Abstract
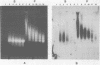
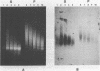
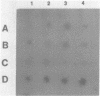
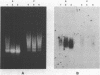
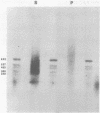
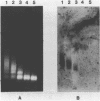
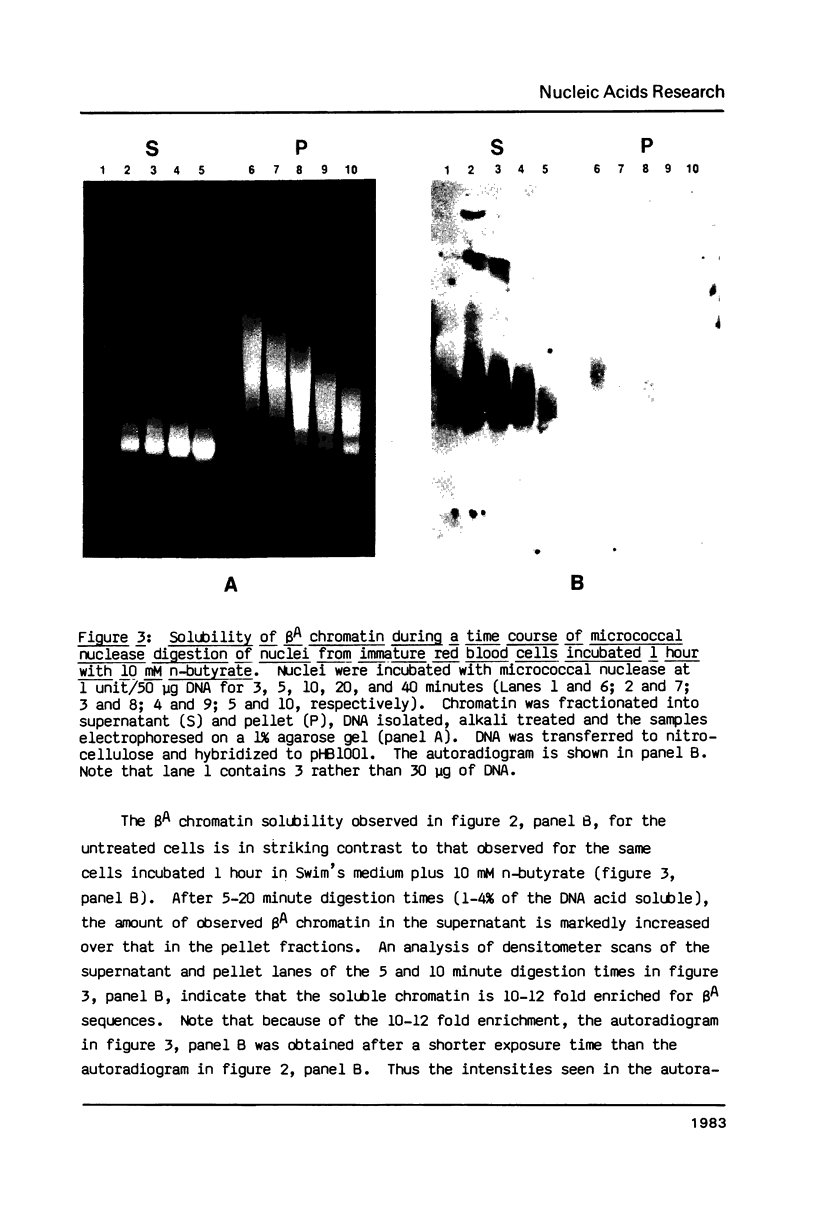
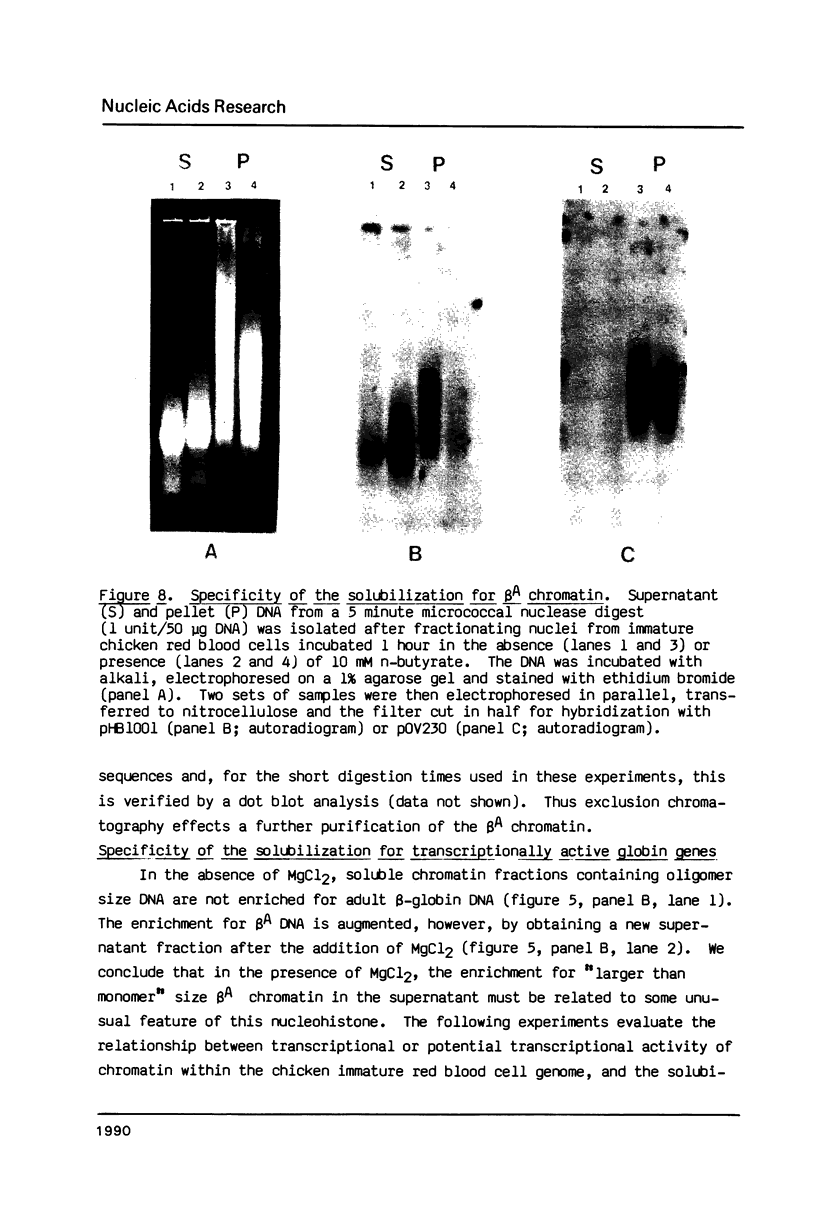
The solubility of adult beta-globin chromatin (beta A chromatin) from immature chicken red blood cells can be controlled by the presence or absence of n-butyrate in a cell incubation medium. In the absence of n-butyrate, only a small percentage (approximately 4%) of the total beta A chromatin is in a soluble chromatin fraction following micrococcal nuclease digestion and centrifugation. This percentage increases to approximately 40-45% of the beta A chromatin if cells are incubated 1 hour in the presence of 10 mM sodium n-butyrate. The highest yield and enrichment of solubilized beta A chromatin is attained when 1-4% of the DNA is rendered acid soluble, and in buffers containing 1.5 - 5 mM MgCl2. The soluble beta A nucleohistone is nucleosome oligomer size (contains DNA 250-600 bases in length) and can be separated from soluble, transcriptionally inert mononucleosomes by agarose A-5m exclusion chromatography. The enhanced solubility appears to be specific for transcriptionally active chromatin. Whereas 40-45% of the beta A chromatin is recovered in the supernatant fraction from n-butyrate incubated immature erythrocytes, nucleohistone containing ovalbumin DNA sequences remains insoluble.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato A. T., Seale R. L. Histone deacetylation is required for the maturation of newly replicated chromatin. J Biol Chem. 1983 Oct 25;258(20):12675–12684. [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Gruss R. J., Allfrey V. G. Manifold effects of sodium butyrate on nuclear function. Selective and reversible inhibition of phosphorylation of histones H1 and H2A and impaired methylation of lysine and arginine residues in nuclear protein fractions. J Biol Chem. 1981 Sep 25;256(18):9612–9621. [PubMed] [Google Scholar]
- Boffa L. C., Vidali G., Mann R. S., Allfrey V. G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978 May 25;253(10):3364–3366. [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Chambers S. A., Rill R. L. Enrichment of transcribed and newly replicated DNA in soluble chromatin released from nuclei by mild micrococcal nuclease digestion. Biochim Biophys Acta. 1984 Jun 16;782(2):202–209. doi: 10.1016/0167-4781(84)90025-3. [DOI] [PubMed] [Google Scholar]
