Abstract
Background
Transmyocardial laser revascularization (TMR) is currently clinically performed with either a CO2 or Ho:YAG laser for the treatment of severe angina. While both lasers provide symptomatic relief, there are significant differences in the laser–tissue interactions specific to each device that may impact their ability to enhance the perfusion of myocardium and thereby improve contractile function of the ischemic heart.
Methods
A porcine model of chronic myocardial ischemia was employed. After collecting baseline functional data with cine magnetic resonance imaging (MRI) and dobutamine stress echo (DSE), 14 animals underwent TMR with either a CO2 or Ho:YAG laser. Transmural channels were created with each laser in a distribution of 1/cm2 in the ischemic zone. Six weeks post-treatment repeat MRI as well as DSE were obtained after which the animals were sacrificed. Histology was preformed to characterize the laser–tissue interaction.
Results
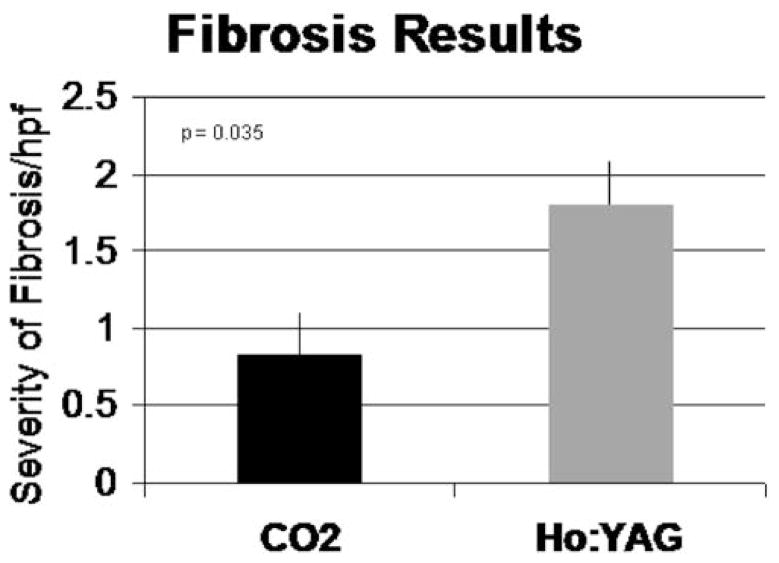
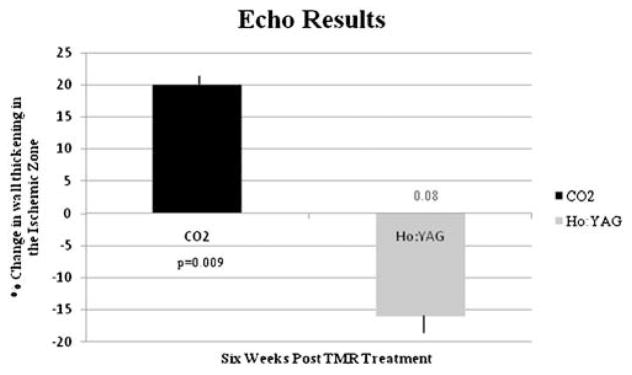
CO2 TMR led to improvement in wall thickening in the ischemic area as seen with cine MRI (40.3% vs. baseline, P < 0.05) and DSE (20.2% increase vs. baseline, P < 0.05). Ho:YAG treated animals had no improvement in wall thickening by MRI (−11.6% vs. baseline, P = .67) and DSE (−16.7% vs. baseline, P = 0.08). Correlative semi-quantitative histology revealed a significantly higher fibrosis index in Ho:YAG treated myocardium versus CO2 (1.81 vs. 0.083, P < 0.05).
Conclusions
In a side-by-side comparison CO2 TMR resulted in improved function of ischemic myocardium as assessed by MRI and echocardiography. Ho:YAG TMR led to no improvement in regional function likely due to concomitant increase in fibrosis in the lasered area.
