Abstract
Mesenchymal stem cells (MSCs) have been isolated from many sources, including adults and fetuses. Previous studies have demonstrated that, compared with their adult counterpart, fetal MSCs with several remarkable advantages may be a better resource for clinical applications. In this study, we successfully isolated a rapidly proliferating cell population from limb bud of aborted fetus and termed them “human limb bud–derived mesenchymal stem cells” (hLB-MSCs). Characteristics of their morphology, phenotype, cell cycle, and differentiation properties were analyzed. These adherent cell populations have a typically spindle-shaped morphology. Flow cytometry analysis showed that hLB-MSCs are positive for CD13, CD29, CD90, CD105, and CD106, but negative for CD3, CD4, CD5, CD11b, CD14, CD15, CD34, CD45, CD45RA, and HLA-DR. The detection of cell cycle from different passages indicated that hLB-MSCs have a similar potential for propagation during long culture in vitro. The most novel finding here is that, in addition to their mesodermal differentiation (osteoblasts and adipocytes), hLB-MSCs can also differentiated into extramesenchymal lineages, such as neural (ectoderm) and hepatic (endoderm) progenies. These results indicate that hLB-MSCs have a high level of plasticity and can differentiate into cell lineages from all three embryonic layers in vitro.
Introduction
Mesenchymal stem cells (MSCs) are nonhematopoietic multipotential cells with spindle shape–like fibroblasts (Lai et al., 2011). Over the past decades, MSCs have drawn intense interest from translational and basic investigators because of their basic characteristics, such as the ability for self-renewal, extensive differentiation potential, high plasticity, and flexibility for genetic modification (Nombela-Arrieta et al., 2011; Tong et al., 2011). As a result, MSCs have been isolated from a broad range of tissues and organs, including bone marrow, liver, and spleen (Chan et al., 2005; Fan et al., 2005; Filioli Uranio et al., 2011; Hu et al., 2003; O'Donoghue et al., 2006; Rzhaninova et al., 2005).
To date, most data about MSCs was from adult tissues, such as bone marrow (BM). Although many studies indicated that adult bone marrow–derived MSCs (BM-MSCs) are one of the most accessible resources and can be rapidly expanded in vitro, it has also been demonstrated that the number and the differentiating potential of BM-MSCs will decrease with age (Alessandri et al., 2004). These limitations have discouraged the use of adult BM-MSCs, indicating that the search for alternative sources of MSCs for clinical applications is important.
Compared with their adult counterpart, the fetal MSCs (fMSCs) have certain advantages. It has been reported that they are less immunogenic at lower stages of differentiation and have higher potential for repopulation and migration (Todorov et al., 2010). However, fMSCs in published studies are mostly derived from tissues or species in the perinatal period, such as umbilical cord, cord blood, placenta, and amniotic fluid (Chen et al., 2011; In't Anker et al., 2004; Kestendjieva et al., 2008; Qiao et al., 2008). Until now, little has been known about their presence in early fetal life, especially in the first trimester of gestation. Taking into account that developmental stage and anatomical locations of the tissue have an effect on the differentiation potential of MSCs, determination of the characteristics of MSCs derived from earlier gestation can provide a new resource and pave the way for future applications (Ramkisoensing et al., 2011).
In this study, the presence of MSCs from human limb bud (hLB-MSCs) was investigated in the first trimester of gestation. Our results showed that hLB-MSCs can be isolated with typically fibroblastoid morphology and a well-defined phenotype. Furthermore, besides the common mesenchymal differentiation (osteoblasts and adipocytes), these cells can even differentiated into extramesenchymal lineages, such as neural cells (ectoderm) and hepatocyte (endoderm). This study demonstrated that hLB-MSCs have transdifferentiation capacity and may be a novel and promising resource for regenerative medicine and tissue engineering.
Materials and Methods
Main materials and reagents
Fetus
After informed consent, one aborted fetus aged about 6 weeks was collected from a healthy pregnant woman who volunteered to terminate her pregnancy by RU486 in the PLA 107 Hospital affiliated with Binzhou Medical College (Yantai, China). The use of fetal tissue for research was approved by the Ethics Committee of Binzhou Medical College.
Cells and cell culture
Dulbecco's modified Eagle's medium (DMEM)-High Glucose (HG) and DMEM-Low Glucose (LG), standard fetal bovine serum (FBS), minimum essential medium-Eagle, Earle's salts base, with nonessential amino acids (MEM-NEAA), and GlutaMAX for Stem Cells culture were purchased from GIBCO-BRL. Cells were rountinely cultured in DMEM (Invitrogen) supplemented with 10% FBS within a humidified incubator containing 5% CO2 at 37°C. L02 cells were obtained from Beijing Cell Bank (The Chinese Academy of Sciences), rountinely cultured in improved MEM (IMEM) (Invitrogen) supplemented with 10% FBS within a humidified incubator containing 5% CO2 at 37°C.
Growth factors
Human epidermal growth factor (hEGF; E9644), human hepatocyte growth factor (hHGF; H9661), and human transforming growth factor-α (hTGF-α; T-9533) were purchased from Sigma-Aldrich.
Antibodies
Monoclonal antibodies against human antigens labeled fluorescently CD3-FITC, CD4-PE, CD5-FITC, CD11b-PE, CD13-APC, CD14-APC, CD15-FITC, CD29-PE, CD34-PE, CD45-PerCP, CD45RA-FITC, CD90-FITC, and HLA-DR-PE were provided by Dr. Jun Zhang (Changhai Hospital, Shanghai) and Dr. Shu-ping Zhao (Taian Central Hospital, Shandong) as a kind gift (Becton Dickinson). Antibodies against human antigens CD106 (Sigma) and CD105 (ZhongShan Biotechnology, Beijing) were a kind gift of Dr. Min-juan Wu. Secondary goat antimouse antibodies and fluorescein isothiocyanate (FITC) were purchased from Santa Cruz Biotechnology.
