Abstract
Toll-like receptor 3 (TLR3) recognizes double-stranded RNA and induces type I IFN-mediated antiviral immunity against a number of viral infections. Type III IFN (IFN-λ) is a newly identified antiviral cytokine that possess similar biological functions as type I IFNs. We thus investigated the role of IFN-λ in TLR3 activation-mediated inhibition of HSV-1 in human primary astrocytes. Human astrocytes express endogenous IFN-λ1 and IFN-λ receptor complex, interleukine-28 receptor α subunit (IL-28Rα) and IL-10Rβ. The activation of TLR3 by PolyI:C treatment significantly induced the expression of IFN-λ1 and IFN-λ2/3 in astrocytes. The induction of IFN-λ contributed to TLR3-mediated HSV-1 inhibition in astrocytes. Investigation of the mechanisms showed that treatment of astrocytes with specific antibody against IFN-λ receptor attenuated the anti-HSV-1 activity of PolyI:C, indicating that endogenous IFN-λ contributes to the anti-HSV-1 effect of TLR3 activation. The anti-HSV-1 effect of endogenous IFN-λ was also confirmed by the finding that recombinant IFN-λ treatment inhibited HSV-1 infection of astrocytes. These results provide direct and compelling evidence that endogenous IFN-λ participates in TLR3-mediated antiviral activity, which may have an important implication in host cell innate immunity against HSV-1 infection in the CNS.
Keywords: IFN-lambda, PolyI:C, Astrocytes, HSV-1, CNS
Introduction
Toll-like receptors (TLRs) are a subset of pattern recognition receptors (PRRs) that mediate host innate immune responses by recognizing various microbial components termed pathogen-associated molecular patterns (PAMPs). Although the CNS is an immune-privileged environment devoid of most immune processes, studies have shown that the CNS cells express high levels of TLR3 and TLR9 (Bsibsi et al. 2002; Kielian 2006; Lee and Lee 2002; McKimmie et al. 2005), two major TLRs in virus-mediated innate immune responses (Jackson et al. 2006; Lafon et al. 2006). Human astrocytes were found to express TLR3, at both cell surface and intracellular compartment (Jack et al. 2005). When activated with the synthetic dsRNA mimic polyriboinosinic:polyribocytidylic acid (PolyI:C), the agonist for TLR3, human astrocytes express a wide range of cytokines and chemokines with a marked bias towards genes of antiviral factors, including type I IFNs and IFN-inducible genes (Rivieccio et al. 2006).
IFN lambda (IFN-λ), classified as type III IFN, is a new member of the IFN family. IFN-λ subfamily contains three cytokines (IFN-λ1, IFN-λ2 and IFN-λ3; also known as interleukine-29, IL-28A and IL-28B, respectively) (Kotenko et al. 2003; Sheppard et al. 2003). IFN-λ was originally described to have the similarity to IFN-α/β (functionally) and the IL-10 family of ligands (structurally) (Kotenko et al. 2003; Sheppard et al. 2003). Although IFN-λ exerts its actions through a receptor complex distinct from IFN-α/β, it exhibits a number of biological functions similar to IFN-α/β, including antiviral activity. IFN-λ has been shown to inhibit replication of a number of viruses in various cell types (Ank and Paludan 2009), e.g. IFN-λ1 exhibited antiviral effect in HT29, A549 and HaCaT cells infected with vesicular stomatitis virus (VSV) and in HT29 and HepG2 cells cells infected with encephalomyocarditis virus (EMCV) (Ank et al. 2006; Kotenko et al. 2003; Sheppard et al. 2003). Because IFN-λ participated in the establishment of an antiviral state, IFN-λ expression has also been shown to be modulated by viral infection and TLR activations in different experimental systems (Ank et al. 2006; Coccia et al. 2004; Kotenko et al. 2003; Onoguchi et al. 2007; Zhou et al. 2009a). IFN-λ was induced by Newcastle disease virus (NDV) infection of mouse embryonic fibroblasts (Onoguchi et al. 2007) and by herpes simplex virus type 2 (HSV-2), EMCV, Sendai virus (SeV), and influenza A virus (IAV) in different types of cells, including A549, Hela, Raji and U937 cells (Ank et al. 2006). IAV infection also induced IFN-λ expression in monocyte-derived dendritic cells (MDDC) and plasmacytoid DC (pDC) (Coccia et al. 2004). TLR9 stimulation by CpG DNA induced IFN-λ expression in pDC, while in MDDC, it is induced by TLR4 (LPS) (Coccia et al. 2004) and TLR3 (PolyI:C) stimulation (Coccia et al. 2004; Kotenko et al. 2003). Our earlier study reported that TLR3 activation in neuronal cells induced IFN-λ expression and exhibited anti-HIV activity (Zhou et al. 2009a). The role of IFN-λ in TLR-mediated antiviral response was also confirmed in an in vivo study, showing that the antiviral activities evoked by TLR3 and TLR9 agonists were significantly attenuated in both IL-28Rα-/- and IFNAR-/- mice (Ank et al. 2008).