Keywords: CO2 laser, Ho:YAG laser, myocardial function, laser–tissue interaction
INTRODUCTION
Patients with significant disabling angina due to severe diffuse coronary artery disease are often not well served by percutaneous coronary interventions or coronary artery bypass grafting. For these patients transmyocardial laser revascularization (TMR) has been utilized since the 1980s following the pioneering efforts of Mirhoseini and Cayton [1] and Okada et al. [2]. The safety and efficacy of TMR have been demonstrated in multiple randomized controlled studies. These trials looked at the use of the CO2 laser or the Ho:YAG laser used for TMR compared with medical management [3–7]. The clinical results of these studies confirmed the angina relief provided by both Ho:YAG and CO2 lasers. The principle mechanism whereby this relief was obtained appears to be angiogenesis; however, an improvement in perfusion and concomitant improvement in myocardial function was not always seen [8].
BACKGROUND
Worldwide there are over 38 countries using TMR laser therapy, with over 45,000 procedures having been performed to date. In the United States, the number of TMR cases continues to increase from year to year (STS National Cardiac Database, www.sts.org). For TMR the FDA has approved two devices: the holmium: yttrium–aluminum–garnet (Ho:YAG) laser system and the carbon dioxide (CO2) laser system.
The Ho:YAG (CardioGenesis Corporation, Foothill Ranch, CA) system creates a pulsed laser beam with a maximum energy output of 20 W. The beam is projected through a 1-mm fiber optic bundle at a rate of 5 pulses per second, each delivering 2 J per pulse [9]. The Ho:YAG laser fiber is advanced manually through the myocardium between pulse rounds. This manual advancement of the fiber through moving myocardium makes it difficult to determine if the channel was created by mechanical or ablative means, and whether thermal dissipation has occurred before the firing of the next laser pulse [8].
The CO2 laser system (PLC Medical Systems, Franklin, MA) delivers a maximal 800 W pulse in 1–99 milliseconds with energies of 8–80 J [9]. Typically a 20 J per pulse beam at 25–30 milliseconds is directed via a system of mirrors to the myocardium. The transmural channel is then created within a single pulse. With the CO2 system the pulse firing is timed with the R-wave of the ECG cycle, when there is maximal ventricular filling and quiescence. Transmurality can be confirmed by transesophageal echocardiography as the laser energy strikes the blood within the ventricle, a characteristic acoustic response is readily observed.
METHODS
Fourteen Yorkshire domestic pigs were randomized for these experiments. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” guide formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1985). This study was approved by the National Heart, Lung and Blood Institute’s Animal Care and Use Committee.
The animals underwent three operations. The first procedure established an ischemic zone. Prior to the second operation baseline functional data was collected with cine magnetic resonance imaging (MRI) and dobutamine stress echocardiography (DSE). Following this imaging the animals were treated by TMR with either the Ho:YAG or CO2 laser. At the time of the third procedure the imaging was repeated and then the animals sacrificed.
Surgical Method
Following the establishment of general endotracheal anesthesia, with the animal in a right lateral decubitis position; a left anterolateral thoracotomy in the third intercostal space was performed. The pericardium was opened and an ameroid constrictor was placed around the proximal circumflex artery. The throacotomy was closed and the animal allowed to recover. Four weeks later a repeat thoracotomy in the 5th intercostal space was performed. The animals were randomized and circumflex territory was then treated by either Ho:YAG or CO2 TMR. Laser channels were created in a distribution of 1/cm2 within the affected ischemic area, 25 channels per animal. Settings for the CO2 laser were 20 J for the single pulse used and for the Ho:YAG2 J for the 10 pulses typically required to traverse the myocardium. At 6 weeks post-treatment, following imaging, the animals were sacrificed and the hearts excised. Histological investigation was then preformed to characterize the laser–tissue interactions.
Echocardiography Methods
Baseline epicardial echocardiography was performed on anesthetized animals using commercially available systems. Short-axis images were obtained to evaluate global and regional contractility. Dobutamine was then given intravenously at a dose of 5 μg/kg/min and titrated to a dose of 15 μg/kg/min to achieve a 100% increase in resting heart rate. Image acquisition was repeated during the dobutamine infusion. Digital images were acquired and stored for later off-line analysis using a specialized workstation (ProSolv Cardiovascular/Fujifilm, Version 3.5, Indianapolis, IN). Wall thickening analysis was performed by measuring end-diastolic wall thickness (EDWT) and end-systolic wall thickness (ESWT) averaged over 3–5 measurements per segment. Wall thickening (WTmm) was calculated as the difference of these measures (EDWT minus WSWT) and the percent wall thickening was calculated as WTmm/EDWT × 100. Echo observers were blinded to the treatment status of the animals to avoid bias.
