Abstract
Background:
Human Streptococcus suis endocarditis occurs infrequently and continues to be a serious illness with high mortality. However, knowledge of the echocardiographic features and clinical outcome of this disease remains unclear.
Methods:
One hundred and fourteen patients were identified in a prospective study, and hospitalized at Queen Sirikit Heart Center and Srinagarind Hospital, Khon Kaen University. Echocardiography was routinely performed in all patients.
Results:
Between January 2010 and December 2011, three cases of S. suis endocarditis were diagnosed. All cases were male and aged 27–53 years. The most common risk factor for contracting S. suis infection was eating undercooked pork. Three patients presented with congestive heart failure. Transthoracic echocardiography demonstrated large, highly mobile vegetations and severe valvular damage. Aortic valve involvement was documented in two patients, and mitral valve involvement in one. One patient presented with embolic stroke and one with arterial occlusion. All patients underwent urgent valve replacement with a good clinical outcome.
Conclusion:
The echocardiographic features of S. suis endocarditis show destructive, extensive valvular damage and early embolization with a fulminant course, needing early surgical intervention with a good clinical outcome.
Keywords: Streptococcus suis, endocarditis, echocardiography
Introduction
Streptococcus suis is an emerging zoonotic pathogen, causing invasive infection in humans who have close contact with infected pigs or contaminated pork-derived products.1,2 The rapid and destructive progression of this pathogen causes valvular damage and consequent congestive heart failure. Transthoracic echocardiography with color flow Doppler imaging has become an important tool for confirming the clinical diagnosis of infective endocarditis and determine the extent of cardiac lesions, and provides accurate and detailed evaluation of the anatomic and physiologic features of infective endocarditis.3–5 The purpose of this study was to examine the echocardiographic features and clinical outcome of S. suis endocarditis.
Materials and Methods
A prospective study of the etiology and characteristics of infective endocarditis at Srinagarind Hospital and Queen Sirikit Heart Center, Khon Kaen University, was performed between January 2010 and December 2011. One hundred and fourteen patients were identified to have a clinical diagnosis of infective endocarditis using Duke criteria.6 Transthoracic echocardiography was performed in all patients, using previously reported techniques.7 The hallmark lesions of infective endocarditis are vegetations occurring on the valves, which can occasionally occur at unusual sites where the endocardium has been disrupted by abnormal flow. All patients also underwent color flow Doppler and hemodynamic assessment. The study was approved by the appropriate ethics committee. All patients gave written informed consent to participate in the study.
Results
Three cases of S. suis endocarditis were diagnosed. The age at echocardiographic diagnosis of infective endocarditis was 27–53 years. The indications for referral included evaluation of fever, infective endocarditis with congestive heart failure, and need for surgical intervention.
Case 1
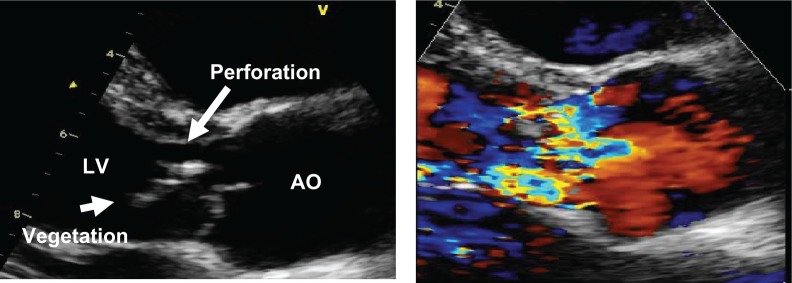
A 27-year-old man was admitted to a Northeast provincial hospital having had malaise, fever, night sweats, weight loss, and progressive dyspnea for a month. He was otherwise in good health and had no known heart disease. He was referred to Srinagarind Hospital for evaluation of fever with heart failure. On physical examination, his blood pressure was 80/60 mmHg, and he had a grade 4/6 diastolic murmur of aortic regurgitation, with orthopnea and dyspnea. No signs of meningitis were noted. Laboratory investigations showed ischemic hepatitis and acute kidney injury. The chest x-ray revealed cardiomegaly and pulmonary edema. Six sets of blood cultures were taken and the results were negative. Transthoracic echocardiography showed that the aortic valve had a large mobile vegetation and perforation of the valve cusps, while color flow Doppler demonstrated severe aortic regurgitation (Fig. 1). The patient was treated with a beta-lactam antibiotic but his severe congestive heart failure did not improve. The patient underwent urgent aortic valve replacement. The perioperative period was uneventful, with continuation of the same antibiotics for 4 weeks after surgery. The serology and valve tissue sent for molecular analysis was positive for S. suis serotype 2 by serotype-specific polymerase chain reaction.