The receptor complex for IFN-λ includes two subunits, IL-28Rα and IL-10Rβ. They functionally form a homodimer that mediates the intracellular signaling and activation of biological activities of IFN-λ. IL-28Rα is more specific for IFN-λ, while IL-10Rβ is an accessory chain that is also part of the receptors for IL-10 family cytokines, such as IL-10, IL-22 and IL-26 (Sheppard et al. 2003). In contrast to the universal expression of type I IFN receptors (IFNAR), there is a more restricted pattern of IFN-λ receptors, especially IL-28Rα expression, suggesting that IFN-λ has a more specialized role in host antiviral defense (Iversen et al. 2010; Megjugorac et al. 2009; Sommereyns et al. 2008).
HSV-1 is a neurotropic virus with the capability of infecting not only neurons, but also microglia and astrocytes (Guo et al. 2010; Li et al. 2011; Marques et al. 2004; Zhou et al. 2009b). As latency is the major obstacle in preventing the eradication of HSV-1, it is important to identify intracellular innate antiviral factors that suppress and eliminate HSV-1 in its target cells. Recent studies have shown that cells in the CNS possess intracellular innate immunity properties, expressing IFNs including IFN-λ (Zhou et al. 2009a) and other viral restriction factors (Paul et al. 2007). HSV-1 recognition is mainly accomplished by several TLRs, including TLR3 and TLR9 (Kurt-Jones et al. 2004; Sergerie et al. 2007). These TLRs are crucial in the innate immune responses, as they recognize and respond to PAMPs, resulting in the activation of intracellular antiviral pathways. Although it is known that TLR3 activation induces type I IFN expression, which is crucial for the protective effect against HSV-1 in the CNS, there is limited information about the role of IFN-λ in TLR3-mediated innate immune response to HSV-1 infection of human astrocytes. We thus examined whether TLR3 activation in astrocytes induces endogenous IFN-λ expression and whether the induction of IFN-λ contributes to TLR3-mediated innate immunity against HSV-1 infection.
MATERIALS AND METHODS
Reagents
The human TLR1-9 agonist kit containing PolyI:C (TLR3), Imiquimod (TLR7), and ODN2206 (TLR9) was purchased from InvivoGen (San Diego, CA). Recombinant human IFN-λ1 and IFN-λ2 were purchased from PeproTech Inc. (Rocky Hill, NJ). Tri-reagent was purchased from Sigma-Aldrich (Saint Louis, MO). AMV transcriptase and RNasin were from Promega Co (Madison, WI). Proteinase K was from Boehringer Ingelheim GmbH (Mannheim, Germany). Anti-IL-10Rβ antibody and normal goat IgG control were purchased from R&D Systems Inc. (Minneapolis, MN).
Primary Astrocytes and Cell Lines
Primary human astrocyte cultures were prepared as previously described (Peterson et al. 1995). Briefly, brain tissues (whole brain or midbrain) from 16-to-20-week-old aborted fetuses were dissociated by trypsinization (0.25%) for 30 min and plated into 75-cm2 Falcon tissue culture flasks in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 2 mM L-Glutamine, 1% non-essential amino acids (NEAA), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated for 21 days with weekly medium changes. On day 21, flasks were shaken, washed and trypsinized with 0.25% trypsin in HBSS for 30 min at 37°C. After adding FBS (final concentration 10%), centrifugation, and washing, cells were seeded into new flasks with DMEM followed by medium change after 24 h. The subculture procedure was repeated four times at a weekly interval. Astrocyte cultures were comprised of cells that were >99% GFAP-positive.
U373 MG (human glioblastoma-astrocytoma cells) was obtained from the American Type Culture Collection. Cells were plated at 0.2×106/ml and grown in DMEM at 37 °C in humidified air with 5% CO2. NT2-N neurons were derived from NT2 (human teratocarcinoma cells) and cultured in DMEM media containing 10% FBS.
HSV-1 Infection
HSV-1 17 syn+ (a wide type strain) was obtained from Dr. James Lokensgard (University of Minnesota). The viral stocks were propagated in rabbit skin fibroblasts, titered on Vero cells, and stored at –80 °C. Both primary astrocytes and U373 cells were plated in 24-well plates and grown to 70% confluency before infected with HSV-1 17 syn+ at a multiplicity of infection (MOI) of 0.01. HSV-1 infection of astrocytes was conducted at 37°C for 1.5 h. The cells were then washed three times and fresh media with 10% FBS were added. After an additional 72 h postinfection, HSV-1 viral DNA extracted from the culture medium (extracellular) or cells (intracellular) was analyzed by PCR analysis. Immunofluorescence staining with antibody to HSV-1 ICP0 and late structural antigens (Chemicon International Inc., Temecula, CA) was also performed to examine HSV-1 protein expression. In addition, infected cells were also observed under a microscope for HSV-1-induced cytopathic effect (CPE).
