this channel may also be closely associated with mGluR1 at the PF-PC synapse [93–95]. In a recent study [27], we suggest that Cav3.1 and mGluR1 are tightly linked together within PC spines. Although, Cav3.1 channels and mGluR1s are also found in PC dendrites, they are both highly expressed at the perisynaptic site around the PSD (Fig. 1f–h). Furthermore, when internal stores are blocked, the mGluR1-mediated augmentation of T-type calcium transients is observed in PC spines, but not their parent dendrites, indicating that the modulation occurs in a microdomain within spines. This evidence suggests either a physical link between Cav3.1 and mGluR1 or that key elements of the modulation pathway are restricted to PC spines. We have also demonstrated that Cav3.1 channels are potentiated by the same unique mGluR1 signaling pathway as for sEPSCs (see “Native Cav3.1 T-Type Channels are Potentiated by mGluR1”). Thus, we propose that activation of mGluR1-mediated pathways can increase intracellular calcium levels within PF-PC spine microdomains through the activation of both voltage-dependent (Cav3.1 and Cav2.1) and voltage-independent (sEPSC) membrane calcium channels (Fig. 2b). The specific physiological roles of both Cav3.1 and Cav2.1 channels are discussed in “PF Inputs Induce Spine-Specific Fast Calcium Signaling Mediated by Cav3.1 T-Type Channels.”
Although direct evidence has not yet linked all of these signaling elements together within a single PC distal dendritic spine, this proposed organization of microdomains below the PSD of the PF-PC synapse is likely to play a major role in calcium signaling and thus in the regulation of synaptic transmission. Indeed, in mice with mutations in myosin Va, the endoplasmic reticulum and associated IP3Rs do not enter into the spine head of the PC [96]. In these mice, basic excitatory transmission is normal and all the synaptic proteins are functional; however, IP3-mediated calcium signaling in spines is altered and LTD induction is impaired [96]. This is the first demonstration in PCs of the requirement for the structural integrity of the subsynaptic scaffolding microdomain.
T-type Channels Promote Bursting Behavior in Reduced Preparations
Recent studies have implicated T-type calcium channels as having a significant role in dendritic calcium spikes and the resultant burst firing within the soma and proximal dendrites of PCs (reviewed in [97]). For over 25 years, it has been accepted that sodium-driven action potentials (APs) are produced at the PC soma while calcium-driven APs are produced in the PC dendrites and that both are generally restricted to their respective compartments. The dendritic calcium spikes originally studied in guinea pig PCs were shown to include both calcium-dependent plateau potentials as well as calcium spikes [1, 98], and P-type calcium channels were thought to generate these calcium spikes [3, 56]. Studying PCs from rat organotypic cerebellar slice cultures revealed that T-type calcium channels underlie the dendritic calcium spikes while P-type channels underlie a plateau potential that is unmasked when potassium channels are blocked [99]. In fact, pharmacological blockade of the P-type current promoted propagation of the low threshold calcium spikes to the PC soma [100]. The robust P-type dendritic currents in PCs have been shown to activate calcium-dependent BK and SK potassium channels, which induces afterhyperpolarizing potentials and alters the frequency of PC firing [94, 95]. It is proposed that dendrosomatic propagation of T-type-dependent calcium spikes is inhibited by this activation of calcium-dependent potassium channels and that these low threshold spikes may underlie the CF-induced complex spike [100, 101]. In separate experiments on acutely dissociated PCs and PCs from acute cerebellar slices, it has been shown that P-type currents are required to sustain the spontaneous firing of PCs, while T-type currents have a substantial contribution to calcium currents generated during interspike intervals of spontaneous bursting PCs [59, 102].
Although this collection of studies implicates T-type calcium channels in generating calcium-dependent bursting in PC dendrites, with potentially large physiological implications, several limitations of these studies should be noted. Firstly, the overall structure and native composition of PCs is not well maintained in most of the in vitro systems used. Acutely dissociated PCs lack the dendritic tree where T-type calcium channels predominate [59] while cerebellar slice cultures are taken from newborn rats and subsequent dendritic growth and channel expression is determined by in vitro culture conditions [63, 99–101]. Indeed, in acute slices from young and mature rodents, T-type calcium currents are not directly involved in the generation of PC bursting behavior [59, 102–104] and except during the CF response, potassium channels likely prevent the generation of calcium spikes in Purkinje cell dendrites [95, 103, 105–107].
T-Type Channels in Local Calcium Signaling in Purkinje Cell Dendrites
PF Inputs Induce Spine-Specific Fast Calcium Signaling Mediated by Cav3.1 T-Type Channels
Miyakawa et al. [108] first demonstrated that bursts of PF stimulation induce two types of calcium transients in PC spines. While low-intensity PF stimulation induces a barely detectable graded response in the distal part of the dendrites (see also [109]), strong PF stimulation induces clear regenerative calcium spikes in PC dendrites. Using confocal and two photon laser microscopy, Eilers et al. [110] and Denk et al. [111] showed that subthreshold PF stimulation induces localized calcium transients in PC spines. Furthermore, both CNQX, an AMPA receptor antagonist, and hyperpolarization decrease the size of the calcium transients, suggesting that they are mediated by voltage-dependent calcium channels. More recently, we found that T-type channels are expressed in PC spines [57] and used ultrafast random access two photon microscopy (RAMP) [112] to demonstrate that bursts of low-intensity PF stimulation induce fast calcium transients that are mainly mediated by Cav3.1 T-type channels in individual spines [27]. At low-stimulation intensity, focal PF stimulation (trains of extracellular stimulation in the molecular layer) induces local calcium influx that is reduced by more than 75% in Cav3.1 KO mice compared to WT mice when internal stores are blocked by heparin. Since P-type channels are also present in PC spines, why are they not activated by low intensity PF stimulation? As shown by Isope and Barbour [113], the individual synaptic efficacy of granule cell to PC connections is widely distributed. Based on the distribution of individual connections, the conversion factor between EPSCs and EPSPs (8.3 μV/pA, see figure 3 of [113]) and a model of dendritic filtering by Roth and Hausser [114], we can estimate that individual synaptic EPSPs range from tens of microvolts to a few millivolts. Although we certainly underestimate the filtering capacity of the spine neck and the drop of potential between the spine and parent dendrite [115, 116], such small EPSPs are below the threshold for activation of Cav2.1 P-type channels (around −45 mV). In fact, for moderate PF stimulations, the first EPSP in the train does not induce any detectable calcium influx, suggesting that temporal summation may be required to even reach the activation threshold for low threshold T-type channels (between −60 and −55 mV; Fig. 3). However, when stimulation intensity is raised and individual PF inputs now summate to locally depolarize PC dendrites, regenerative P-type calcium transients can be observed in several spiny branchlets (Supplementary figure 6 in [27] and unpublished data). Interestingly, these calcium spikes do not propagate to the soma and Rancz and Hausser have also demonstrated that calcium-activated BK channels can restrict the spread of calcium spikes in PC dendrites [105].