Figure 1.
Transthoracic echocardiography shows large, mobile vegetation on the aortic valve with perforation of the RCC (left), severe aortic regurgitation (right).
Case 2
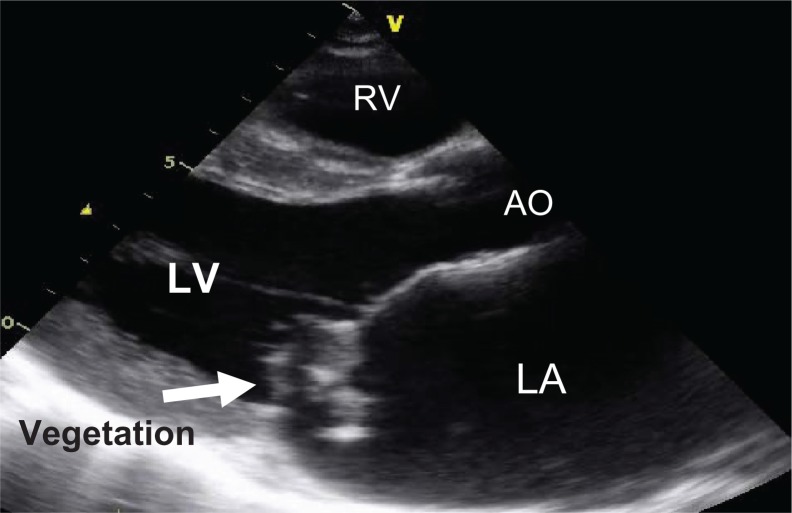
A 53-year-old man presented to the clinic, having had fever and fatigue for a month. One week before admission he had had dyspnea and orthopnea, and had developed left hemiplegia. On physical examination, his blood pressure was 87/57 mmHg, and he had atrial fibrillation, dyspnea, and orthopnea, but no signs of meningitis. He had a murmur of mitral regurgitation at the apex. Crepitations were heard in both lung fields. A chest x-ray revealed cardiomegaly and pulmonary congestion. Three sets of blood cultures grew Streptococcus viridans. Transthoracic echocardiography demonstrated large, highly mobile vegetations on the anterior mitral valve leaflet with severe mitral regurgitation (Fig. 2). The patient was treated with ceftriaxone but remained in a state of cardiogenic shock. He underwent urgent mitral valve replacement. The perioperative period was uneventful, with continuation of the same antibiotics for 4 weeks after surgery. The serology and valve tissue sent for molecular analysis was positive for S. suis serotype 2 by serotype-specific polymerase chain reaction.
Figure 2.
A parasternal long-axis view shows large vegetations on the mitral valve leaflet.
Case 3
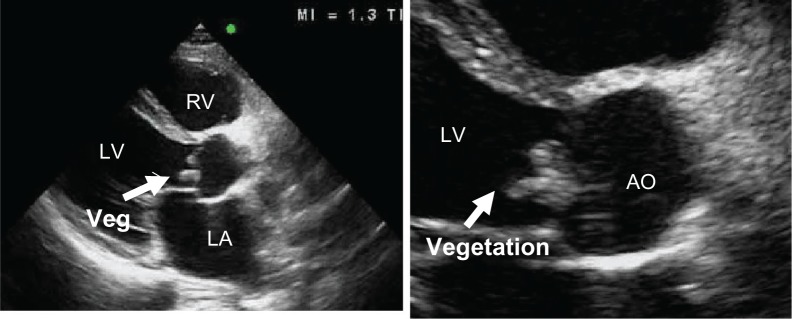
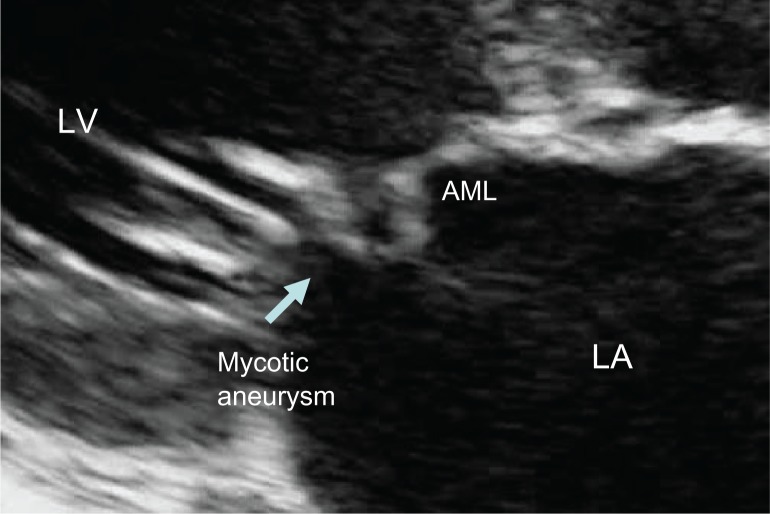
A 52-year-old man presented to the clinic, having had fever and fatigue for 3 months. Two weeks before admission, he had developed dyspnea on exertion, orthopnea, and a painful left foot. On physical examination, all vital signs were normal. He was dyspneic, orthopneic, and mildly pale. Ecchymoses of the distal left foot were observed, with an absent dorsalis pedis pulse. A diastolic blowing murmur was demonstrated at the left parasternal border. No signs of meningitis were noted. Three sets of blood cultures grew S. suis. Transthoracic echocardiography showed large vegetations on the aortic valve (Fig. 3) with a mycotic aneurysm at the atrial surface of the anterior mitral leaflet (Fig. 4). Color flow Doppler demonstrated severe aortic regurgitation and moderate mitral regurgitation. The patient underwent urgent aortic and mitral valve replacement and recovered uneventfully.
Figure 3.
A parasternal long-axis view shows large, highly mobile vegetations on the aortic cusps.
Figure 4.
Transthoracic echocardiography shows mycotic aneurysm on the anterior mitral leaflet (AML).
Echocardiographic findings