Immunofluorescence Assay
Primary astrocytes were cultured on 8-chamber slides (Lab-Tek, Nunc.). After treatment and infection, cells were washed with 1× ice-cold PBS (with Ca2+ and Mg2+) twice, then fixed at 4°C in 4% paraformaldehyde plus 4% sucrose in PBS for 30 min. Subsequently, the cells were permeated in cold methanol (100%) for 10 min followed by 0.2% Triton X-100 for 10 min. Cells were blocked in Block Solution (Pierce, Rockford, IL) for 1h at room temperature, and then incubated with goat polyclonal antibody against HSV-1 gD (1:200) for 1h. After three times wash with PBS, cells were incubated with FITC-conjugated donkey anti-goat IgG (1:250) or Alexa Fluor 568 donkey anti-goat IgG (1:200) for 1h. For additional Hoechst staining, 1 μg/ml of Hoechst was used to incubate the cells for 1 min. Cells were then viewed under a fluorescence microscope (Olympus IX71).
RNA Extraction and Real Time RT-PCR
Total RNA was extracted from cell culture with Tri-Reagent (Sigma-Aldrich, Saint Louis, MO) and reverse transcription was performed using the AMV transcriptase and RNasin (Promega Co., Madison, WI, USA) following the manufacturer's instructions. The real time RT-PCR using Brilliant SYBR Green Master Mix (Bio-Rad Laboratories, Hercules, CA, USA) for this study was described previously (Zhou et al. 2009a). The oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA) and sequences will be available upon request. All values were expressed as the increase relative to the expression of GAPDH mRNA. The mean value of the replicates for each sample was calculated and expressed as the cycle threshold (CT; cycle number at which each PCR reaches a predetermined fluorescence threshold, set within the linear range of all reactions). The levels of gene expression were then calculated as the difference between the CT of the sample for the target gene and the mean of that sample for the endogenous control (comparative CT method).
Knockdown of IRF3 and IRF7
Lentiviral plasmids with sequence-verified shRNA for gene silencing of IRF3 and IRF7 were gifts from Dr. Rongtuan Lin (McGill University, Canada). Primary astrocytes were plated in 24-well plates at 1.5×105 per well 1 day before transfection. Cells were then transfected with the plasmids using FuGene HD (Roche, MN) at a ratio of 1:3 (μg:μl) prepared in OPTI-MEM. After 48 h, cells were used for PolyI:C treatment. For negative knockdown, cells were transfected with the non-target shRNA control vector, pLKO.1 (SHC002, Sigma).
Data Analysis
Where appropriate, data were expressed as the mean ± SD from at least three independent experiments. For comparison of the mean of the two groups, statistical significance was measured by Student's t test. To compare the difference between multiple groups, statistical significance was analyzed using a one-way analysis of variance (ANOVA) followed by post Newman-Keul's test. Calculations were performed with Stata Statistical Software (StataCorp, College Station, TX). Statistical significance was defined as P<0.05.
RESULTS
IFN-λ and IFN-λ Receptor Expression in Human Astrocytes
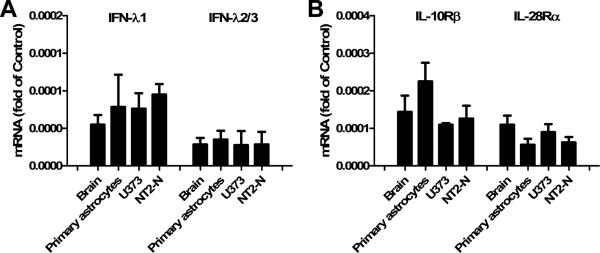
Our previous study showed that neuronal cells express endogenous IFN-λ (Zhou et al. 2009a). Here, we further examined whether human astrocytes (primary astrocytes and U373 cells) express IFN-λ. Because human IFN-λ2 and IFN-λ3 genes are almost identical, we used the same primers for both IFN-λ2 and IFN-λ3. As shown in Fig. 1A, human brain, primary astrocytes, U373 astrocytoma cells and NT2-N cells expressed IFN-λ1 and IFN-λ2/3 mRNAs (Fig. 1A). Since the biological functions of IFN-λ depend mainly on IFN-λ receptors, we also examined the expression of IFN-λ receptors (IL-28Rα and IL-10Rβ) in human astrocytes. As shown in Fig. 1B, primary human astrocytes and U373 cells expressed mRNAs for both IL-28Rα and IL-10Rβ. Therefore, in addition to neurons, astrocytes also express IFN-λ and both IFN-λ receptors.
Fig. 1. Expression of IFN-λ and IFN-λ receptors (IL-10Rβ and IL-28Rα) in astrocytes.
RNA extracted from brain tissue and cultured cells as indicated was subjected to the real-time RT-PCR for IFN-λ1, IFN-λ2/3 (A) and IL-10Rβ/IL-128Rα (B). Data is expressed as the target mRNA ratio to the internal control GAPDH. Value is the mean ± SD of three independent experiments (*, p<0.05; **, p<0.01). NT2-N cells were used as a positive control that express IFN-λ and IFN-λ receptors.