Methods
Construction of hLB-MSC populations
The cells were prepared following the procedures previously described by Liu et al. (2004). Briefly, strictly aseptic fetal chorion was observed under a dissecting microscope to assure that it was intact, plump, and not deformed. Attached vessels and fat tissue were cut off. The fetal limb bud tissue was preserved, washed with D-Hanks' solution (Biosis Biotechnology Co., Ltd., Shanghai, China) several times, pipetted to a penicillin vial, ground with ophthalmic scissors, and digested with 0.25% trypsin-EDTA at 37°C for 2 min. DMEM-LG containing 10% FBS was added to terminate digestion, and the tissue was dispersed gently to a single-cell suspension. A Pasteur pipette was inserted vertically to the bottom of a 15-mL centrifuge tube, followed by addition of Percoll lymphocyte separation solution (P4937, Sigma) and density-gradient centrifugation (1.077g/mL) according to the manufacturer's instructions. A 4-mL Percoll solution was gently added to the surface, of which 8 mL of cell suspension was gently superpositioned along the tube wall and centrifuged at 2000 rpm at room temperature for 25 min. The white cloud-like karyocyte layer in the separation medium was pipetted to a centrifuge tube, DMEM-LG containing 10% FBS was added, and the mixture was centrifuged at 800 rpm for 4 min. The supernatant was discarded, and cells were harvested and plated in tissue culture flasks for purification in expansion culture medium.
Expansion culture medium consisted of DMEM-LG and 10% FBS supplemented with MEM-NEAA (a 200-fold dilution of the stock; GIBCO), GlutaMAX (a 200-fold dilution of the stock; GIBCO), 100 units of penicillin, and 1000 units of streptomycin.
Morphological and biological behaviors of hLB-MSCs
Morphological and biological behaviors of hLB-MSCs were observed under a phase-contrast microscope every 2 days.
Related cell-surface antigens phentotype characterization of hLB-MSCs
The 7th-passage cells in doubling time phase were prepared to a 106 cells/mL single-cell suspension. The volume of each sample was 100 μL, to which anti- CD3-FITC, CD4-PE,CD5-FITC, CD11b-PE, CD13-APC, CD14-APC, CD15-FITC, CD29-PE, CD34-PE, CD45-PerCP, CD45RA-FITC, CD90-FITC, HLA-DR-PE, CD105–FITC, and CD106-FITC labeled fluorescence monoclonal antibodies were added, cultured at room temperature for 30 min, washed with phosphate-buffered saline (PBS) three times, stained with propidium iodide (PI; Becton Dickinson) for 15 min, and then assayed by fluroescence-activated cell sorting (FACS).
Analysis of cell cycle in hLB-MSCs by flow cytometry
The 25th-passage cells in doubling time phase were washed with PBS (Biosis Biotechnology, China) three times and digested to a single-cell suspension. They were washed with PBS for three times, centrifuged, suspended in 70% alcohol, fixed at 4°C overnight, washed with PBS three times, and then rested in 100 μg/mL RNase at room temperature for 30 min, stained with 20 μg/mL PI, and assayed by flow cytometry.
In vitro cell differentiation studies
Differentiation into adipocyte and osteogenic cells (mesodermal differentiation)
Methods were based on the protocol described by Aurich et al. with minor modifications (Aurich et al., 2007). For adipocyte differentiation, cells of 5th to 13th passage were used. The suspension was inoculated at a density of 0.8×104/cm2. At the initial induction, cells were serum deprived for 24 h, then treated with adipogenic medium. Adipogenic medium consisted of DMEM-LG and 5% FBS with 0.5 mmol/L 3-isobutyl-1-methylxanthine (IMBX; I5879, Sigma), 0.5 μmol/L dexamethasone (Sigma), 1.0×10−7 μmol/L recombined human insulin (Invitrogen), MEM-NEAA (a 200-fold dilution of the stock), and GlutaMAX (a 200-fold dilution of the stock). Induced cells were collected on days 0, 7, and 14. Adipogenesis was assessed by Oil Red (Shenggong Biotechnology, Shanghai) staining. The reverse transcription polymerase chain reaction (RT-PCR) for the adipocyte-specific gene adipsin (AD) was also detected. Primers of AD are listed in Table 1.
Table 1.
Specific Primers Used for Reverse-Transcription Polymerase Chain Reaction
| Gene | Accession | Sequence | Product (bp) |
|---|---|---|---|
| AD | NM_001928 | 5′-GTC ACC CAA GCA ACA AAG TCC-3′ 5′-GCC TCC TGC GTT CAA GTC AT-3′ |
271 |
| BGLAP | NM_199173 | 5′-CTG ACC ACA TCG GCT TTC-3′ 5′-TCT GGA GTT TAT TTG GGA GC-3′ |
210 |
| NF | NM_021076 | 5′-GTG AAG AGT GTC GGA TTG GC-3′ 5′-CTG GTG ACT TGG CTT CCT T-3′ |
336 |
| Alb | NM_000477 | 5′-AAC TCC TTT TCT TTG TCA-3′ 5′-TCT CAT CAT TTT CCA CTT-3′ |
435 |
| AFP | NM_001134 | 5′-GCG TTT CTC GTT GCT TAC-3′ 5′-CAG GGT TTA CTG GAG TCA TT-3′ |
199 |
| CK8 | NM_002273 | 5′-AGG ATG CCA ACG CCA AGT-3′ 5′-GTC TCC TGT TCC CAG TGC CA-3′ |
508 |
| CYP2B6 | NM_000767 | 5′-AGG ACC TCA TCG ACA CCT-3′ 5′-CCT GTT CAA TCT CCC TGT-3′ |
207 |
| TAT | BC020707 | 5′-TCC AGA TGA GCA GCA AAG-3′ 5′-AAG AAG CAA TCT CCT CCC-3′ |
357 |
| GAPDH | AF261085 | 5′-GGA TTT GGT CGT ATT GGG-3′ 5′-CAG TTG GTG GTG CAG GAG-3′ |
440 |
AD, adipsin; BGLAP, bone gamma-carboxyglutamate protein; NF, neurofilament; Alb, albumin; AFP, alpha fetoprotein; CK8, cytokeratin; CYP2B6, cytochrome P450, family 2, subfamily B, polypeptide 6; TAT, tyrosine aminotransferase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; bp, base pairs.