- Cousens L. S., Alberts B. M. Accessibility of newly synthesized chromatin to histone acetylase. J Biol Chem. 1982 Apr 10;257(7):3945–3949. [PubMed] [Google Scholar]
- Covault J., Chalkley R. The identification of distinct populations of acetylated histone. J Biol Chem. 1980 Oct 10;255(19):9110–9116. [PubMed] [Google Scholar]
- Davie J. R., Candido E. P. Acetylated histone H4 is preferentially associated with template-active chromatin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3574–3577. doi: 10.1073/pnas.75.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. R., Saunders C. A. Chemical composition of nucleosomes among domains of calf thymus chromatin differing in micrococcal nuclease accessibility and solubility properties. J Biol Chem. 1981 Dec 10;256(23):12574–12580. [PubMed] [Google Scholar]
- Davie J. R., Saunders C. A., Walsh J. M., Weber S. C. Histone modifications in the yeast S. Cerevisiae. Nucleic Acids Res. 1981 Jul 10;9(13):3205–3216. doi: 10.1093/nar/9.13.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Fambrough D. M., Smith E. L., Bonner J. Calf and pea histone IV. 3. Complete amino acid sequence of pea seedling histone IV; comparison with the homologous calf thymus histone. J Biol Chem. 1969 Oct 25;244(20):5669–5679. [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieva E. I., Pashev I. G., Tsanev R. G. Distribution of acetylated forms of nucleosomal histones in fractionated chromatin. Arch Biochem Biophys. 1982 Jun;216(1):88–92. doi: 10.1016/0003-9861(82)90191-6. [DOI] [PubMed] [Google Scholar]
- Gershey E. L., Vidali G., Allfrey V. G. Chemical studies of histone acetylation. The occurrence of epsilon-N-acetyllysine in the f2a1 histone. J Biol Chem. 1968 Oct 10;243(19):5018–5022. [PubMed] [Google Scholar]
- Goldsmith M. E. Release of a globin gene enriched chromatin fraction from chicken erythrocyte nuclei following DNase II digestion. Nucleic Acids Res. 1981 Dec 11;9(23):6471–6485. doi: 10.1093/nar/9.23.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Glover C., Johmann C. A., Keevert J. B., Mathis D. J., Samuelson M. Histones and chromatin structure in Tetrahymena macro- and micronuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):493–503. doi: 10.1101/sqb.1978.042.01.052. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J. M., Garrard W. T., Bagi G., Wilson R. F., Bonner J. Partial purification of the template-active fraction of chromatin: a preliminary report. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2193–2197. doi: 10.1073/pnas.71.