Fig. 3.
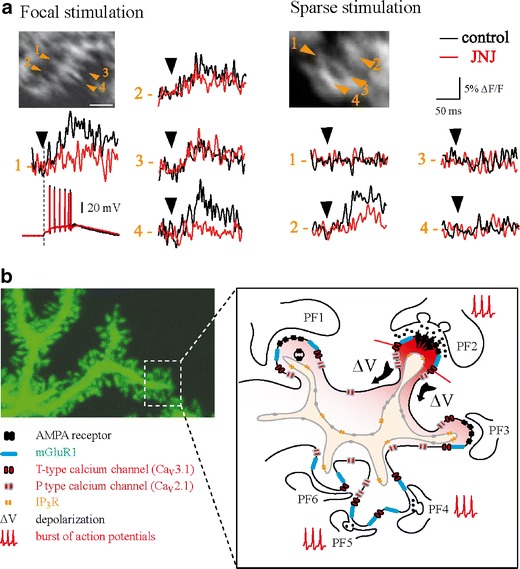
Spine-restricted fast calcium signaling is mediated by T-type channels. a Variation of calcium fluorescence in individual spines following parallel fiber stimulation in control conditions (black) and during application of an antagonist of the mGluR1 receptor (red, 1.5 μM JNJ16259685; for detailed description, see [27]). In all experiments, calcium release by internal stores was pharmacologically blocked using heparin and cyclopiazonic acid. Left panel an example of the EPSPs recorded at the soma. Note that when multiple spines are activated in one spiny branchlet (“focal stimulation”), two types of voltage-dependent calcium transients are observed: in one population of spines (spines 2 and 3), the calcium transients are not blocked by the mGluR1 antagonist, suggesting that depolarization is the sole mediator of T-type channel opening. In a second population (spines 1 and 4), the calcium transients are highly affected by the mGluR1 antagonist, suggesting that they directly receive synaptic input. Right panel However, when one spine is active in the spiny branchlet (“sparse stimulation”), the calcium transient is almost completely controlled by mGluR1 activation (spine 2). We postulate that this situation resembles physiological conditions. From Hildebrand et al. [27] with permission. b Model of fast calcium signaling in Purkinje cell dendritic spines. When a burst of parallel fiber action potentials release enough glutamate to activate both AMPA and mGluR1 receptors, the combination of local depolarization and potentiation of T-type channel activation mediated by mGluR1 leads to an enhanced calcium transient in the activated spine. Spine specificity is promoted by: (1) the fact that in non-synaptically activated spines, the depolarization is smaller and does not reach the threshold for T-type channel activation (e.g., PF1 and PF3) and (2) the high number of silent synapses (e.g., PF4 and PF5; Isope and Barbour [113]). Spiny branchlet picture courtesy of Boris Barbour
We have also shown that mGluR1 gates the T-type calcium transients by shifting the activation curve towards hyperpolarized potentials and increasing maximal conductance. Blockade of mGluR1 leads to a significant decrease in synaptic calcium transients produced by low intensity burst stimulation of PFs. Interestingly, when focusing on individual spiny branchlets, we observed that focal stimulation of PF inputs can induce T-type calcium transients in most spines of a given branchlet, leading to depolarization of the entire branchlet (Fig. 3). In this particular case, “unlocking” by mGluR1 is not necessary for the activation of T-type channels (although mGluR1-mediated potentiation is still observed in most spines). However, at the periphery of the excited PF beam, it is possible to identify spiny branchlets wherein T-type calcium transients are elicited in single spines and are almost fully blocked by mGluR1 antagonist (Fig. 3). We postulate that T-type channel signaling is designed to mediate local calcium signaling selectively in spines that receive a PF input. In vivo experiments have revealed a large dynamic range for granule cell activity: although the activity of granule cells is notably sparse, they are also capable of bursting at frequencies exceeding 1 kHz [117–119]. We hypothesize that mGluR1 activation will both amplify the discrepancy between activated spines and their neighbors as well as detect incoming bursts of PF inputs.
Climbing Fiber Signaling: Decoupling Somatic and Dendritic Excitability
During the 1990s, the laboratory of William Ross first demonstrated that distal CF-evoked calcium transients are highly variable compared to proximal ones ([108]; see also figure 1 of [109]), suggesting that regenerative calcium spikes do not always propagate to spiny branchlets. Furthermore, they showed that evoked inhibition by activation of molecular layer interneurons is able to block the CF-activated calcium transient in identified dendritic branches or even the full dendritic tree [120]. Ross and colleagues also showed that the shape of the complex spike recorded at the soma of PCs is not modified by the extent of the dendritic calcium transient, suggesting that sodium channel input is the major component of the plateau depolarization typical of the complex spike waveform. This finding was confirmed in a recent article using simultaneous dendritic and somatic patch-clamp recordings [121]. Since a direct projection of the CF onto molecular interneurons has now been demonstrated both in vitro and in vivo [122, 123], it appears that synaptic connections between stellate and basket cells might dampen the extent of the calcium transient mediated by the CF input. Finally, the group of Stephane Dieudonné showed that T-type calcium channels are also involved in CF calcium signaling that is dependent on PC dendritic excitability ([124]; see also [125]).