For osteogenic differentiation, DMEM containing 200 mM ascorbic acid 2-phosphate, 1 mM dexamethason, 10 mM glycerol-3 phosphate (all Sigma), and 10% fetal calf serum (FCS; Invitrogen) was used. The medium was changed every 3–4 days over a period of 14 days. Morphological changes were observed under microscopy at a 7-day interval. The expression of bone gamma-carboxyglutamate protein (BGLAP) was analyzed by RT-PCR and immunocytochemistry (ICC).
Differentiation into neurocyte-like cells (ectodermal differentiation)
According to an established protocol with minor modifications, cells were serum deprived for 24 h at the initial induction, then treated with neurogenic medium (Vourc'h et al., 2004). Medium changes were performed twice weekly. Neurogenic medium consisted of DMDM-LG and 5% FBS with 2.0×10−5 mol/L β-mercaptoethanol (2ME, Sigma), MEM-NEAA (a 200-fold dilution of the stock), and GlutaMAX (a 200-fold dilution of the stock). Morphological changes were observed under the microscope. Induced cells were collected on days 0, 7, and 14. Neurogenesis was assessed by RT-PCR and ICC for neurocyte specific gene neurofilament (NF). Primers of NF are listed in Table 1.
Differentiation into hepatocyte-like cells (endodermal differentiation)
For hepatogenic differentiation, 5th- to 13th-passage cells were used. The suspension was inoculated at a density of 1.0×104/cm2. Induction proceduces were based on a “two-step method” described in previous publications with modifications (Lee et al., 2004). At the initial induction, cells were serum deprived for 1 day in DMEM-LG supplemented with 20 ng/mL hEGF. Then, differentiation was induced by treating hLB-MSCs with differentiation medium, consisting of DMDM-LG and 10% FBS supplemented with 10 ng/mL hEGF, 25 ng/mL hHGF, 5 ng/mL hTGF-α, 1 μmol/L dexamethasone, MEM-NEAA (a 200-fold dilution of the stock), and GlutaMAX (a 200-fold dilution of the stock). Morphological changes were observed every 7 days. Medium changes were performed twice weekly. After a 21-day differentiation, hepatogenesis was assessed by RT-PCR for the expression of liver-associated specific genes at the indicated time points. Primers used are listed in Table 1.
Total RNA extraction and RT-PCR
hLB-MSCs cells were induced toward three lineages for defined time periods. Total RNA was extracted from hLB-MSCs, differentiated cells, and undifferentiated cells using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions and reverse-transcribed using conventional protocols. PCR amplification was performed using the primers (Table 1). All primer sequences were determined using established GenBank [National Center for Biotechnology Information (NCBI)] sequences. Duplicate PCR reactions were amplified using primers designed glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a control for assessing PCR efficiency and for subsequent analysis by agarose gel electrophoresis. Untreated hLB-MSCs cells were examined as a negative control. L02 was analyzed as a positive control for the hepatogenic lineage.
Results
Morphology and biological behaviors
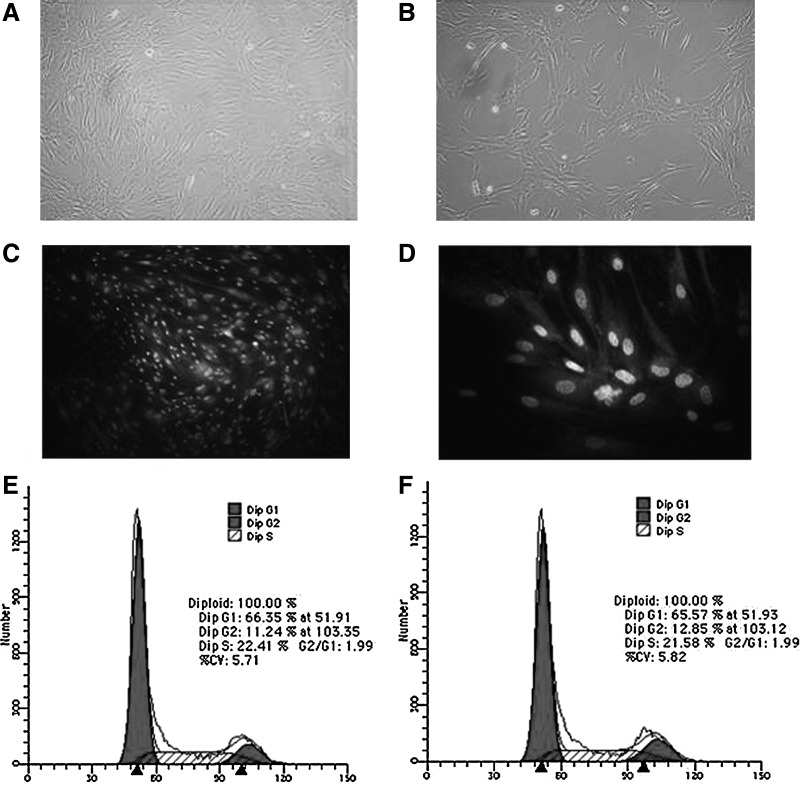
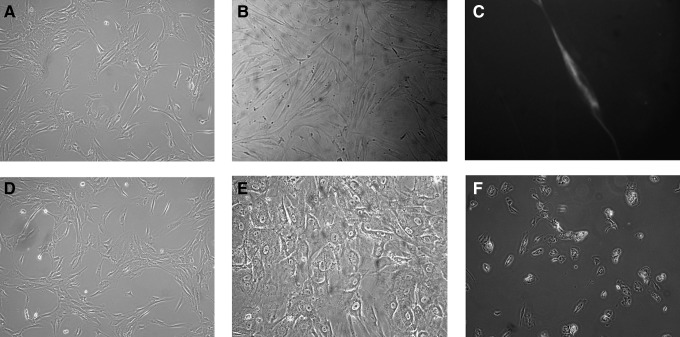
hLB-MSCs were isolated from mononuclear cells, obtained by Percoll density-gradient centrifugation. The eluted cells were harvested and cultured in the presence of 10% FBS. Observation by microscopy showed that cells separated from primary tissue proliferated rapidly in expansion culture medium. The special medium for MSCs was changed every 2 days to deplete blood cells, fibroblasts, or other miscellaneous cells. After primary cultured cells were passed for more than four passages, their morphology became identical to fibroblast-like and was marked by “P1.” After the first passage, cells from samples expanded in the same monolayer manner, with homogeneous fibroblast-like cells. Single-layered cells were grown in a reticular-like manner when the density was low, and in a cluster radial- or vortex-like manner when the density was high; their morphology was consistent with that reported in the literature for MSCs (Fig. 1A–D) (Hass et al., 2011; Hua et al., 2009). So far, hLB-MSCs had been passaged up to 35 passages and maintained the undifferentiated state as spindle-shaped, fibroblastic morphology. hLB-MSCs could be cryopreservated in a 50% culture medium in the proportion 40% serum:10% dimethyl sulfoxide (DMSO). The 7th-passage cells were used for phenotype detection and in vitro differentiation.