MRI Methodology
Left ventricular size and function was imaged using a steady state free precession cine MRI on a 1.5 T Siemens scanner and using a custom 8 element cardiac phased array coil (Nova Medical Systems). Left ventricular volumes and wall thickening were measured from a stack of parallel short axis images. Long axis images were also obtained in the 3 chamber, 4 chamber, and 2 chamber views. Typical imaging parameters aimed for a temporal resolution of at least 45 milliseconds across the cardiac cycle and 1.4 mm × 2.2 mm in plane. Imaging parameters had to be adjusted to account for animal growth through the course of the protocol and thus were optimized on a study-by-study basis. Images were analyzed on a Siemens Leonardo workstation. An experienced investigator drew epicardial and endocardial contours on end systolic and end diastolic images with computer assisted planimetry. Left ventricular mass, wall thickness, and wall thickening were calculated on a per sector basis. MRI investigators were also blinded to the treatment status of the animals.
Histological Analysis Methodology
After euthanization, the ischemic (left circumflex artery territory) myocardium was cut into 5 mm × 5-thick pieces, either collected in cassettes and fixed with 10% buffered formalin for paraffin embedding, or in OCT for frozen sections with no fixation. Paraffin embedded sections were stained with H&E and Masson trichrome for morphological analysis. Fibrotic scoring of the ischemic myocardium was performed by counting the fibrotic foci found under the 40× microscopic field of a whole slide (average the counts from a total of five slides, scoring 0–3).
STATISTICAL ANALYSIS
Analyses were performed with a two-tail, two sample equal variant T-test used for normally distributed continuous variables. The appropriateness of this analysis was confirmed by performing a marginal homogeneity test that compared the baseline with the long-term follow-up. P < 0.05 was considered to indicate statistical significance. Statistically it was found that the cell therapy data showed there was a significant difference between the pre- and post- treatment for the CO2 laser and not the Ho:YAG laser. When comparing the post-treatment data there was only significance found for the fibrotic score data. The analysis indicates that there was no improvement in function with the Ho:YAG laser by the echocardiography or MRI results. Conversely there was improvement in function with the CO2 laser system.
RESULTS
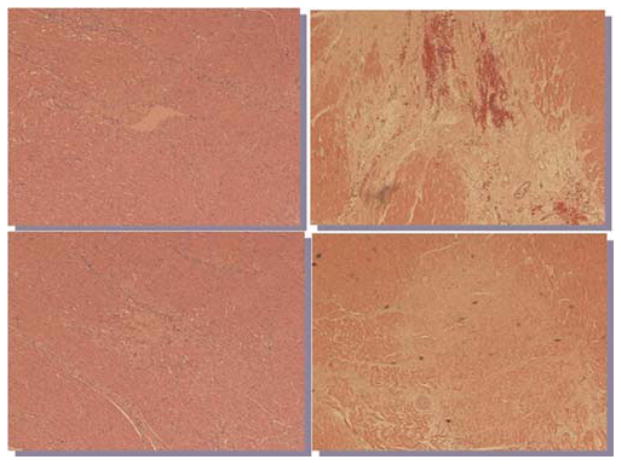
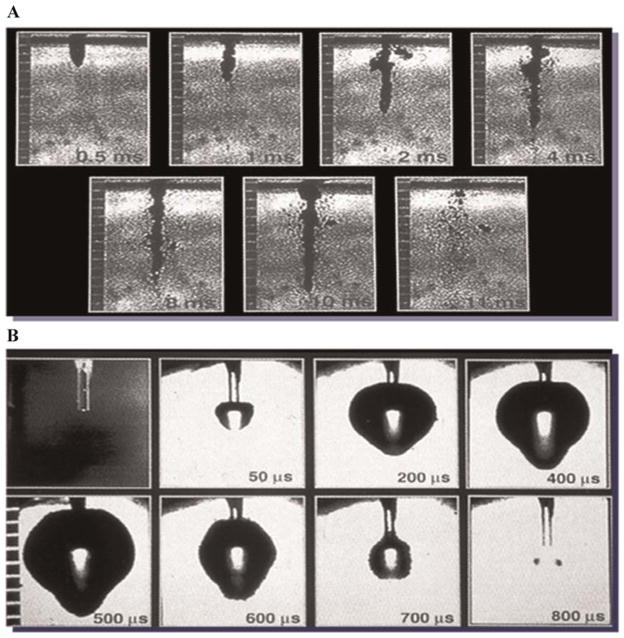