An emerging hypothesis of CF signaling in PCs is that the stereotyped somatic complex spike mediated by sodium channels is decoupled from the dendritic calcium transient that is highly variable and strongly modulated both by intrinsic dendritic conductances and molecular layer interneuron inhibition. How could such a large depolarization by climbing input in the main trunk of PC dendrites decline so rapidly and lead to only small depolarizations in spines? Since both low threshold (Kv1 and Kv4.3) and high threshold (Kv3) voltage-dependent potassium channels have been identified in PC dendrites [106, 126–128], one hypothesis suggests that these channels can dampen CF EPSPs. Indirect experiments demonstrate that Kv1 or Kv4 blockade favors the initiation of calcium spikes in the dendrites, suggesting that low threshold potassium channels control dendritic excitability [106, 129]. We hypothesize that high levels of low-threshold potassium channels can dramatically decrease the large CF-EPSP as it travels up to more distal parts of the PC dendrites (as far as 150 μm in adult mice). Furthermore, after calcium spike occurrence, high levels of calcium-activated potassium channels (BK and SK) will help repolarize dendrites and limit spike propagation [95, 105]. These findings help explain the absence of propagation of calcium transients from the dendrites toward the somatic compartment. We further postulate that CF dendritic calcium spike gating regulates the source (T-type vs P-type) and amplitude of CF-mediated calcium signaling and provides for new mechanistic possibilities concerning activity-dependent learning in PCs.
Physiological Consequences and Future Directions
Based on the above discussions, we propose a new picture of voltage-dependent calcium signaling within PC dendrites and make hypotheses that may stimulate new experiments on information processing in the cerebellar cortex.
Cav3.1 T-type channels are highly expressed in PC dendrites and spines. T-type channels are not inactivated at rest and regular spiking at the soma does not interfere with channel availability in spines [27, 54]. Low threshold potassium channels such as Kv1 and Kv4.3 channels probably clamp the cell to hyperpolarized potentials [106, 126–128]. As recently described [130], the activity of inhibitory oscillatory networks could also enhance the deinactivation of T-type channels in distal dendrites in some physiological conditions.
Parallel fiber synaptic input induces fast T-type calcium transients in spines following either conjunctive activation of a group of neighboring synapses or by mGluR1 activation, which selectively unlocks T-type channels in individual spines. This effect is specific to PF bursts and occurs during the burst itself with no time lag. The T-type channel conductances contribute to synaptic charge and boost individual synaptic inputs. LTP at PF synapses is mediated by an unknown mGluR1-mediated calcium-dependent pathway [131, 132] and we postulate that T-type channels could be the source of calcium in this plasticity.
Although 85% of PF-PC synapses are electrically silent in the adult rat when recorded at the soma, it is likely that sparse glutamate receptor activation at a synaptic site would not induce sufficient charge to be detected at the soma due to dendritic filtering. Indeed, it was demonstrated that all spines possess at least a small number of AMPA receptors [133]. The functional coupling between mGluR1 and T-type channels could then provide a mechanism for inducing calcium inputs in spines where the efficacy of transmission is low because of a small number of receptors. This pathway could potentially lead to the awakening of synaptic transmission to the soma via LTP.
We postulate that P-type channels are activated only when a large number of PF inputs impinge simultaneously on the PC dendritic tree, or during simultaneous CF activation. Conjunction of CF inputs and PF inputs could lead to the inactivation of low threshold potassium channels (Kv1, Kv4.3) and promote a supralinear calcium increase in spines as shown by Wang and Hausser [109] and LTD induction. T-type channel unlocking in the early phase followed by subsequent IP3-mediated calcium release from internal stores might combine to reach a high level of calcium both in local microdomains and in the whole spiny branchlets. This second level of voltage-dependent calcium signaling is projected to propagate to groups of spiny branchlets and could trigger release of retrograde signaling via endocannabinoids [105] or glutamate [134]. The two levels of calcium signaling might be tightly regulated in PC dendrites by potassium channels and/or the depolarization state of the PC.
Acknowledgements
T.P.S. is supported by an operating grant from the Canadian Institutes of Health Research and a Canada Research Chair in Biotechnology and Genomics-Neurobiology. M.E.H. is supported by an Industrial Research and Development Fellowship from the Natural Sciences and Engineering Research Council of Canada. P.I is supported by two operating grants from the Agence Nationale pour la Recherche (ANR; CeCoMod and MicroCer) and by the Centre National pour la Recherche Scientifique.
Conflict of Interest
The authors have no competing financial interests in relation to the subject of this review.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AP
Action potential
- CF
Climbing fiber
- CNS
Central nervous system
- HVA
High-voltage activated
- IP3R
Inositol trisphosphate receptors
- KO
Knock-out
- LTD
Long-term depression
- Ni2+
Nickel
- PCs
Purkinje cells
- PF
Parallel fiber
- RAMP
Random access two photon microscopy
- sEPSC
Slow excitatory postsynaptic current
- sEPSP
Slow excitatory postsynaptic potential
- PSD
Postsynaptic density
- WT
Wild-type
Footnotes
Philippe Isope and Michael E. Hildebrand contributed equally to the manuscript.
Contributor Information
Philippe Isope, Email: philippe.isope@inci-cnrs.unistra.fr.