FIG. 1.
The morphology and cell cycle analysis of hLB-MSCs from the 7th and 25th passages. (A, passage 7; B, passage 25). The morphological appearances of hLB-MSCs from different passages are similar. The cells are both spindle-shaped and fibroblast-like. (C and D) 4′,6-Diamidino-2-phenylindole (DAPI) staining of hLB-MSCs. hLB-MSCs with smooth nuclear are positive of DAPI staining. The cells were considered normal on the basis of typical morphology. (E, passage 7; F, passage 25). Cell cycle analysis of hLB-MSCs from the 7th and 25th passages indicated that hLB-MSCs have similar potentials of propagation during long culture in vitro.
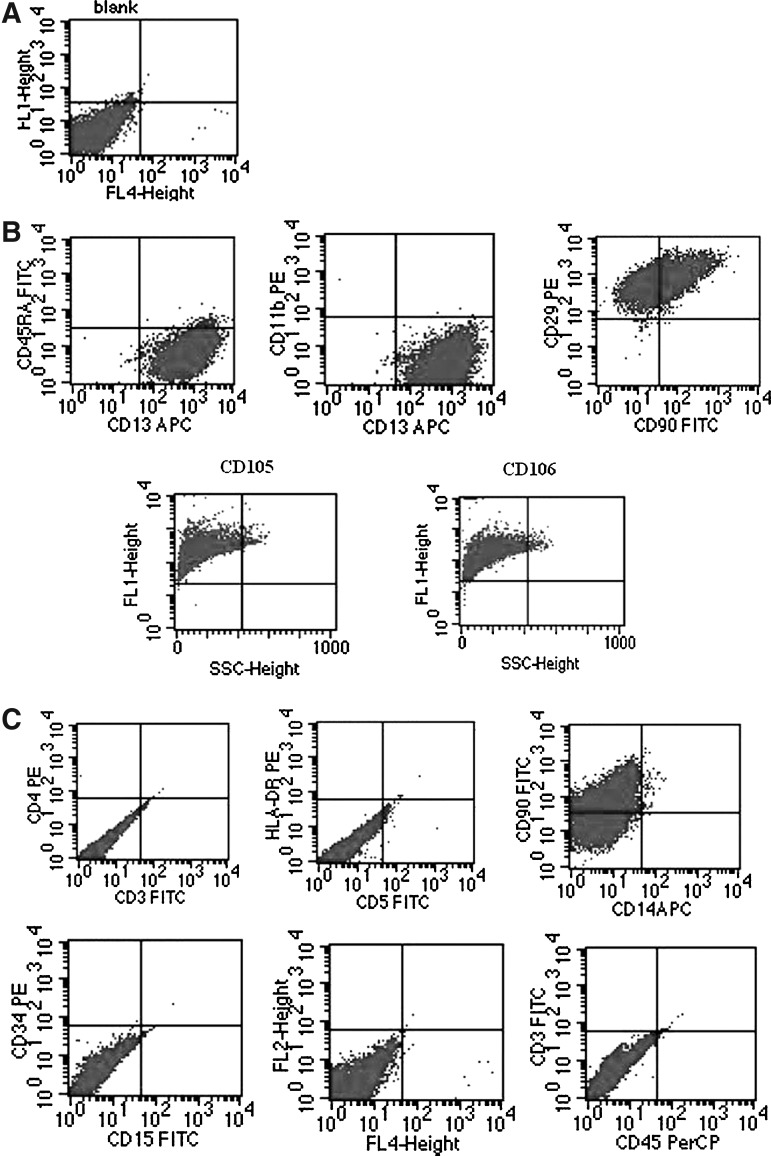
hLB-MSC surface phenotype
To investigate the expression of cell membrane proteins markers, FACS was performed. As shown in Figure 2, hLB-MSCs cells were negative for the expression of CD3, CD4, CD5, CD11b, CD14, CD15, CD34, CD45, CD45RA, and HLA-DR (markers of endothelial/hematopoietic stem cell, granulocytes/macrophages, etc.), but were positive for the expression of CD13, CD29, CD90, CD105, and CD106, which are generally considered for markers of MSCs. These data confirmed that the isolated cells were MSCs.
FIG. 2.
Immunophenotypic characteristics of hLB-MSCs detected by flow cytometry. (A) Negative control. (B) Flow cytometry analysis showed that the hLB-MSCs stained homogeneously strong with markers for mesenchymal progenitors, such as CD13, CD29, CD90, CD105, and CD106. (C) The analyzed cells were negative for the markers of hematopoietic cells (CD34, CD45), as well as CD3, CD4, CD5, CD11b, CD14, CD15, and CD45RA.