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzen P. C., Rho J. H., Bekhor I. Nuclear matrix DNA from chicken erythrocytes contains beta-globin gene sequences. Proc Natl Acad Sci U S A. 1984 Jan;81(2):304–307. doi: 10.1073/pnas.81.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski A., Takamatsu H., Laskowski M., Sr An endonuclease activity of chicken erythrocyte nuclei and mononucleosomes. J Biol Chem. 1980 Nov 10;255(21):10542–10545. [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Levy-Wilson B. Modulations of prolactin and growth hormone gene expression and chromatin structure in cultured rat pituitary cells. Nucleic Acids Res. 1983 Feb 11;11(3):823–835. doi: 10.1093/nar/11.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Partial purification of transcriptionally active nucleosomes from trout testis cells. Nucleic Acids Res. 1978 Nov;5(11):4155–4163. doi: 10.1093/nar/5.11.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie A. J., Candido E. P., Dixon G. H. Enzymatic modifications and their possible roles in regulating the binding of basic proteins to DNA and in controlling chromosomal structure. Cold Spring Harb Symp Quant Biol. 1974;38:803–819. doi: 10.1101/sqb.1974.038.01.084. [DOI] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. A. Histone acetylation in baker's yeast. Maintenance of the hyperacetylated configuration in log phase protoplasts. J Biol Chem. 1982 Feb 25;257(4):1565–1568. [PubMed] [Google Scholar]
- Nelson D. A., Perry M., Sealy L., Chalkley R. DNAse I preferentially digests chromatin containing hyperacetylated histones. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1346–1353. doi: 10.1016/0006-291x(78)90337-6. [DOI] [PubMed] [Google Scholar]
- Nelson D., Covault J., Chalkley R. Segregation of rapidly acetylated histones into a chromatin fraction released from intact nuclei by the action of micrococcal nuclease. Nucleic Acids Res. 1980 Apr 25;8(8):1745–1763. doi: 10.1093/nar/8.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas R. H., Wright C. A., Cockerill P. N., Wyke J. A., Goodwin G. H. The nuclease sensitivity of active genes. Nucleic Acids Res. 1983 Feb 11;11(3):753–772. doi: 10.1093/nar/11.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M., Chalkley R. Histone acetylation increases the solubility of chromatin and occurs sequentially over most of the chromatin. A novel model for the biological role of histone acetylation. J Biol Chem. 1982 Jul 10;257(13):7336–7347. [PubMed] [Google Scholar]