Michael E. Hildebrand, Email: mhildebrand@neuromed.com
Terrance P. Snutch, Email: snutch@msl.ubc.ca
References
- 1.Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 3.Usowicz MM, Sugimori M, Cherksey B, Llinas R. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron. 1992;9(6):1185–1199. doi: 10.1016/0896-6273(92)90076-p. [DOI] [PubMed] [Google Scholar]
- 4.Tank DW, Sugimori M, Connor JA, Llinas RR. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988;242(4879):773–777. doi: 10.1126/science.2847315. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83(1):117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 6.McRory JE, Santi CM, Hamming KS, Mezeyova J, Sutton KG, Baillie DL, et al. Molecular and functional characterization of a family of rat brain T-type calcium channels. J Biol Chem. 2001;276(6):3999–4011. doi: 10.1074/jbc.M008215200. [DOI] [PubMed] [Google Scholar]
- 7.Iftinca M, McKay BE, Snutch TP, McRory JE, Turner RW, Zamponi GW. Temperature dependence of T-type calcium channel gating. Neuroscience. 2006;142(4):1031–1042. doi: 10.1016/j.neuroscience.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P. Alternatively spliced alpha(1G) (Ca(V)3.1) intracellular loops promote specific T-type Ca(2+) channel gating properties. Biophys J. 2001;80(3):1238–1250. doi: 10.1016/S0006-3495(01)76100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell KL, Cain SM, Ng C, Sirdesai S, David LS, Kyi M, et al. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci. 2009;29(2):371–380. doi: 10.1523/JNEUROSCI.5295-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh R, Nakabayashi Y, Kano M. Pharmacological properties of two types of calcium channel in embryonic chick skeletal muscle cells in culture. Neurosci Lett. 1991;122(2):233–236. doi: 10.1016/0304-3940(91)90866-r. [DOI] [PubMed] [Google Scholar]
- 11.Kaneda M, Wakamori M, Ito C, Akaike N. Low-threshold calcium current in isolated Purkinje cell bodies of rat cerebellum. J Neurophysiol. 1990;63(5):1046–1051. doi: 10.1152/jn.1990.63.5.1046. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J. 1999;77(6):3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamponi GW, Bourinet E, Snutch TP. Nickel block of a family of neuronal calcium channels: subtype- and subunit-dependent action at multiple sites. J Membr Biol. 1996;151(1):77–90. doi: 10.1007/s002329900059. [DOI] [PubMed] [Google Scholar]
- 14.McDonough SI, Bean BP. Mibefradil inhibition of T-type calcium channels in cerebellar purkinje neurons. Mol Pharmacol. 1998;54(6):1080–1087. doi: 10.1124/mol.54.6.1080. [DOI] [PubMed] [Google Scholar]
- 15.Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36(7):879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- 16.Viana F, Van den Bosch L, Missiaen L, Vandenberghe W, Droogmans G, Nilius B, et al. Mibefradil (Ro 40-5967) blocks multiple types of voltage-gated calcium channels in cultured rat spinal motoneurones. Cell Calcium. 1997;22(4):299–311. doi: 10.1016/s0143-4160(97)90068-3. [DOI] [PubMed] [Google Scholar]
- 17.Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, et al. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T) window. J Neurosci. 2010;30(1):99–109. doi: 10.1523/JNEUROSCI.4305-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yunker AM. Modulation and pharmacology of low voltage-activated (“T-Type”) calcium channels. J Bioenerg Biomembr. 2003;35(6):577–598. doi: 10.1023/b:jobb.0000008025.65675.37. [DOI] [PubMed] [Google Scholar]
- 19.Tao J, Hildebrand ME, Liao P, Liang MC, Tan G, Li S, et al. Activation of corticotropin-releasing factor receptor 1 selectively inhibits CaV3.2 T-type calcium channels. Mol Pharmacol. 2008;73(6):1596–1609. doi: 10.1124/mol.107.043612. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe JT, Wang H, Howard J, Garrison JC, Barrett PQ. T-type calcium channel regulation by specific G-protein betagamma subunits. Nature. 2003;424(6945):209–213. doi: 10.1038/nature01772. [DOI] [PubMed] [Google Scholar]
- 21.Welsby PJ, Wang H, Wolfe JT, Colbran RJ, Johnson ML, Barrett PQ. A mechanism for the direct regulation of T-type calcium channels by Ca2+/calmodulin-dependent kinase II. J Neurosci. 2003;23(31):10116–10121. doi: 10.1523/JNEUROSCI.23-31-10116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todorovic SM, Jevtovic-Todorovic V, Meyenburg A, Mennerick S, Perez-Reyes E, Romano C, et al. Redox modulation of T-type calcium channels in rat peripheral nociceptors. Neuron. 2001;31(1):75–85. doi: 10.1016/s0896-6273(01)00338-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen RS, Best PM. A small peptide inhibitor of the low voltage-activated calcium channel Cav3.1. Mol Pharmacol. 2009;75(5):1042–1051. doi: 10.1124/mol.108.052654. [DOI] [PubMed] [Google Scholar]
- 24.Hildebrand ME, David LS, Hamid J, Mulatz K, Garcia E, Zamponi GW, et al. Selective inhibition of Cav3.3 T-type calcium channels by Galphaq/11-coupled muscarinic acetylcholine receptors. J Biol Chem. 2007;282(29):21043–21055. doi: 10.1074/jbc.M611809200. [DOI] [PubMed] [Google Scholar]
- 25.Iftinca M, Hamid J, Chen L, Varela D, Tadayonnejad R, Altier C, et al. Regulation of T-type calcium channels by Rho-associated kinase. Nat Neurosci. 2007;10(7):854–860. doi: 10.1038/nn1921. [DOI] [PubMed] [Google Scholar]