Cell cycle in hLB-MSCs
The 7th- and 25th-passage cells of hLB-MSCs in doubling time were analyzed by FACS. For both passages, the DNA content of G2–M phase cells was two-fold that of G0–G1 phase cells, suggesting the karyotype was homogenic. Their curve form at either G0–G1 phase or G2–M phase was smooth without peak shoulders or false diploid. The proportions of S phase cells in 7th and 25th passage were 22.41% and 21.58%, respectively (Fig. 1E and F). The results indicated that hLB-MSCs from different passages have similar potential of propagation and could maintain a vigorous proliferating ability during long culture in vitro.
In vitro cell differentiation studies
Identification of adipogenic and osteogenic differentiation (mesodermal differentiation)
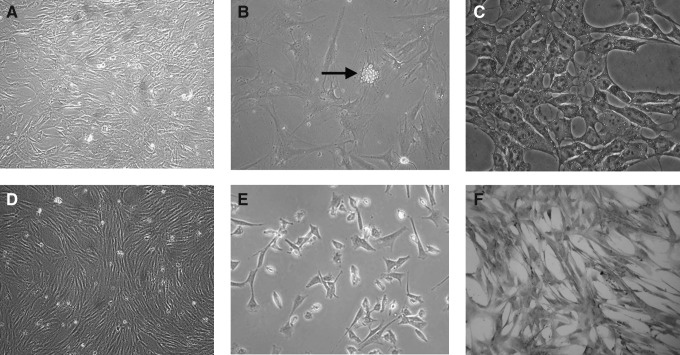
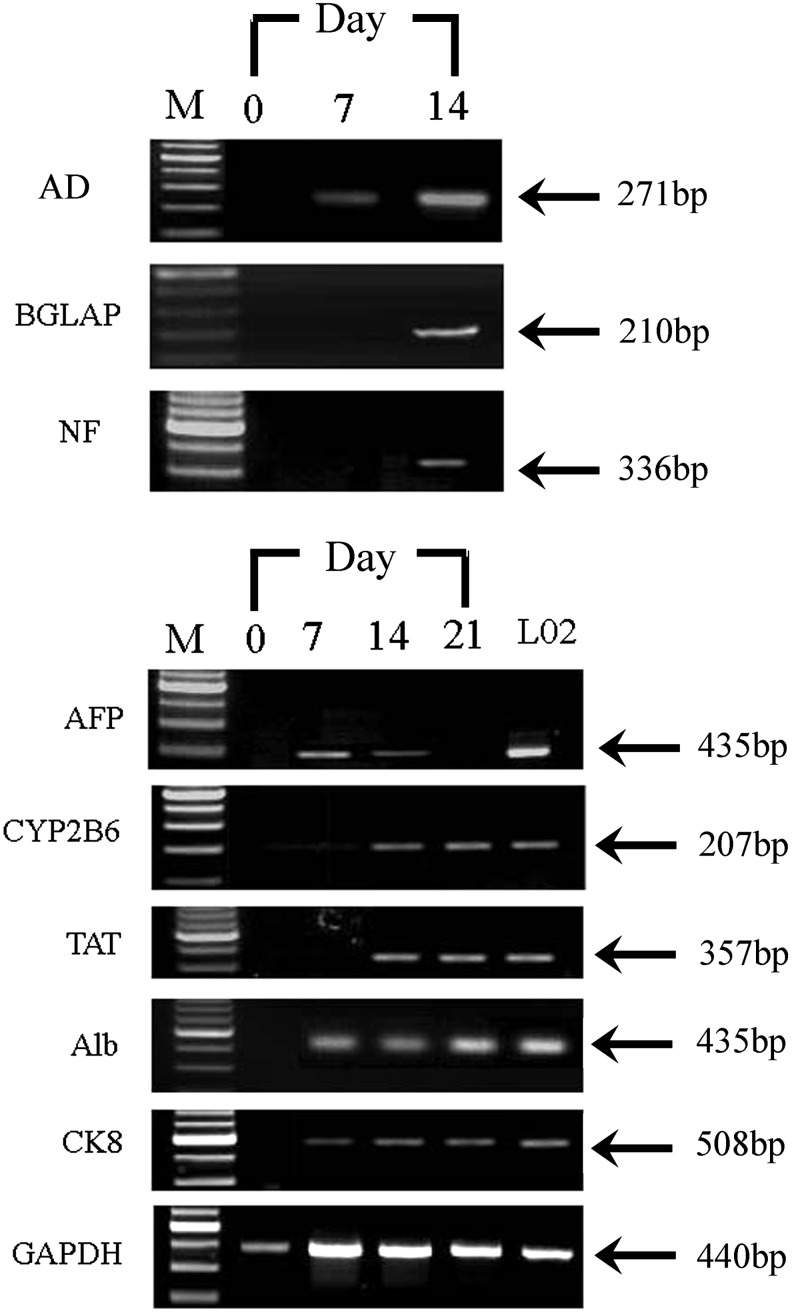
To evaluate the differentiation capability of fetal limb bud MSCs into various mesenchymal lineages, adipogenic and osteogenic differentiation protocols were performed by changing the induction medium as described above. For adipogenic differentiation, small fat droplets in the cytoplasm could be observed in the cytoplasm after 14 days of induction. Induced cells gradually became larger in an expanded cell morphology. Lipid droplets that stained red with Oil Red accumulated in the cytoplasm of positive cells, and the number of stained cells increased in a time-dependent manner (Fig. 3A –C). In contrast, no fat droplet was detected in the control. Expression of adipsin, a specific gene of adipocytes, was detected in induced cells by RT-PCR but undetectable in uninduced hLB-MSCs (see Fig. 5). These findings suggested that hLB-MSCs could be induced to differentiate into adipocyte-like cells.
FIG. 3.