TLR3 Activation Induced IFN-λ Expression in Human Astrocytes
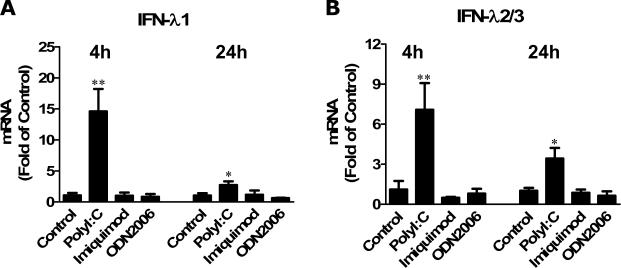
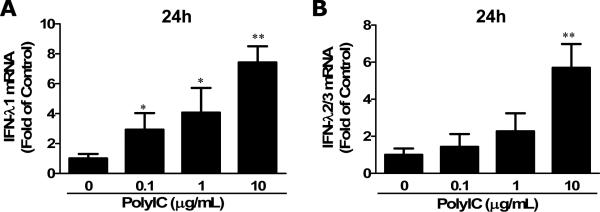
TLR3 activation has been shown to induce the activation of neuronal cells in terms of type I IFN and IFN-λ expression (Zhou et al. 2009a; Zhou et al. 2009c). We then examined whether the activation of TLRs could induce IFN-λ expression in primary human astrocytes. We used the TLR agonists (PolyI:C, Imiquimod and ODN2006) to activate TLR3, TLR7 and TLR9, respectively. As shown in Fig. 2, the treatment of astrocytes with Imiquimod and ODN2006 had little effect on IFN-λ1 and IFN-λ2/3 expression (Fig. 2). In contrast, PolyI:C (10μg/ml) treatment of primary astrocytes induced the expression of IFN-λ1 and IFN-λ2/3. The higher expressionn of IFN-λ was observed at 4h after treatment with PolyI:C (Fig. 2). We also examined the dose effect of PolyI:C on IFN-λ expression, demonstrating that PolyI:C-mediated induction of IFN-λ in astrocytes was dose-dependent (Fig. 3). These data indicate that human astrocytes express functional TLR3, which could be activated by PolyI:C, inducing IFN-λ expression.
Fig. 2. TLR3 activation induced IFN-λ expression.
Human primary astrocytes were treated with PolyI:C (10μg/ml), Imiquimod (1μg/ml) or ODN2006 (5μM) for 4h or 24h. RNA extracted from cells was subjected to the real-time RT-PCR for IFN-λ1 and IFN-λ2/3. Data is expressed as IFN-λ mRNA ratio to the internal standard GAPDH. Value is the mean ± SD of three independent experiments (*, p<0.05; **, p<0.01).
Fig. 3. Dose-effects of PolyI:C treatment on IFN-λ expression.
Human primary astrocytes were treated with or without PolyI:C at indicated concentrations for 24h. Total cellular RNA was extracted for the real time RT-PCR. Data is expressed as IFN-λ mRNA ratio to the internal standard GAPDH. Value is the mean ± SD of three independent experiments (*, p<0.05; **, p<0.01).
Induction of Endogenous IFN-λ Contributes to HSV-1 Inhibition
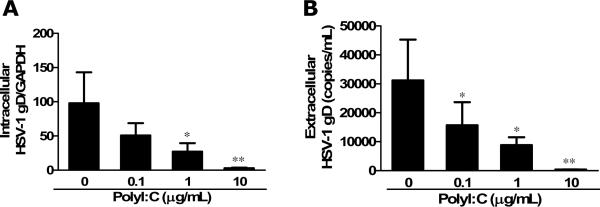
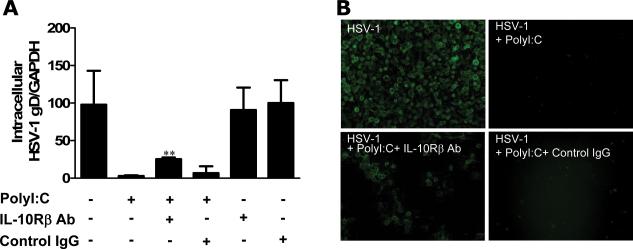
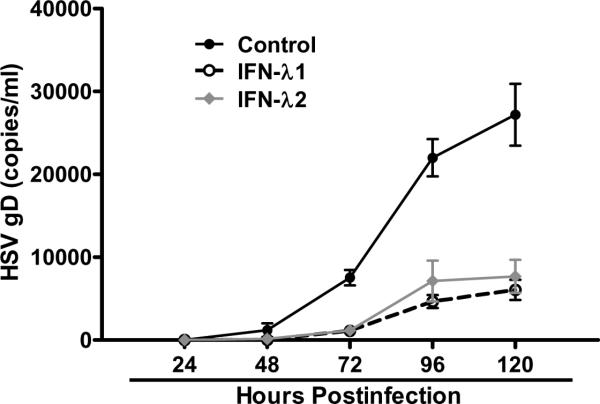
TLR3 activation has been shown to have anti-viral activities in astrocytes and type I IFN plays an important role in the effect (Rivieccio et al. 2006). However, it is unclear whether IFN-λ is involved in the TLR3 action.. We next examined whether the induction of endogenous IFN-λ by PolyI:C treatment contributes to the TLR3-mediated anti-HSV-1 activity in astrocytes. We observed that PolyI:C in a dose–dependent fashion inhibited HSV-1 infection of astrocytes, which was evidenced by decreased HSV-1 gD gene expression at both intracellular (Fig. 4A) and extracellular (Fig. 4B) levels. When the cells were treated with PolyI:C together with antibody to IFN-λ receptor (IL-10Rβ), the antiviral effect of PolyI:C was partially reversed (Fig. 5A), while control IgG had little effect on the antiviral potency of PolyI:C (Fig. 5A). Immunofluorescence staining also demonstrated that IL-10Rβ antibody could partially block the anti-HSV-1 activity of PolyI:C (Fig. 5B). Because of the lack of commercial antibody to IL-28Rα, we were unable to demonstrate whether to block both subunits of IFN-λ receptor could abolish the anti-HSV-1 activity of TLR3 activation. The anti-HSV-1 effect of IFN-λ was also demonstrated in astrocytes treated with recombinant IFN-λ1or IFN-λ2 (Fig. 6). These data indicate IFN-λ does contribute to the anti-HSV-1 effect of PolyI:C.