Vegetative lesions using transthoracic echocardiography demonstrated significant valvular regurgitation and severely damaged valves in all three cases. Aortic valve vegetations were found in two cases and in one case in the mitral valve position. All cases showed large, highly mobile vegetations. A mycotic aneurysm involving an anterior mitral leaflet was found in one of the cases; this aneurysm occurred in association with infective endocarditis of the aortic valve. The mechanism of its formation was severely destructive to the aortic valve resulting in an aortic regurgitation jet striking the anterior mitral leaflet of the mitral valve, creating a secondary site of infection leading to development of an aneurysm.8
Surgical findings
In this study, all three patients with S. suis endocarditis underwent urgent surgical intervention due to large, mobile vegetations with severe valvular damage, having progressed to congestive heart failure and cardiogenic shock. The surgical findings were in agreement with the transthoracic echocardiography and Doppler echocardiography assessments.
Clinical outcome
All three of these patients with S. suis endocarditis underwent surgery, with the perioperative period being uneventful. None of the patients died after surgery or during the follow-up period.
Discussion
S. suis endocarditis is an infective process which occurs infrequently in humans (mostly involving meningitis).9–12 It is associated with significant, severe valvular damage, often causing congestive heart failure and cardiogenic shock, needing urgent cardiac surgery. Even though our study deals with a small series of patients, to our knowledge, this is the most extensive description of the echocardiographic features of S. suis endocarditis. The high resolution of transthoracic echocardiography allows accurate diagnosis of this infrequent disease. Accurate detection of valvular vegetations and damage using Doppler hemodynamic assessment is also possible. Preoperative catheterization and transesophageal echocardiography were deemed unnecessary due to good imaging from transthoracic echocardiography in all three patients. All of the findings gleaned from echocardiography were confirmed at surgery.
A likely explanation for fulminant life-threatening S. suis endocarditis in humans is S. suis serotype 2, which has a series of potential virulence factors, including capsular polysaccharide, extracellular protein factor, muramidase-released protein, suilysin, several adhesions,13–16 and subsequent severe destruction of the valves. The portal of entry of the organism in all patients might have been eating raw pork, which is the most common risk factor in this region. We describe the first reported cases showing the echocardiographic features of S. suis endocarditis showing the destructive, extensive valvular damage and a fulminant course, with early embolization needing urgent surgery.
Acknowledgments
We are grateful to Professor Fournier for his assistance with the molecular techniques used in this work, which was supported by research grants from the Centers for Disease Control and Prevention, Thailand. We thank Bryan Roderick Hamman and Janice Loewen-Hamman for assistance with the English language presentation of the manuscript.