The mesenchymal differentiation potential of hLB-MSCs. For adipogenic differentiation, small lipid droplets in the cytoplasm could be observed in the cytoplasm stained red with Oil Red after a 14 days of induction. (A) Untreated cells. (B) Phase-contrast photograph (black arrow represents lipid droplets in the cytoplasm). (C) Cells stained positively with Oil Red. For osteogenic differentiation, hLB-MSCs changed from a fibroblast to a cobblestone morphology stained positively for BGLAP. (D) Untreated cells. (E) Phase-contrast picture (cells lost their typical fibroblast appearance turning into a rounder, more cuboidal shape). (F) Cells stained positively with BGLAP.
FIG. 5.
Gene expression of tissue-specific genes in hLB-MSCs before and after differentiation. Specific genes were detected from the cDNA of undifferentiated and differentiated hLB-MSCs. Adipogenic differentiation was evaluated by expression of AD, which was detected as early as day 7. BGLAP, an osteogenic marker gene, was only expressed in the MSCs for osteogenic differentiation at day 14. Neuronal differentiation medium induced the expression of NF. For hepatogenic differentiation, AFP were detected in the early stage of induction. In contrast, TAT and CYP2B6 were positive only in the late maturation course. Regarding Alb and CK8, their expression was continued throughout the differentiated process. L02 cells were analyzed as a positive control for the hepatogenic lineage. The endogenous ‘‘housekeeping’’ gene GAPDH was used as an internal control.
For osteogenic differentiation, cells started to change morphologically as early as 5 days in an inducing culture medium. The cells lost their typical fibroblast appearance and showed a rounder, more cuboidal shape. The ability of hLB-MSCs to differentiate into osteocytes was demonstrated by staining for BGLAP. Intense black staining was detected between osteogenic cells (Fig. 3D–F). The cells cultured in the control medium were always negative for BGLAP. Expression of BGLAP, a specific gene of osteoblasts, was detected in induced cells by RT-PCR but undetectable in uninduced hLB-MSCs (see Fig. 5). These findings suggested that hLB-MSCs could be induced to differentiate into osteoblast-like cells.
Identification of neurogenic differentiation (ectodermal differentiation)
To determine whether hLB-MSCs contain the capablity to differentiate into neural lineages, the cells were induced based on the method of Vourc'h et al. (2004). Additional characterization of these differentiated cells was identified by specific staining and expression analysis of lineage-specific markers by RT-PCR and ICC. After exposure to neural induction medium, hLB-MSCs exhibited obvious morphological changes: they retracted their cytoplasm, formed a compact cell body, and exhibited multiple extensions. At day 14, the neural-like cells had a sharp and elongated shape with secondary branches that expressed the neural marker NF (Fig. 4A–C). The expression of NF was also detected by RT-PCR in the treated group (Fig. 5). In contrast, hLB-MSCs from untreated control cultures did not have any morphological changes and did not express the neural marker. These data suggested that hLB-MSCs had the potential of differentiate into neurocyte-like cells.
FIG. 4.
The extramesenchymal differentiation potential of hLB-MSCs. After exposure in neural induction medium for 14 days, the morphological appearance of hLB-MSCs changed dramatically. (A) Control cells growing in the regular medium. (B) Phase-contrast photograph (cells with retracted bodies, long processes, and networks after induction). (C) Positive cells stained with NF. For hepatogenic differentiation, hLB-MSCs were induced in appropriate induction medium for 21 days. (D) Untreated cells. (E and F) Phase-contrast photograph (cells showed the transition from fibroblast-like morphology to round or polygonal shape). (E) Magnification, 100×. (F) Magnification, 200×.
Identification of hepatogenic differentiation (endodermal differentiation)
To assess their hepatic differentiation potiential, hLB-MSCs were induced in the hepatogenic-differentiation medium described above. At the initial induction, cells started to shrink and turned from a long spindle shape into a short and thick shape. Cell morphology underwent a marked change with maintenance of induction. At day 7 of induction, 60% cells the cells began to show the transition from bipolar fibroblast-like morphology to a round or polygonal shape. With the continuous exposure to hepatic differentiating medium, most of the hLB-MSCs had turned into oval cells that were increasingly similar to hepatocytes in appearance at day 21 (Fig. 4D–F). We further confirmed the hepatic differentiation of induced cells using RT-PCR for specific markers of hepatocyte [alpha-fetoprotein (AFP), albumin (Alb), cytokeratin-8 (CK8), CYP2B6, and tyrosine aminotransferase (TAT)]. RT-PCR showed that the expression of AFP can be detected in the early stage of induction. In contrast, TAT and CYP2B6 were positive only in the late maturation course. Regarding Alb and CK8, their expression continued throughout the differentiation process (Fig. 5). The expression levels of these marker genes were either minimal or negligible in undifferentiated cells. These findings indicated that hLB-MSCs can be induced to differentiate into hepatocyte-like cells.
Discussion
Stem cells provide an available resource for the development of cell-based therapies and tissue repair in regenerative medicine (Fukuchi et al., 2004). However, some obvious limitations of specific stem cells from certain origins may impede their future clinical applications. For embryonic stem cells (ESCs), controversies about the possibility of tumorigenesis make them a less interesting candidate for practical therapy. Similarly, induced pluripotent stem cells (iPSCs), which are still at the experimental level, are in the same situation (Cooper and Viswanathan, 2011).
The plasticity, self-renewal, and multilineage potential of MSCs make them an attractive therapeutic tool for various kinds of diseases (Kennea et al., 2009). Since first reported, human MSCs have been obtained almost in every tissue. Because variability exists within MSC populations due to differences in the species, tissue source, and the in vitro culture conditions used, the International Society for Cellular Therapy (ISCT) released minimum criteria for defining MSCs. According to these standards, MSCs must be plastic-adherent during in vitro culture, express CD73, CD90, and CD105, and lack expression of the hematopoietic lineage markers CD45, CD34, and HLA-DR. Moreover, MSCs must differentiate into at least osteoblasts, adipocytes, and chondroblasts in vitro (Lange et al., 2005; Majore et al., 2009).