Fig. 4. Effect of TLR3 activation on HSV-1 infection.
Human primary astrocytes were treated with or without PolyI:C as indicated concentrations for 24h prior to HSV-1 infection. HSV-1 replication was quantified at 96h postinfection. (A) Intracellular HSV-1 gD gene expression was measured using GAPDH as an internal standard and expressed as ratio to GAPDH expression. (B) Extracellular HSV-1 gD gene expression was examined by using serial dilutions of HSV-1 gD standards with known copy numbers. All value is the mean ± SD of three independent experiments (*, p<0.05; **, p<0.01).
Fig. 5. Effect of antibody against IFN-λ receptor (IL-10Rβ) on the anti-HSV-1 activity of PolyI:C.
Human primary astrocytes were treated with PolyI:C (10μg/ml) together with anti-IL-10Rβ antibody (10μg/ml) for 24h prior to HSV-1 infection. HSV-1 replication was quantified at 96h postinfection. (A) HSV-1 gD gene expression was determined by PCR using GAPDH as an internal standard and expressed as ratio to GAPDH expression. All value is the mean ± SD of three independent experiments (*, p<0.05; **, p<0.01). (B) Immunofluorescence assay using anti-HSV-1 antibody. Representative data from three independent experiments are shown.
Fig. 6. Effect of recombinant IFN-λ on HSV-1 infection.
Human primary astrocytes were treated with recombinant human IFN-λ1 or IFN-λ2 (100ng/ml) for 24h and then infected with HSV-1 (MOI 0.01). Supernatant from the cultures was collected at indicated time points postinfection and subjected to DNA extraction. Extracellular HSV-1 gD gene expression was examined by using serial dilutions of HSV-1 gD standards with known copy number. All value is the mean ± SD of three independent experiments.
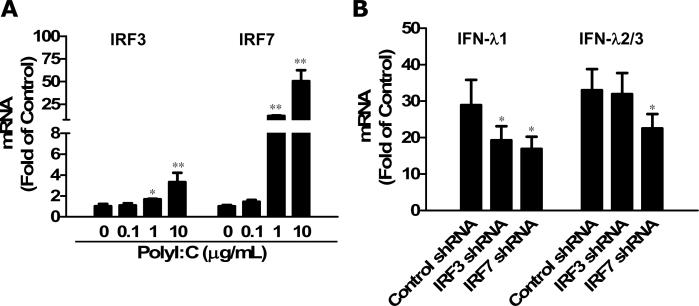
TLR3-mediated IFN-λ Induction is Regulated by IRF3 and IRF7
The IFN regulatory factors (IRFs), especially IRF3 and IRF7 have been implicated in the control of type I IFN expression. However, the regulation of IFN-λ expression induced by viral infections or other stimuli remains unclear. To determine the mechanisms for the anti-HSV-1 effect of TLR3 activation, we examined the effect of TLR3 activation on IRF3 and IRF7 expression in astrocytes. As shown in Fig. 7A, PolyI:C treatment of astrocytes induced the expression of IRF3 and IRF7 in a dose-dependent fashion. To determine the role of IRF3 and IRF7 in TLR3-mediated IFN-λ induction, we examined whether the knockdown of IRF3 or IRF7 could compromise the action of PolyI:C treatment on the activation of IFN-λ. Interestingly, IRF3 knockdown attenuated only IFN-λ1 expression, while IRF7 knockdown impaired both IFN-λ1 and IFN-λ2/3 expression (Fig. 7B). This result suggests that although both IRF3 and IRF7 are involved in the TLR3-mediated IFN-λ induction, they may differentially regulate the expression of IFN-λ1 and IFN-λ2/3.