Footnotes
Author Contributions
Conceived and designed the experiments: OP, ST, PM, AK. Analysed the data: OP, ST, PM, AK. Wrote the first draft of the manuscript: OP, ST, PM, AK. Contributed to the writing of the manuscript: OP, ST, PM, AK. Agree with manuscript results and conclusions: OP, ST, PM, AK. Jointly developed the structure and arguments for the paper: OP, ST, PM, AK. Made critical revisions and approved final version: OP, ST, PM, AK. All authors reviewed and approved of the final manuscript.
Funding
Author(s) disclose no funding sources.
Competing Interests
Authors disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis. 2007;7:201–9. doi: 10.1016/S1473-3099(07)70001-4. [DOI] [PubMed] [Google Scholar]
- 2.Benslimani A, Fenollar F, Lepidi H, Raoult D. Bacterial zoonoses and infective endocarditis, Algeria. Emerg Infect Dis. 2005;11:216–24. doi: 10.3201/eid1102.040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffe WM, Morgan DE, Pearlman AS, Otto CM. Infective endocarditis, 1983–1988: echocardiographic findings and factors influencing morbidity and mortality. J Am Coll Cardiol. 1990;15:1227–33. doi: 10.1016/s0735-1097(10)80005-1. [DOI] [PubMed] [Google Scholar]
- 4.Sanfilippo AJ, Picard MH, Newell JB, et al. Echocardiographic assessment of patients with infectious endocarditis: prediction of risk for complications. J Am Coll Cardiol. 1991;18:1191–9. doi: 10.1016/0735-1097(91)90535-h. [DOI] [PubMed] [Google Scholar]
- 5.Murphy JG, Foster-Smith K. Management of complications of infective endocarditis with emphasis on echocardiographic findings. Infect Dis Clin North Am. 1993;7:153–65. [PubMed] [Google Scholar]
- 6.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200–9. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 7.Tajik AJ, Seward JB, Hagler DJ, Mair DD, Lie JT. Two-dimensional real-time ultrasonic imaging of the heart and great vessels: technique, image orientation, structure identification, and validation. Mayo Clin Proc. 1978;53:271–3. [PubMed] [Google Scholar]
- 8.Reid CL, Chandraratna AN, Harrison E, et al. Mitral valve aneurysm: clinical features, echocardiographic-pathologic correlations. J Am Coll Cardiol. 1983;2:460–4. doi: 10.1016/s0735-1097(83)80272-1. [DOI] [PubMed] [Google Scholar]
- 9.Avihingsanon A, Suankratay C, Tantawichien T, Nunthapisud P. Two cases of human endocarditis in Thailand due to Streptococcus suis. Chula Med J. 1999;43:317–25. [Google Scholar]
- 10.Chotmongkol V, Janma J, Kawamatawong T. Streptococcus suis meningitis: report of a case. J Med Assoc Thai. 1999;82:922–4. [PubMed] [Google Scholar]
- 11.Leelarasamee A, Nilakul C, Tien-Grim S, Srifuengfung S, Susaengrat W. Streptococcus suis toxic shock syndrome and meningitis. J Med Assoc Thai. 1997;80:63–8. [PubMed] [Google Scholar]
- 12.Phuapradit P, Boongird P, Boonyakarnkul S. Meningitis caused by Streptococcus suis in humans. Intern Med. 1987;3:120–2. [Google Scholar]
- 13.de Greeff A, Buys H, Verhaar R, Dijkstra J, van Alphen L, Smith HE. Contribution of fibronectin-binding protein to pathogenesis of suis serotype 2. Infect Immun. 2002;70:1319–25. doi: 10.1128/IAI.70.3.1319-1325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King SJ, Allen AG, Maskell DJ, Dowson CG, Whatmore AM. Distribution, genetic diversity, and variable expression of the gene encoding hyaluronate lyase within the Streptococcus suis population. J Bacteriol. 2004;186:4740–7. doi: 10.1128/JB.186.14.4740-4747.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Martinez G, Gottschalk M, et al. Identification of a surface protein of Streptococcus suis and evaluation of its immunogenic and protective capacity in pigs. Infect Immun. 2006;74:305–12. doi: 10.1128/IAI.74.1.305-312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith HE, Wisselink HJ, Stockhofe-Zurwieden N, Vecht U, Smits MM. Virulence markers of Streptococcus suis Type 1 and 2. Adv Exp Med Biol. 1997;64:3659–65. doi: 10.1007/978-1-4899-1825-3_152. [DOI] [PubMed] [Google Scholar]