Until now the majority of literature about MSCs has concerned adult tissues, but some striking advantages of fMSCs have attracted more and more attention. First, a principal prerequisite for the use of MSCs for cell-based treatment is their proliferative capacity. It has been demonstrated that fMSCs are readily expandable in vitro, with population doublings every 24–30 h, compared to at best 48–72 h for their adult counterparts (Guillot et al., 2007; O'Donoghue and Chan, 2006). This greater proliferative capacity provides them with their rapid expansion in vitro. Consequently, sufficient amounts of therapeutic cells can be obtained during a short period. Second, findings confirmed that MSCs from fetal sources can also undergo a greater number of passages before they reach senescence than MSCs from adult tissue do (Klingemann et al., 2008). This means that characteristics of undifferentiated fMSCs can be maintained during the course of in vitro culture. The molecular mechanisms that contribute to the discrepancy of aging process between MSCs from fetal sources and MSCs from adult tissue are not clear. At least, on the basis of available data, the longer telomeres and greater telomerase activity in fetal MSCs may be one possible reason (Zhang et al., 2011). More recently, the differences between MSCs derived from fetal and adult tissues have been examined by analyzing their global gene expression profiles and epigenetic alterations (Frost et al., 2011; Götherström et al., 2005). The increased expression of transcripts promoting cell proliferation suggests that fMSCs have a growth advantage. Previous studies suggested that epigenetic regulation, such as DNA methylation and histone acetylation, can also influence the properties of MSCs significantly (Li et al., 2011; Zhang et al., 2009). It can be expected that the elucidation of biological mechanisms will make MSC-based medical treatments more encouraging in future.
MSCs differentiation into cell types of the mesodermal lineage has been extensively investigated (Ling et al., 2008). Accumulated data have demonstrated that, besides their mesodermal differentiation, MSCs can also differentiate into extramesenchymal lineages, such as muscle, neurons, hepatocytes, and insulin-producing cells (Bianco et al., 2008; Tamaki et al., 2007; Zheng et al., 2008). The capacity of adult stem cells to cross lineage boundaries and differentiate into cells of other tissues is termed plasticity. Some transcription factors involved in pluripotency, such as Oct4 and Nanog, were also deteted in fMSCs, implying that they may be have a number of primitive properties similar to hESCs. The high plasticity of fMSCs and their expression of pluripotency-related markers led to the speculation that they may be an intermediate phenotype between embryonic and adult stem cells (Liu et al., 2009; Pierantozzi et al., 2011). Despite controversy about their plasticity, especially in vivo, these advancements make it possible that a broader range of human diseases could be treated with fMSC-based therapy.
At present, there is little information about the characteristics and functional properties of MSCs in the fetus as early as first-trimester of gestation (Zhang et al., 2009; Zhang et al., 2011). The first report was from the study of Campagnoli et al. (2001), in which MSCs from first-trimester human fetal blood, liver, and bone marrow had been isolated (Campagnoli et al., 2001). Subsequently, it was confirmed that these human first-trimester fetal MSCs could considerably ameliorate skeletal pathology in a mouse model through intrauterine transplantation (Guillot et al., 2008). Similar to the previous studies, hLB-MSCs isolated in an early fetus in the present study have typical characteristics defined by ISCT, such as fibroblast-like morphology, the expression of MSC markers, and the ability to differentiate into mesodermal lineages. Findings in this study demonstrated that MSCs can also be isolated from other tissues in a first-trimester human fetus. Another noted finding is the transdifferentiation capacity of hLB-MSCs. We confirmed that hLB-MSCs can differentiated into neurogenic and hepatogenic linkages, which originate from the ectoderm and endoderm of embryos, respectively. For MSCs, the potential of differentiation into three germ layers of MSCs was reported more recently. After induction, decidua-derived mesenchymal stem cells (DMSCs) from human term placentas have successfully differentiated into linkages of mesoderm (osteoblasts, adipocytes, and chondroblasts), ectoderm (neurogenic cells), and endoderm (pulmonary cells) (Macias et al., 2010). For future investigations, there is high interest in better understanding the mechanisms underlying pluripotent-like characteristics of these cells.
In summary, we isolated and characterized typical MSCs from the limb bud of a first-trimester fetus. More importantly, in addition to traditional mesodermal differentiation, the isolated hLB-MSCs are also capable of a differentiating potential into neurocytes and hepatocytes. In the future, this early fetal tissue–derived MSCs can be used as novel seed cells for biomedical practice.
Acknowledgments
We greatly thank Prof. Hong-yang Wang (Second Military Medicine University, Shanghai) for experimental design and constructive suggestion. We also thank Dr. Jun Zhang (Changhai Hospital, Shanghai), Ms. Wei Zhang (Changhai Hospital, Shanghai), Dr. Shu-ping Zhao (Taian Central Hospital, Shandong), and Dr. Yang-fang Li (Chinese Academy of Medical Sciences, Beijing) for providing antibodies and instrumental and technological support. This work was partly supported by grants from the National Natural Science Foundation of China (No.31000564) and the Foundation of Shandong Educational Committee (No.J10LF12 and No.G08LG53).
Author Disclosure Statement
The authors declare that there is no conflict of interest that would prejudice the impartiality of this work.