- 26.Kim T, Kim S, Yun HM, Chung KC, Han YS, Shin HS, et al. Modulation of Ca(v)3.1 T-type Ca2+ channels by the ran binding protein RanBPM. Biochem Biophys Res Commun. 2009;378(1):15–20. doi: 10.1016/j.bbrc.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrand ME, Isope P, Miyazaki T, Nakaya T, Garcia E, Feltz A, et al. Functional coupling between mGluR1 and Cav3.1 T-type calcium channels contributes to parallel fiber-induced fast calcium signaling within Purkinje cell dendritic spines. J Neurosci. 2009;29(31):9668–9682. doi: 10.1523/JNEUROSCI.0362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams SR, Toth TI, Turner JP, Hughes SW, Crunelli V. The ‘window’ component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. J Physiol. 1997;505(Pt 3):689–705. doi: 10.1111/j.1469-7793.1997.689ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33(6):947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 30.Llinas R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 32.Diana MA, Otsu Y, Maton G, Collin T, Chat M, Dieudonne S. T-type and L-type Ca2+ conductances define and encode the bimodal firing pattern of vestibulocerebellar unipolar brush cells. J Neurosci. 2007;27(14):3823–3838. doi: 10.1523/JNEUROSCI.4719-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki S, Rogawski MA. T-type calcium channels mediate the transition between tonic and phasic firing in thalamic neurons. Proc Natl Acad Sci USA. 1989;86(18):7228–7232. doi: 10.1073/pnas.86.18.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson MP, Mochizuki T, Xie J, Fischler W, Manger JP, Talley EM, et al. Thalamic Cav3.1 T-type Ca2+ channel plays a crucial role in stabilizing sleep. Proc Natl Acad Sci USA. 2005;102(5):1743–1748. doi: 10.1073/pnas.0409644102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, et al. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31(1):35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 36.McKay BE, McRory JE, Molineux ML, Hamid J, Snutch TP, Zamponi GW, et al. Ca(V)3 T-type calcium channel isoforms differentially distribute to somatic and dendritic compartments in rat central neurons. Eur J Neurosci. 2006;24(9):2581–2594. doi: 10.1111/j.1460-9568.2006.05136.x. [DOI] [PubMed] [Google Scholar]
- 37.Molineux ML, McRory JE, McKay BE, Hamid J, Mehaffey WH, Rehak R, et al. Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc Natl Acad Sci USA. 2006;103(14):5555–5560. doi: 10.1073/pnas.0601261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magee JC, Christofi G, Miyakawa H, Christie B, Lasser-Ross N, Johnston D. Subthreshold synaptic activation of voltage-gated Ca2+ channels mediates a localized Ca2+ influx into the dendrites of hippocampal pyramidal neurons. J Neurophysiol. 1995;74(3):1335–1342. doi: 10.1152/jn.1995.74.3.1335. [DOI] [PubMed] [Google Scholar]
- 39.Markram H, Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci USA. 1994;91(11):5207–5211. doi: 10.1073/pnas.91.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22(9):3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christie BR, Schexnayder LK, Johnston D. Contribution of voltage-gated Ca2+ channels to homosynaptic long-term depression in the CA1 region in vitro. J Neurophysiol. 1997;77(3):1651–1655. doi: 10.1152/jn.1997.77.3.1651. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299(5610):1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- 43.Tringham EW, Payne CE, Dupere JR, Usowicz MM. Maturation of rat cerebellar Purkinje cells reveals an atypical Ca2+ channel current that is inhibited by omega-agatoxin IVA and the dihydropyridine (-)-(S)-Bay K8644. J Physiol. 2007;578(Pt 3):693–714. doi: 10.1113/jphysiol.2006.121905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulik A, Nakadate K, Hagiwara A, Fukazawa Y, Lujan R, Saito H, et al. Immunocytochemical localization of the alpha 1A subunit of the P/Q-type calcium channel in the rat cerebellum. Eur J Neurosci. 2004;19(8):2169–2178. doi: 10.1111/j.0953-816X.2004.03319.x. [DOI] [PubMed] [Google Scholar]
- 45.Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, et al. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci. 1995;15(10):6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, et al. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993;123(4):949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liljelund P, Netzeband JG, Gruol DL. L-Type calcium channels mediate calcium oscillations in early postnatal Purkinje neurons. J Neurosci. 2000;20(19):7394–7403. doi: 10.1523/JNEUROSCI.20-19-07394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regan LJ. Voltage-dependent calcium currents in Purkinje cells from rat cerebellar vermis. J Neurosci. 1991;11(7):2259–2269. doi: 10.1523/JNEUROSCI.11-07-02259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meacham CA, White LD, Barone S, Jr, Shafer TJ. Ontogeny of voltage-sensitive calcium channel alpha(1A) and alpha(1E) subunit expression and synaptic function in rat central nervous system. Brain Res Dev Brain Res. 2003;142(1):47–65. doi: 10.1016/s0165-3806(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama CT, Westenbroek RE, Hell JW, Soong TW, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of the neuronal class E calcium channel alpha 1 subunit. J Neurosci. 1995;15(10):6419–6432. doi: 10.1523/JNEUROSCI.15-10-06419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crepel F, Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol. 1986;372:1–23. doi: 10.1113/jphysiol.1986.sp015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bossu JL, Fagni L, Feltz A. Voltage-activated calcium channels in rat Purkinje cells maintained in culture. Pflugers Arch. 1989;414(1):92–94. doi: 10.1007/BF00585632. [DOI] [PubMed] [Google Scholar]
- 53.Hirano T, Hagiwara S. Kinetics and distribution of voltage-gated Ca, Na and K channels on the somata of rat cerebellar Purkinje cells. Pflugers Arch. 1989;413(5):463–469. doi: 10.1007/BF00594174. [DOI] [PubMed] [Google Scholar]
- 54.Mouginot D, Bossu JL, Gahwiler BH. Low-threshold Ca2+ currents in dendritic recordings from Purkinje cells in rat cerebellar slice cultures. J Neurosci. 1997;17(1):160–170. doi: 10.1523/JNEUROSCI.17-01-00160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19(5):1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe S, Takagi H, Miyasho T, Inoue M, Kirino Y, Kudo Y, et al. Differential roles of two types of voltage-gated Ca2+ channels in the dendrites of rat cerebellar Purkinje neurons. Brain Res. 1998;791(1–2):43–55. doi: 10.1016/s0006-8993(98)00048-1. [DOI] [PubMed] [Google Scholar]