- Perry M., Chalkley R. The effect of histone hyperacetylation on the nuclease sensitivity and the solubility of chromatin. J Biol Chem. 1981 Apr 10;256(7):3313–3318. [PubMed] [Google Scholar]
- Perry M., Nelson D., Moore M., Chalkley R. Histone deacetylation in nuclei isolated from hepatoma tissue culture cells. Inhibition by sodium butyrate. Biochim Biophys Acta. 1979 Feb 27;561(2):517–525. doi: 10.1016/0005-2787(79)90159-x. [DOI] [PubMed] [Google Scholar]
- Reeves R., Candido E. P. Turnover of histone acetyl groups in cultured cells is inhibited by sodium butyrate. FEBS Lett. 1978 Jul 1;91(1):117–120. doi: 10.1016/0014-5793(78)80030-1. [DOI] [PubMed] [Google Scholar]
- Reiser J., Renart J., Stark G. R. Transfer of small DNA fragments from polyacrylamide gels to diazobenzyloxymethyl-paper and detection by hybridization with DNA probes. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1104–1112. doi: 10.1016/0006-291x(78)90656-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Small D., Idzerda R., McKnight G. S., Vogelstein B. The association of transcriptionally active genes with the nuclear matrix of the chicken oviduct. Nucleic Acids Res. 1983 Aug 11;11(15):5113–5130. doi: 10.1093/nar/11.15.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha E., Davie J. R., van Holde K. E., Weintraub H. Differential salt fractionation of active and inactive genomic domains in chicken erythrocyte. J Biol Chem. 1984 Jul 10;259(13):8558–8563. [PubMed] [Google Scholar]
- Sanders L. A., Schechter N. M., McCarty K. S. A comparative study of histone acetylation, histone deacetylation, and ribonucleic acid synthesis in avian reticulocytes and erythrocytes. Biochemistry. 1973 Feb 27;12(5):783–791. doi: 10.1021/bi00729a001. [DOI] [PubMed] [Google Scholar]
- Sanders M. M. Fractionation of nucleosomes by salt elution from micrococcal nuclease-digested nuclei. J Cell Biol. 1978 Oct;79(1):97–109. doi: 10.1083/jcb.79.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Modulation of nucleosome structure by histone subtypes in sea urchin embryos. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6803–6807. doi: 10.1073/pnas.78.11.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbilt J. N., Bloom K. S., Anderson J. N. Endogenous nuclease. Properties and effects on transcribed genes in chromatin. J Biol Chem. 1982 Nov 10;257(21):13009–13017. [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali G., Gershey E. L., Allfrey V. G. Chemical studies of histone acetylation. The distribution of epsilon-N-acetyllysine in calf thymus histones. J Biol Chem. 1968 Dec 25;243(24):6361–6366. [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]