Fig 7. Induced IRF3 and IRF7 regulated IFN-λ expression mediated by TLR3 activation.
(A) Effects of TLR3 activation on IRF3 and IRF7 expression. Human primary astrocytes were treated with or without PolyI:C at the indicated concentrations for 24h. Total cellular RNA was extracted for the real time RT-PCR. Data is expressed as the fold to the untreated control (designated as 1). Value is the mean ± SD of three independent experiments (*, p<0.05; **, p<0.01). (B) shRNA-mediated knockdown of IRF3 and IRF7 impaired IFN-λ expression induced by PolyI:C treatment. Human primary astrocytes were transfected with control vector, IRF3 shRNA or IRF7 shRNA, respectively. Forty-eight hours posttransfection, cells were treated with or without PolyI:C (10μg/ml) for additional 24h. RNA was extracted and IFN-λ mRNA levels were determined by real-time PCR. Data is expressed as the fold to the untreated control. Value is the mean ± SD of three independent experiments. (*, p<0.05).
DISCUSSION
The expression of TLRs in astrocytes has been well documented (Bowman et al. 2003; Bsibsi et al. 2007; Farina et al. 2005; Jack et al. 2005; Suh et al. 2009; van Noort and Bsibsi 2009). Human astrocytes were found to express multiple functional TLRs with particularly high levels of TLR3, regardless of the species or age of the brains from which the astrocytes were isolated (Farina et al. 2005; Jack et al. 2005; Suh et al. 2009). Given the preferential expression of TLR3 in astrocytes, it is likely that astrocytes may be particularly important for antiviral responses in the CNS. TLR3 responds to viral dsRNA and induces type I IFN expression, thus playing a key role in antiviral immune responses. TLR-mediated antiviral activity in the CNS is highlighted by a recent report showing that TLR-deficient patients appear to be specifically prone to HSV-1-induced encephalitis (Zhang et al. 2007). TLR activation could also stimulate IFN-λ expression on the immune cells (Coccia et al. 2004; Siren et al. 2005; Yang et al. 2005). Our early study showed that TLR3 activation in human neurons induced IFN-λ expression (Zhou et al. 2009a). In this study, we showed that among the TLR ligands tested, only PolyI:C significantly enhanced the expression of all three members of IFN-λ family. This induction of IFN-λ expression in astrocytes is at least partially responsible for TLR activation-mediated anti-HSV-1 activity, which was evidenced by the observation that antibody to IL-10Rβ, one of IFN-λ receptor subunits, could partially reverse the inhibitory effect of PolyI:C on HSV-1 replication in astrocytes. Because of the lack of commercial antibody to IL-28Rα, we were unable to demonstrate a more potent blockade effect on the inhibition of HSV-1 replication by PolyI:C. Nevertheless, it is unexpected that to completely block IFN-λ receptors would abolish PolyI:C effect on HSV-1, as TLR3 activation also induces type I IFN expression, which also contributes to HSV-1 inhibition.
Although it is known that the induction of type I IFN is mainly regulated by IRF3 and IRF7, it remains to be determined whether IFN-λ is regulated by IRF3 and IRF7. We demonstrated that the knockdown of IRF3 or IRF7 in astrocytes compromised PolyI:C-mediated IFN-λ induction (Fig. 7B). However, IRF3 seemed to have an effect on IFN-λ1 only while IRF7 has a role in both IFN-λ1 and IFN-λ2/3 induction (Fig. 7B). These findings are supported by Osterlund et al.'s finding showing that IFN-λ1 gene is regulated by virus-activated (murine SV) IRF3 and IRF7, whereas IFN-λ2/3 gene expression is mainly controlled by IRF7 in MDDCs (Osterlund et al. 2007). The differential effect of IRF3 and IRF7 on IFN-λ is similar to the regulation of type I IFNs by IRF3 and IRF7. IRF7 is expressed at low level under normal physiological condition and plays an important role at later phase of viral restriction by activation of both late phase IFN-α and IFN-β gene expression. On the contrary, IRF3 regulates the expression of IFN-β gene, initiating the early antiviral responses upon activation (Honda et al. 2005; Sakaguchi et al. 2003; Solis et al. 2006). IRF7 knockout impairs both IFN-α and IFN-β induction by viral infection (HSV, ECMV and VSV), while IRF3 knockout mainly impairs IFN-β expression in mice pDcs (Honda et al, 2005; Solis et al, 2006).