Figure 1 illustrates typical channels created with either the CO2 or Ho:YAG devices. While evidence of angiogenesis is seen following treatment with both lasers, there is significantly more fibrosis noted in the myocardium that underwent Ho:YAG TMR. This tissue response correlates with the energy distribution and characteristics of the two lasers as demonstrated in the series flash photography of both lasers firing [10] (Fig. 2). When the CO2 laser is fired, the single pulse creates a relatively straight and clean channel within the tissue. When the Ho:YAG laser is fired, with each pulse, explosive ablations are created with a wider expenditure of energy from the fiber tip and require multiple pulses with fiber advancement to complete a transmural channel. Our results indicate that when the CO2 laser was used, the severity of developed fibrosis on semi-quantitative scale was 0.8 hpf, compared to 1.8 hpf of the Ho:YAG at 6 weeks post-treatment (Fig. 3). The Ho:YAG laser created over twice as much scar tissue as the CO2 laser.
Fig. 1.
Histologic sections of laser channel 6 weeks after TMR treatment of myocardium (H&E 100×). A, B: CO2 laser. C, D: Ho:YAG laser.
Fig. 2.
Stop action photography of laser pulse with (A) CO2 and (B) Ho:YAG.
Fig. 3.
XXX.
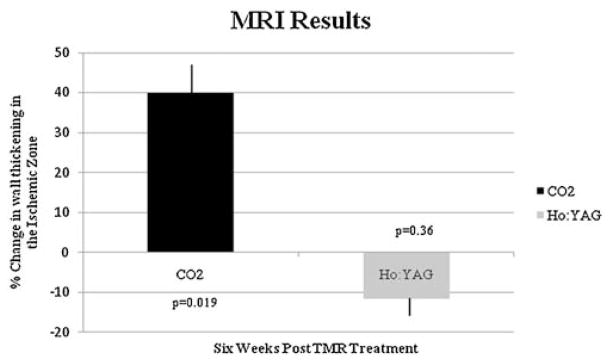
Functional assessment by non-invasive imaging demonstrated that this scarring effect had a negative impact. The stress echocardiography results showed a 20% improvement in wall thickening following treatment with the CO2 laser, conversely there was a −16% change in wall thickening for the Ho:YAG laser at 6 weeks post-trial (P = 0.003) (Fig. 4). MRI results showed a 40% change in segmental wall function in the ischemic zone for the CO2 laser, while the Ho:YAG laser showed a −10% change (P = 0.002) (Fig. 5). MRI was used as a secondary confirmation of the treatment effectiveness and to confirm stability of the model.
Fig. 4.
Change in contractility over time in the ischemic area after treatment with either a CO2 or Ho:YAG laser as assessed by DSE.
Fig. 5.
Change in contractility over time in the ischemic area after treatment with either a CO2 or Ho:YAG laser as assessed by cine MRI.
DISCUSSION
When compared side-by-side, these results indicate a difference between the CO2 and the Ho:YAG laser systems when TMR is performed. This is likely due to differences in the laser–tissue interactions for these different wavelengths of light.
Although the channel created when using the Ho:YAG can be less than the diameter of the channel created by the CO2 laser; it causes up to six times as much damage to the tissue. This creates more necrotic tissue in an already ischemic heart. Following this necrosis, collagen is laid down in the lasered area and Ho:YAG TMR does not accentuate the growth of angiogenesis in viable myocardium and can potentially further impair already ischemic tissue.