- 57.Isope P, Murphy TH. Low threshold calcium currents in rat cerebellar Purkinje cell dendritic spines are mediated by T-type calcium channels. J Physiol. 2005;562(Pt 1):257–269. doi: 10.1113/jphysiol.2004.074211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gruol DL, Deal CR, Yool AJ. Developmental changes in calcium conductances contribute to the physiological maturation of cerebellar Purkinje neurons in culture. J Neurosci. 1992;12(7):2838–2848. doi: 10.1523/JNEUROSCI.12-07-02838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci. 2003;23(29):9650–9663. doi: 10.1523/JNEUROSCI.23-29-09650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKay BE, Turner RW. Physiological and morphological development of the rat cerebellar Purkinje cell. J Physiol. 2005;567(Pt 3):829–850. doi: 10.1113/jphysiol.2005.089383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci. 2008;11(7):790–798. doi: 10.1038/nn.2137. [DOI] [PubMed] [Google Scholar]
- 62.Tringham EW, Dupere JR, Payne CE, Usowicz MM. Protease treatment of cerebellar purkinje cells renders omega-agatoxin IVA-sensitive Ca2+ channels insensitive to inhibition by omega-conotoxin GVIA. J Pharmacol Exp Ther. 2008;324(2):806–814. doi: 10.1124/jpet.107.130641. [DOI] [PubMed] [Google Scholar]
- 63.Cavelier P, Beekenkamp H, Shin HS, Jun K, Bossu JL. Cerebellar slice cultures from mice lacking the P/Q calcium channel: electroresponsiveness of Purkinje cells. Neurosci Lett. 2002;333(1):64–68. doi: 10.1016/s0304-3940(02)00962-x. [DOI] [PubMed] [Google Scholar]
- 64.Craig PJ, Beattie RE, Folly EA, Banerjee MD, Reeves MB, Priestley JV, et al. Distribution of the voltage-dependent calcium channel alpha1G subunit mRNA and protein throughout the mature rat brain. Eur J Neurosci. 1999;11(8):2949–2964. doi: 10.1046/j.1460-9568.1999.00711.x. [DOI] [PubMed] [Google Scholar]
- 65.Kase M, Kakimoto S, Sakuma S, Houtani T, Ohishi H, Ueyama T, et al. Distribution of neurons expressing alpha 1G subunit mRNA of T-type voltage-dependent calcium channel in adult rat central nervous system. Neurosci Lett. 1999;268(2):77–80. doi: 10.1016/s0304-3940(99)00385-7. [DOI] [PubMed] [Google Scholar]
- 66.Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19(6):1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yunker AM, Sharp AH, Sundarraj S, Ranganathan V, Copeland TD, McEnery MW. Immunological characterization of T-type voltage-dependent calcium channel CaV3.1 (alpha 1G) and CaV3.3 (alpha 1I) isoforms reveal differences in their localization, expression, and neural development. Neuroscience. 2003;117(2):321–335. doi: 10.1016/s0306-4522(02)00936-3. [DOI] [PubMed] [Google Scholar]
- 68.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59(3):392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Staub C, Vranesic I, Knopfel T. Responses to Metabotropic Glutamate Receptor Activation in Cerebellar Purkinje Cells: Induction of an Inward Current. Eur J Neurosci. 1992;4(9):832–839. doi: 10.1111/j.1460-9568.1992.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 70.Canepari M, Ogden D. Evidence for protein tyrosine phosphatase, tyrosine kinase, and G-protein regulation of the parallel fiber metabotropic slow EPSC of rat cerebellar Purkinje neurons. J Neurosci. 2003;23(10):4066–4071. doi: 10.1523/JNEUROSCI.23-10-04066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canepari M, Auger C, Ogden D. Ca2+ ion permeability and single-channel properties of the metabotropic slow EPSC of rat Purkinje neurons. J Neurosci. 2004;24(14):3563–3573. doi: 10.1523/JNEUROSCI.5374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uemura T, Mori H, Mishina M. Direct interaction of GluRdelta2 with Shank scaffold proteins in cerebellar Purkinje cells. Mol Cell Neurosci. 2004;26(2):330–341. doi: 10.1016/j.mcn.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, et al. Signaling complex formation of phospholipase Cbeta4 with metabotropic glutamate receptor type 1alpha and 1, 4, 5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20(11):2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- 74.Roche KW, Ly CD, Petralia RS, Wang YX, McGee AW, Bredt DS, et al. Postsynaptic density-93 interacts with the delta2 glutamate receptor subunit at parallel fiber synapses. J Neurosci. 1999;19(10):3926–3934. doi: 10.1523/JNEUROSCI.19-10-03926.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bockers TM, Segger-Junius M, Iglauer P, Bockmann J, Gundelfinger ED, Kreutz MR, et al. Differential expression and dendritic transcript localization of Shank family members: identification of a dendritic targeting element in the 3′ untranslated region of Shank1 mRNA. Mol Cell Neurosci. 2004;26(1):182–190. doi: 10.1016/j.mcn.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 76.Yawata S, Tsuchida H, Kengaku M, Hirano T. Membrane-proximal region of glutamate receptor delta2 subunit is critical for long-term depression and interaction with protein interacting with C kinase 1 in a cerebellar Purkinje neuron. J Neurosci. 2006;26(14):3626–3633. doi: 10.1523/JNEUROSCI.4183-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28(2):499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 78.Hirano T. Cerebellar regulation mechanisms learned from studies on GluRdelta2. Mol Neurobiol. 2006;33(1):1–16. doi: 10.1385/MN:33:1:001. [DOI] [PubMed] [Google Scholar]
- 79.Yuzaki M. The delta2 glutamate receptor: a key molecule controlling synaptic plasticity and structure in Purkinje cells. Cerebellum. 2004;3(2):89–93. doi: 10.1080/14734220410028921. [DOI] [PubMed] [Google Scholar]