The receptor complex for IFN-λ includes two subunits, IL-28Rα and IL-10Rβ. In our previous study, we identified the expression of IFN-λ receptors (IL-28Rα and IL-10Rβ) in human neurons (Zhou et al. 2009a). The coexpression of both IL-28Rα and IL-10Rβ is essential for them to form a homodimer and functionally mediate the intracellular signaling and activation of biological activities of IFN-λ. Although IL-28Rα is more specific for IFN-λ and IL-10Rβ is an accessory chain, part of the receptors for IL-10 family cytokines, such as IL-10, IL-22 and IL-26 (Sheppard et al. 2003), neither IL-10Rβ nor IL-28Rα alone is efficient to render cells responsive to IFN-λ (Kotenko et al. 2003). IFN-λ functionally resembles type I IFNs, including antiviral protection in vitro (Kotenko et al. 2003; Robek et al. 2005; Sheppard et al. 2003) as well as in vivo (Ank et al. 2006). Generally, IFN-λ is less effective than type I IFNs and exhibits antiviral activity in limited cell lines (Ank et al. 2006; Ma et al. 2009; Meager et al. 2005). Despite the overlapping antiviral functions of IFN-λ with type I IFNs, which leads to the debate the issue why higher organisms possess two very similar systems for combating viral infections, the more restricted pattern of IFN-λ receptor (IL-28Rα) expression suggests that IFN-λ has a more specialized role in host antiviral defense against certain viruses in a subset of cells (Iversen et al. 2010; Megjugorac et al. 2010; Sommereyns et al. 2008). In a most recent report, it was found that new world hantaviruses can activate IFN-λ production in type I IFN-deficient Vero E6 cells and the interferon-stimulated genes (ISGs) induction is due to IFN-λ (Prescott et al. 2010).
Taken together, given the essential functions of type I IFNs in the host cell-mediated innate immunity, our finding that human astrocytes possess TLR3-mdiated anti-HSV-1 innate immune response via IFN-λ induction is highly significant. Although the precise cellular and molecular mechanisms by which IFN-λ is regulated upon viral and/or other cellular factors remain to be determined, TLR3/IFN-λ pathway may have a crucial role in innate protection against HSV-1 infection in the CNS.
ACKNOWLEDGEMENTS
We thank Dr. Rongtuan Ling (McGill University, Canada) for providing us the lentiviral plasmids encoding IRF3 and IRF7 shRNA and Dr. James Lokensgard (University of Minnesota) for providing us the HSV-1 17 syn+ .
This work was support by the National Institute on Drug Abuse (Grants DA012815, DA027550 and DA022177).
REFERENCES
- Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180(4):2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- Ank N, Paludan SR. Type III IFNs: new layers of complexity in innate antiviral immunity. Biofactors. 2009;35(1):82–87. doi: 10.1002/biof.19. [DOI] [PubMed] [Google Scholar]
- Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80(9):4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43(3):281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Bajramovic JJ, Van Duijvenvoorden E, Persoon C, Ravid R, Van Noort JM, Vogt MH. Identification of soluble CD14 as an endogenous agonist for Toll-like receptor 2 on human astrocytes by genome-scale functional screening of glial cell derived proteins. Glia. 2007;55(5):473–482. doi: 10.1002/glia.20473. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61(11):1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34(3):796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol. 2005;159(1-2):12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Guo YJ, Zhao L, Li XF, Mei YW, Zhang SL, Tao JY, Zhou Y, Dong JH. Effect of Corilagin on anti-inflammation in HSV-1 encephalitis and HSV-1 infected microglias. Eur J Pharmacol. 2010;635(1-3):79–86. doi: 10.1016/j.ejphar.2010.02.049. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434(7034):772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Iversen MB, Ank N, Melchjorsen J, Paludan SR. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-kappaB than type I IFNs. J Virol. 2010;84(9):4579–4586. doi: 10.1128/JVI.02591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175(7):4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Rossiter JP, Lafon M. Expression of Toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J Neurovirol. 2006;12(3):229–234. doi: 10.1080/13550280600848399. [DOI] [PubMed] [Google Scholar]
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83(5):711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4(1):69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101(5):1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon M, Megret F, Lafage M, Prehaud C. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci. 2006;29(3):185–194. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee S. Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy. 2002;1(2):181–191. doi: 10.2174/1568010023344698. [DOI] [PubMed] [Google Scholar]
- Li J, Hu S, Zhou L, Ye L, Wang X, Ho J, Ho W. Interferon lambda inhibits herpes simplex virus type I infection of human astrocytes and neurons. Glia. 2011;59(1):58–67. doi: 10.1002/glia.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Jiang D, Qing M, Weidner JM, Qu X, Guo H, Chang J, Gu B, Shi PY, Block TM, Guo JT. Antiviral effect of interferon lambda against West Nile virus. Antiviral Res. 2009;83(1):53–60. doi: 10.1016/j.antiviral.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques CP, Hu S, Sheng W, Cheeran MC, Cox D, Lokensgard JR. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia. 2004;47(4):358–366. doi: 10.1002/glia.20045. [DOI] [PubMed] [Google Scholar]