Previous publications have investigated the differences in myocardial damage between the CO2 and the Ho:YA Glaser. Kitade et al. found that when the Ho:YAG laser was fired, a layer of 760 ± 288 μm of thermal damage was created. When the CO2 laser was fired, a layer of 249 ± 83 μm of thermal damage was created. This study also found that the CO2 laser was more appropriate for end-stage myocardial ischemia then a Ho:YAG laser; in terms of the damage created [11]. Another study investigated the vascular response of TMR as a relation to the scar size left by the laser [12]. They reported that the angiogenic response to TMR is limited to the channel scar and related to the scar size. With a smaller scar channel (CO2 laser) there is greater angiogenesis in the scar. Additional experiments of histologically tracking laser tissue interactions over time revealed that the acute thermal injury and initial scarring at 2–3 weeks was greater with a Ho:YAG laser than with a CO2 laser [13]. There was scar contracture over time and at 6 weeks there was little difference between the groups. Moving beyond these histologic reports, Eckstein et al. [14] concluded from their trial that a Ho:YAG laser did not provide acute improvement of myocardial perfusion.
The channel created by the single pulse of the CO2 laser creates less trauma and fibrosis in the surrounding tissue and may engender an angiogenic response that allows for recovery of myocardial function in the ischemic area. Krabatsch et al. [15] found that the CO2 laser did not cause significant collateral damage to the myocardium and therefore resulted in minimal tissue fibrosis and more healthy tissue remained. In contrast, the Ho:YAG laser requires multiple blast-like pulses to traverse the myocardium, such firing damages the surrounding tissue creating more scar. This scarring of the myocardium leads to fibrosis that negatively impacts the contractile function of the heart. This is not to say that the Ho:YAG laser does not relieve angina but the mechanism of action is unlikely to be due to improved perfusion and function. Previous clinical studies have shown little to no perfusion benefits with Ho:YAG TMR [3,16–18]. In contrast, the CO2 laser offers a superior environment for stimulating angiogenesis and subsequent blood flow as has been reported clinically [4,5,19,20].
Confirmatory of our findings is a recent report of a cohort of patients 12 years after being treated with Ho:YAG TMR. The study found that cases of angina returned in treated patients after 3 years [21]. This was not seen in a report of CO2 treated TMR patients out to 7 years post-treatment [22].
It has been suggested that the scar tissue generated from the revascularization techniques can have a beneficial outcome. The new scar tissue can help to redistribute cardiac wall stress, penetrating all three layers of cardiac muscle acting as redistributing points to reduce interfascicular tension [23]. We have previously seen that focal injury caused by laser kinetic energy that leads to scarring and angiogenesis can have an impact on myocardial function and that the balance of fibrosis and perfusion is important [24]. One limitation of the present study is that unlike these reports we did not focus on the histologic findings but on the functional results. As noted many previous reports have discussed the different laser–tissue interactions with these two wavelengths of light and resultant tissue level findings. Our inclusion of histologic findings was not to be the foundation of the study therefore semi-quantitative methods were employed. Accurately tracking the laser injury histologically over time is difficult and it may be more clinically relevant to determine if the laser–tissue interaction with either scarring or angiogenesis led to changes in myocardial contractility. We endeavored to describe the ensuing functional impact.
CONCLUSION
We conclude that with the CO2 laser treatment for TMR; there was an improvement in the function of ischemic myocardium. With the Ho:YAG laser treatment there was no functional improvement following revascularization; likely due to increased fibrosis. This experiment indicates that there are differing laser–tissue interactions with the different TMR laser systems. Although both lasers treat cardiac ischemia, the CO2 laser does so with a greater rate of angiogenesis and less damage to the myocardium.
Acknowledgments
Contract grant sponsor: Division of Intramural Research; Contract grant sponsor: National Heart, Lung and Blood Institute, NIH, Bethesda, MD.
Special acknowledgements would like to be given to Dr. Yifu Zhou for the statistical analysis review. This research was funded by the Division of Intramural Research; National Heart, Lung and Blood Institute, NIH, Bethesda, MD.