- 80.Uemura T, Kakizawa S, Yamasaki M, Sakimura K, Watanabe M, Iino M, et al. Regulation of long-term depression and climbing fiber territory by glutamate receptor delta2 at parallel fiber synapses through its C-terminal domain in cerebellar Purkinje cells. J Neurosci. 2007;27(44):12096–12108. doi: 10.1523/JNEUROSCI.2680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shiraishi Y, Mizutani A, Yuasa S, Mikoshiba K, Furuichi T. Differential expression of Homer family proteins in the developing mouse brain. J Comp Neurol. 2004;473(4):582–599. doi: 10.1002/cne.20116. [DOI] [PubMed] [Google Scholar]
- 82.Mizutani A, Kuroda Y, Futatsugi A, Furuichi T, Mikoshiba K. Phosphorylation of Homer3 by calcium/calmodulin-dependent kinase II regulates a coupling state of its target molecules in Purkinje cells. J Neurosci. 2008;28(20):5369–5382. doi: 10.1523/JNEUROSCI.4738-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, et al. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21(4):707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 84.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, et al. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21(4):717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 85.Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81(3):1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 86.Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11(4):771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 87.Khodakhah K, Ogden D. Functional heterogeneity of calcium release by inositol trisphosphate in single Purkinje neurones, cultured cerebellar astrocytes, and peripheral tissues. Proc Natl Acad Sci USA. 1993;90(11):4976–4980. doi: 10.1073/pnas.90.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396(6713):753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 89.Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396(6713):757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- 90.Batchelor AM, Madge DJ, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre-Purkinje cell pathway in rat cerebellar slices. Neuroscience. 1994;63(4):911–915. doi: 10.1016/0306-4522(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 91.Kina S, Tezuka T, Kusakawa S, Kishimoto Y, Kakizawa S, Hashimoto K, et al. Involvement of protein-tyrosine phosphatase PTPMEG in motor learning and cerebellar long-term depression. Eur J Neurosci. 2007;26(8):2269–2278. doi: 10.1111/j.1460-9568.2007.05829.x. [DOI] [PubMed] [Google Scholar]
- 92.Kitano J, Nishida M, Itsukaichi Y, Minami I, Ogawa M, Hirano T, et al. Direct interaction and functional coupling between metabotropic glutamate receptor subtype 1 and voltage-sensitive Cav2.1 Ca2+ channel. J Biol Chem. 2003;278(27):25101–25108. doi: 10.1074/jbc.M303266200. [DOI] [PubMed] [Google Scholar]
- 93.Canepari M, Ogden D. Kinetic, pharmacological and activity-dependent separation of two Ca2+ signalling pathways mediated by type 1 metabotropic glutamate receptors in rat Purkinje neurones. J Physiol. 2006;573(Pt 1):65–82. doi: 10.1113/jphysiol.2005.103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol. 2003;548(Pt 1):53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24(40):8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miyata M, Finch EA, Khiroug L, Hashimoto K, Hayasaka S, Oda SI, et al. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 2000;28(1):233–244. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 97.Cavelier P, Bossu JL. Dendritic low-threshold Ca2+ channels in rat cerebellar Purkinje cells: possible physiological implications. Cerebellum. 2003;2(3):196–205. doi: 10.1080/14734220310016141. [DOI] [PubMed] [Google Scholar]
- 98.Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pouille F, Cavelier P, Desplantez T, Beekenkamp H, Craig PJ, Beattie RE, et al. Dendro-somatic distribution of calcium-mediated electrogenesis in purkinje cells from rat cerebellar slice cultures. J Physiol. 2000;527(Pt 2):265–282. doi: 10.1111/j.1469-7793.2000.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cavelier P, Pouille F, Desplantez T, Beekenkamp H, Bossu JL. Control of the propagation of dendritic low-threshold Ca2+ spikes in Purkinje cells from rat cerebellar slice cultures. J Physiol. 2002;540(Pt 1):57–72. doi: 10.1113/jphysiol.2001.013294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cavelier P, Desplantez T, Beekenkamp H, Bossu JL. K+ channel activation and low-threshold Ca2+ spike of rat cerebellar Purkinje cells in vitro. NeuroReport. 2003;14(2):167–171. doi: 10.1097/00001756-200302100-00001. [DOI] [PubMed] [Google Scholar]
- 102.Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci. 2004;24(14):3511–3521. doi: 10.1523/JNEUROSCI.0290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons. J Neurosci. 2003;23(7):2600–2607. doi: 10.1523/JNEUROSCI.23-07-02600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci. 2002;22(24):10603–10612. doi: 10.1523/JNEUROSCI.22-24-10603.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rancz EA, Hausser M. Dendritic calcium spikes are tunable triggers of cannabinoid release and short-term synaptic plasticity in cerebellar Purkinje neurons. J Neurosci. 2006;26(20):5428–5437. doi: 10.1523/JNEUROSCI.5284-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khavandgar S, Walter JT, Sageser K, Khodakhah K. Kv1 channels selectively prevent dendritic hyperexcitability in rat Purkinje cells. J Physiol. 2005;569(Pt 2):545–557. doi: 10.1113/jphysiol.2005.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Womack MD, Khodakhah K. Characterization of large conductance Ca2+-activated K+ channels in cerebellar Purkinje neurons. Eur J Neurosci. 2002;16(7):1214–1222. doi: 10.1046/j.1460-9568.2002.02171.x. [DOI] [PubMed] [Google Scholar]