- McKimmie CS, Johnson N, Fooks AR, Fazakerley JK. Viruses selectively upregulate Toll-like receptors in the central nervous system. Biochem Biophys Res Commun. 2005;336(3):925–933. doi: 10.1016/j.bbrc.2005.08.209. [DOI] [PubMed] [Google Scholar]
- Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. Biological activity of interleukins-28 and -29: comparison with type I interferons. Cytokine. 2005;31(2):109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Megjugorac NJ, Gallagher GE, Gallagher G. Modulation of human plasmacytoid DC function by IFN-lambda1 (IL-29). J Leukoc Biol. 2009;86(6):1359–1363. doi: 10.1189/jlb.0509347. [DOI] [PubMed] [Google Scholar]
- Megjugorac NJ, Gallagher GE, Gallagher G. IL-4 enhances IFN-lambda1 (IL-29) production by plasmacytoid DCs via monocyte secretion of IL-1Ra. Blood. 2010;115(21):4185–4190. doi: 10.1182/blood-2009-09-246157. [DOI] [PubMed] [Google Scholar]
- Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, Fujita T. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007;282(10):7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J Immunol. 2007;179(6):3434–3442. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]
- Paul S, Ricour C, Sommereyns C, Sorgeloos F, Michiels T. Type I interferon response in the central nervous system. Biochimie. 2007;89(6-7):770–778. doi: 10.1016/j.biochi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Chao CC. Human astrocytes inhibit intracellular multiplication of Toxoplasma gondii by a nitric oxide-mediated mechanism. J Infect Dis. 1995;171(2):516–518. doi: 10.1093/infdis/171.2.516. [DOI] [PubMed] [Google Scholar]
- Prescott J, Hall P, Acuna-Retamar M, Ye C, Wathelet MG, Ebihara H, Feldmann H, Hjelle B. New World hantaviruses activate IFNlambda production in type I IFN-deficient vero E6 cells. PLoS One. 2010;5(6):e11159. doi: 10.1371/journal.pone.0011159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivieccio MA, Suh HS, Zhao Y, Zhao ML, Chin KC, Lee SC, Brosnan CF. TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for viperin/cig5. J Immunol. 2006;177(7):4735–4741. doi: 10.4049/jimmunol.177.7.4735. [DOI] [PubMed] [Google Scholar]
- Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79(6):3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Negishi H, Asagiri M, Nakajima C, Mizutani T, Takaoka A, Honda K, Taniguchi T. Essential role of IRF-3 in lipopolysaccharide-induced interferon-beta gene expression and endotoxin shock. Biochem Biophys Res Commun. 2003;306(4):860–866. doi: 10.1016/s0006-291x(03)01049-0. [DOI] [PubMed] [Google Scholar]
- Sergerie Y, Boivin G, Gosselin D, Rivest S. Delayed but not early glucocorticoid treatment protects the host during experimental herpes simplex virus encephalitis in mice. J Infect Dis. 2007;195(6):817–825. doi: 10.1086/511987. [DOI] [PubMed] [Google Scholar]
- Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4(1):63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- Siren J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005;174(4):1932–1937. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- Solis M, Goubau D, Romieu-Mourez R, Genin P, Civas A, Hiscott J. Distinct functions of IRF-3 and IRF-7 in IFN-alpha gene regulation and control of anti-tumor activity in primary macrophages. Biochem Pharmacol. 2006;72(11):1469–1476. doi: 10.1016/j.bcp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4(3):e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HS, Brosnan CF, Lee SC. Toll-like receptors in CNS viral infections. Curr Top Microbiol Immunol. 2009;336:63–81. doi: 10.1007/978-3-642-00549-7_4. [DOI] [PubMed] [Google Scholar]
- van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res. 2009;175:139–148. doi: 10.1016/S0079-6123(09)17509-X. [DOI] [PubMed] [Google Scholar]
- Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Marodi L, Davidson D, Speert D, Roifman C, Garty BZ, Ozinsky A, Barrat FJ, Coffman RL, Miller RL, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann F, Jouanguy E, Casanova JL. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda Is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23(5):465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Sancho-Shimizu V, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev. 2007;220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wang X, Wang YJ, Zhou Y, Hu S, Ye L, Hou W, Li H, Ho WZ. Activation of toll-like receptor-3 induces interferon-lambda expression in human neuronal cells. Neuroscience. 2009a;159(2):629–637. doi: 10.1016/j.neuroscience.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Guo YJ, Liu X, Mei YW. Cell cycle inhibitor enhances the resolution of HSV-1-induced proinflammatory response in murine microglial cells. Neurol Res. 2009b;31(9):910–916. doi: 10.1179/174313209X383222. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ye L, Wan Q, Zhou L, Wang X, Li J, Hu S, Zhou D, Ho W. Activation of Toll-like receptors inhibits herpes simplex virus-1 infection of human neuronal cells. J Neurosci Res. 2009c;87(13):2916–2925. doi: 10.1002/jnr.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]