References
- 1.Mirhoseini M, Cayton MM. Revascularization of the heart by laser. J Microsurg. 1981;2(4):253–260. doi: 10.1002/micr.1920020406. [DOI] [PubMed] [Google Scholar]
- 2.Okada M, Ikuta H, Shimizu K, Horii H, Nakamura K. Alternatives method of myocardial revascularization by laser: Experimental and clinical study. Kobe J Med Sci. 1986;32(5):151–161. [PubMed] [Google Scholar]
- 3.Burkhoff D, Schmidt S, Schulman SP, Myers J, Resar J, Becker LC, Weiss J, Jones JW. Transmyocardial laser revascularisation compared with continued medical therapy for treatment of refractory angina pectoris: A prospective randomised trial. ATLANTIC Investigators. Angina Treatments-Lasers and Normal Therapies in Comparison. Lancet. 1999;354(9182):885–890. doi: 10.1016/s0140-6736(99)08113-1. [DOI] [PubMed] [Google Scholar]
- 4.Frazier OH, March RJ, Horvath KA. Transmyocardial revascularization with a carbon dioxide laser in patients with end-stage coronary artery disease. N Engl J Med. 1999;341(14):1021–1028. doi: 10.1056/NEJM199909303411402. [DOI] [PubMed] [Google Scholar]
- 5.Schofield PM, Sharples LD, Caine N, Burns S, Tait S, Wistow T, Buxton M, Wallwork J. Transmyocardial laser revascularisation in patients with refractory angina: A randomised controlled trial. Lancet. 1999;353(9152):519–524. doi: 10.1016/s0140-6736(98)11478-2. [DOI] [PubMed] [Google Scholar]
- 6.Aaberge L, Nordstrand K, Dragsund M, Saatvedt K, Endresen K, Golf S, Geiran O, Abdelnoor M. Transmyocardial revascularization with CO2 laser in patients with refractory angina pectoris. Clinical results from the Norwegian randomized trial. J Am Coll Cardiol. 2000;35(5):1170–1177. doi: 10.1016/s0735-1097(00)00519-2. [DOI] [PubMed] [Google Scholar]
- 7.Allen KB, Dowling RD, DelRossi AJ, Realyvasques F, Lefrak EA, Pfeffer TA, Fudge TL, Mostovych M, Schuch D, Szentpetery S, Shaar CJ. Transmyocardial laser revascularization combined with coronary artery bypass grafting: Amulti-center, blinded, prospective, randomized, controlled trial. J Thorac Cardiovasc Surg. 2000;119(3):540–549. doi: 10.1016/s0022-5223(00)70134-6. [DOI] [PubMed] [Google Scholar]
- 8.Horvath KA. Transmyocardial laser revascularization. J Card Surg. 2008;23(3):266–276. doi: 10.1111/j.1540-8191.2008.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen KB, Horvath KA. Results of Prosepective Randomized Trials. Malden, MA: Blackwell Futura; 2006. [Google Scholar]
- 10.Horvath KA. Mechanisms and results of transmyocardial laser revascularization. Cardiology. 2004;101(1–3):37–47. doi: 10.1159/000075984. [DOI] [PubMed] [Google Scholar]
- 11.Kitade T, Okada M, Tsuji Y, Nakamura M, Matoba Y. Experimental investigations on relationships between myocardial damage and laser type used in transmyocardial laser revascularization (TMLR) Kobe J Med Sci. 1999;45(3–4):127–136. [PubMed] [Google Scholar]
- 12.Fisher PE, Khomoto T, DeRosa CM, Spotnitz HM, Smith CR, Burkhoff D. Histologic analysis of transmyocardial channels: Comparison of CO2 and holmium: YAG lasers. Ann Thorac Surg. 1997;64:466–472. doi: 10.1016/S0003-4975(97)00519-5. [DOI] [PubMed] [Google Scholar]