- 108.Miyakawa H, Lev-Ram V, Lasser-Ross N, Ross WN. Calcium transients evoked by climbing fiber and parallel fiber synaptic inputs in guinea pig cerebellar Purkinje neurons. J Neurophysiol. 1992;68(4):1178–1189. doi: 10.1152/jn.1992.68.4.1178. [DOI] [PubMed] [Google Scholar]
- 109.Wang SS, Denk W, Hausser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3(12):1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 110.Eilers J, Augustine GJ, Konnerth A. Subthreshold synaptic Ca2+ signalling in fine dendrites and spines of cerebellar Purkinje neurons. Nature. 1995;373(6510):155–158. doi: 10.1038/373155a0. [DOI] [PubMed] [Google Scholar]
- 111.Denk W, Sugimori M, Llinas R. Two types of calcium response limited to single spines in cerebellar Purkinje cells. Proc Natl Acad Sci USA. 1995;92(18):8279–8282. doi: 10.1073/pnas.92.18.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Otsu Y, Bormuth V, Wong J, Mathieu B, Dugue GP, Feltz A, et al. Optical monitoring of neuronal activity at high frame rate with a digital random-access multiphoton (RAMP) microscope. J Neurosci Methods. 2008;173(2):259–270. doi: 10.1016/j.jneumeth.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 113.Isope P, Barbour B. Properties of unitary granule cell–> Purkinje cell synapses in adult rat cerebellar slices. J Neurosci. 2002;22(22):9668–9678. doi: 10.1523/JNEUROSCI.22-22-09668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roth A, Hausser M. Compartmental models of rat cerebellar Purkinje cells based on simultaneous somatic and dendritic patch-clamp recordings. J Physiol. 2001;535(Pt 2):445–472. doi: 10.1111/j.1469-7793.2001.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proc Natl Acad Sci USA. 2006;103(47):17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bloodgood BL, Giessel AJ, Sabatini BL. Biphasic synaptic Ca influx arising from compartmentalized electrical signals in dendritic spines. PLoS Biol. 2009;7(9):e1000190. doi: 10.1371/journal.pbio.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arenz A, Silver RA, Schaefer AT, Margrie TW. The contribution of single synapses to sensory representation in vivo. Science. 2008;321(5891):977–980. doi: 10.1126/science.1158391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428(6985):856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- 119.Jorntell H, Ekerot CF. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J Neurosci. 2006;26(45):11786–11797. doi: 10.1523/JNEUROSCI.2939-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Callaway JC, Lasser-Ross N, Ross WN. IPSPs strongly inhibit climbing fiber-activated [Ca2+]i increases in the dendrites of cerebellar Purkinje neurons. J Neurosci. 1995;15(4):2777–2787. doi: 10.1523/JNEUROSCI.15-04-02777.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Davie JT, Clark BA, Hausser M. The origin of the complex spike in cerebellar Purkinje cells. J Neurosci. 2008;28(30):7599–7609. doi: 10.1523/JNEUROSCI.0559-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10(6):735–742. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- 123.Jorntell H, Ekerot CF. Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J Neurosci. 2003;23(29):9620–9631. doi: 10.1523/JNEUROSCI.23-29-09620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Otsu Y, Isope P, Feltz A, Tsujita M, Araishi K, Watanabe M, et al. Gating of complex spike calcium transient by metabotropic glutamate receptors in cerebellar Purkinje cells. 6th FENS Forum of European Neuroscience. Geneva: Federation of European Neuroscience Societies; 2008. [Google Scholar]
- 125.Cavelier P, Lohof AM, Lonchamp E, Beekenkamp H, Mariani J, Bossu JL. Participation of low-threshold Ca2+ spike in the Purkinje cells complex spike. NeuroReport. 2008;19(3):299–303. doi: 10.1097/WNR.0b013e3282f4cb96. [DOI] [PubMed] [Google Scholar]
- 126.McKay BE, Molineux ML, Mehaffey WH, Turner RW. Kv1 K+ channels control Purkinje cell output to facilitate postsynaptic rebound discharge in deep cerebellar neurons. J Neurosci. 2005;25(6):1481–1492. doi: 10.1523/JNEUROSCI.3523-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martina M, Yao GL, Bean BP. Properties and functional role of voltage-dependent potassium channels in dendrites of rat cerebellar Purkinje neurons. J Neurosci. 2003;23(13):5698–5707. doi: 10.1523/JNEUROSCI.23-13-05698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79(2):1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- 129.Midtgaard J, Lasser-Ross N, Ross WN. Spatial distribution of Ca2+ influx in turtle Purkinje cell dendrites in vitro: role of a transient outward current. J Neurophysiol. 1993;70(6):2455–2469. doi: 10.1152/jn.1993.70.6.2455. [DOI] [PubMed] [Google Scholar]
- 130.Middleton SJ, Racca C, Cunningham MO, Traub RD, Monyer H, Knopfel T, et al. High-frequency network oscillations in cerebellar cortex. Neuron. 2008;58(5):763–774. doi: 10.1016/j.neuron.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jorntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52(2):227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 132.Wang X, Chen G, Gao W, Ebner T. Long-term potentiation of the responses to parallel fiber stimulation in mouse cerebellar cortex in vivo. Neuroscience. 2009;162(3):713–722. doi: 10.1016/j.neuroscience.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Masugi-Tokita M, Tarusawa E, Watanabe M, Molnar E, Fujimoto K, Shigemoto R. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci. 2007;27(8):2135–2144. doi: 10.1523/JNEUROSCI.2861-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Duguid IC, Pankratov Y, Moss GW, Smart TG. Somatodendritic release of glutamate regulates synaptic inhibition in cerebellar Purkinje cells via autocrine mGluR1 activation. J Neurosci. 2007;27(46):12464–12474. doi: 10.1523/JNEUROSCI.0178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