- 13.Huikeshoven M, Beliën JA, Tukkie R, Beek JF. The vascular response induced by transmyocardial laser revascularization is determined by the size of the channel scar: Results of CO2, holmium and excimer lasers. Lasers Surg Med. 2004;35(1):35–40. doi: 10.1002/lsm.20070. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein FS, Scheule AM, Pauncz Y, Schmid ST, Zucker M, Ziemer G. Transmyocardial laser revascularization with the Holmium:YAG laser does not improve myocardial perfusion in the acutely ischemic heart: An experimental study measuring myocardial perfusion by a thermal imaging camera. Thorac Cardiovasc Surg. 1999;47(5):293–297. doi: 10.1055/s-2007-1013161. [DOI] [PubMed] [Google Scholar]
- 15.Krabatsch T, Petzina R, Baretti R, Hausmann H, Hetzer R. Extent of myocardial tissue damage during transmyocardial laser revascularization with the CO2 heart laser. J Clin Laser Med Surg. 2001;19(5):251–259. doi: 10.1089/10445470152611982. [DOI] [PubMed] [Google Scholar]
- 16.Milano A, Pratali S, Tartarini G, Mariotti R, DeCarlo M, Paterni G, Boni G, Borotolotti U. Early results of transmyocardial revascularization with a holmium laser. Ann Thorac Surg. 1998;65(3):700–704. doi: 10.1016/s0003-4975(97)01380-5. [DOI] [PubMed] [Google Scholar]
- 17.Allen KB, Dowling RD, Fudge TL, Schoettle GP, Selinger SL, Gangahar DM, Angell WW, Petracek MR, Shaar CJ, O’Neill WO. Comparison of transmyocardial revascularization with medical therapy in patients with refractory angina. N Engl J Med. 1999;341(14):1029–1036. doi: 10.1056/NEJM199909303411403. [DOI] [PubMed] [Google Scholar]
- 18.Jones JW, Schmidt SE, Richman BW, Miller CC, III, Sapire KJ, Burkhoff D, Baldwin JC. Holmium:YAG laser transmyocardial revascularization relieves angina and improves functional status. Ann Thorac Surg. 1999;67(6):1596–1601. doi: 10.1016/s0003-4975(99)00368-9. [DOI] [PubMed] [Google Scholar]
- 19.Horvath KA, Mannting FR, Cummings N, Shernan SK, Cohn LH. Transmyocardial laser revascularization: Operative techniques and clinical results at two years. J Thorac Cardiovasc Surg. 1996;111(7):1047–1053. doi: 10.1016/s0022-5223(96)70381-1. [DOI] [PubMed] [Google Scholar]
- 20.Frazier OH, Cooley DA, Kadipasaoglu KA, Pehlivanoglu S, Lindenmeir M, Barasch E, Conger JL, Wilansky S, Moore WH. Myocardial revascularization with laser: Preliminary findings. Circulation. 1995;92:58–62. doi: 10.1161/01.cir.92.9.58. [DOI] [PubMed] [Google Scholar]
- 21.Pratali S, Chiaramonti F, Milano A, Bortolotti U. Transmyocardial laser revascularization 12 years later. Interact Cardiovasc Thorac Surg. 2010;11:480–481. doi: 10.1510/icvts.2010.243618. [DOI] [PubMed] [Google Scholar]
- 22.Horvath KA, Aranki SF, Cohn LH, March RJ, Frazier OH, Kadipasaoglu KA, Boyce SW, Lytle BW, Landolfo KP, Lowe JE, Hattler B, Griffith BP, Lansing AM. Sustained angina relief 5 years after transmyocadial laser revascularization with a CO2 laser. Circulation. 2001;104:I-81–I-84. doi: 10.1161/hc37t1.094774. [DOI] [PubMed] [Google Scholar]
- 23.Cardarelli M. A proposed alternative mechanism of action for transmyocardial revascularization prefaced by a review of the accepted explanations. Tex Heart Inst J. 2006;33(4):424–426. [PMC free article] [PubMed] [Google Scholar]
- 24.Horvath KA, Belkind N, Wu I, Greene R, Doukas J, Lomasney JW, McPherson DD, Fullerton DA. Functional comparison of transmyocardial revascularization by mechanical and laser means. Ann Thorac Surg. 2001;72(6):1997–2002. doi: 10.1016/s0003-4975(01)03243-x. [DOI] [PubMed] [Google Scholar]