References
- Alessandri G. Pagano S. Bez A., et al. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet. 2004;364:1872–1883. doi: 10.1016/S0140-6736(04)17443-6. [DOI] [PubMed] [Google Scholar]
- Aurich I. Mueller L.P. Aurich H., et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56:405–415. doi: 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P. Robey P.G. Simmons P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnoli C. Roberts I.A. Kumar S., et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- Chan J. O'Donoghue K. de la Fuente J., et al. Human fetal mesenchymal stem cells as vehicles for gene delivery. Stem Cells. 2005;23:93–102. doi: 10.1634/stemcells.2004-0138. [DOI] [PubMed] [Google Scholar]
- Chen J. Lu Z. Cheng D., et al. Isolation and characterization of porcine amniotic fluid-derived multipotent stem cells. PLoS One. 2011;6:e19964. doi: 10.1371/journal.pone.0019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. Viswanathan C. Establishment of a mesenchymal stem cell bank. Stem Cells Int. 2011;2011:905621. doi: 10.4061/2011/905621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C.G. Tang F.W. Zhang Q.J., et al. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant. 2005;14:311–321. doi: 10.3727/000000005783983070. [DOI] [PubMed] [Google Scholar]
- Filioli Uranio M. Valentini L. Lange-Consiglio A., et al. Isolation, proliferation, cytogenetic, and molecular characterization and in vitro differentiation potency of canine stem cells from foetal adnexa: A comparative study of amniotic fluid, amnion, and umbilical cord matrix. Mol. Reprod. Dev. 2011;78:361–373. doi: 10.1002/mrd.21311. [DOI] [PubMed] [Google Scholar]
- Frost J. Monk D. Moschidou D., et al. The effects of culture on genomic imprinting profiles in human embryonic and fetal mesenchymal stem cells. Epigenetics. 2011;6:52–62. doi: 10.4161/epi.6.1.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi Y. Nakajima H. Sugiyama D., et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- Götherström C. West A. Liden J., et al. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica. 2005;90:1017–1026. [PubMed] [Google Scholar]
- Guillot P.V. Gotherstrom C. Chan J., et al. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646–654. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- Guillot P.V. Abass O. Bassett J.H., et al. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood. 2008;111:1717–1725. doi: 10.1182/blood-2007-08-105809. [DOI] [PubMed] [Google Scholar]
- Hass R. Kasper C. Böhm S., et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. Liao L. Wang Q., et al. Isolation and identification of mesenchymal stem cells from human fetal pancreas. J. Lab. Clin. Med. 2003;141:342–349. doi: 10.1016/S0022-2143(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Hua J. Yu H. Dong W., et al. Characterization of mesenchymal stem cells (MSCs) from human fetal lung: Potential differentiation of germ cells. Tissue Cell. 2009;41:448–455. doi: 10.1016/j.tice.2009.05.004. [DOI] [PubMed] [Google Scholar]
- In't Anker P.S. Scherjon S.A. Kleijburg-van der Keur C., et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- Kennea N.L. Waddington S.N. Chan J., et al. Differentiation of human fetal mesenchymal stem cells into cells with an oligodendrocyte phenotype. Cell Cycle. 2009;8:1069–1079. doi: 10.4161/cc.8.7.8121. [DOI] [PubMed] [Google Scholar]
- Kestendjieva S. Kyurkchiev D. Tsvetkova G., et al. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol. Int. 2008;32:724–732. doi: 10.1016/j.cellbi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Klingemann H. Matzilevich D. Marchand J. Mesenchymal stem cells—Sources and clinical applications. Transfus. Med. Hemother. 2008;35:272–277. doi: 10.1159/000142333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai R.C. Choo A. Lim S.K. Derivation and characterization of human ESC-derived mesenchymal stem cells. Methods Mol. Biol. 2011;698:141–150. doi: 10.1007/978-1-60761-999-4_11. [DOI] [PubMed] [Google Scholar]
- Lange C. Schroeder J. Stute N., et al. High-potential human mesenchymal stem cells. Stem Cells Dev. 2005;14:70–80. doi: 10.1089/scd.2005.14.70. [DOI] [PubMed] [Google Scholar]
- Lee K.D. Kuo T.K. Whang-Peng J., et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- Li Z. Liu C. Xie Z., et al. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS One. 2011;6:e20526. doi: 10.1371/journal.pone.0020526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L. Ni Y. Wang Q., et al. Transdifferentiation of mesenchymal stem cells derived from human fetal lung to hepatocyte-like cells. Cell Biol. Int. 2008;32:1091–1098. doi: 10.1016/j.cellbi.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Liu S. Liu H. Pan Y., et al. Human embryonic germ cells isolation from early stages of post-implantation embryos. Cell Tissue Res. 2004;318:525–531. doi: 10.1007/s00441-004-0990-7. [DOI] [PubMed] [Google Scholar]
- Liu T.M. Wu Y.N. Guo X.M., et al. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev. 2009;18:1013–1022. doi: 10.1089/scd.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias M.I. Grande J. Moreno A., et al. Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am. J. Obstet. Gynecol. 2010;203:495.e9–495.e23. doi: 10.1016/j.ajog.2010.06.045. [DOI] [PubMed] [Google Scholar]
- Majore I. Moretti P. Hass R., et al. Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord. Cell Commun. Signal. 2009;7:6. doi: 10.1186/1478-811X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela-Arrieta C. Ritz J. Silberstein L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donoghue K. Chan J. Human fetal mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2006;1:371–386. doi: 10.2174/157488806778226768. [DOI] [PubMed] [Google Scholar]
- Pierantozzi E. Gava B. Manini I., et al. Pluripotency regulators in human mesenchymal stem cells: Expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 2011;20:915–923. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- Qiao C. Xu W. Zhu W., et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol. Int. 2008;32:8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Ramkisoensing A.A. Pijnappels D.A. Askar S.F., et al. Human embryonic and fetal mesenchymal stem cells differentiate toward three different cardiac lineages in contrast to their adult counterparts. PLoS One. 2011;6:e24164. doi: 10.1371/journal.pone.0024164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzhaninova A.A. Gornostaeva S.N. Goldshtein D.V. Isolation and phenotypical characterization of mesenchymal stem cells from human fetal thymus. Bull. Exp. Biol. Med. 2005;139:134–140. doi: 10.1007/s10517-005-0231-4. [DOI] [PubMed] [Google Scholar]
- Tamaki T. Okada Y. Uchiyama Y., et al. Clonal multipotency of skeletal muscle-derived stem cells between mesodermal and ectodermal lineage. Stem Cells. 2007;25:2283–2290. doi: 10.1634/stemcells.2006-0746. [DOI] [PubMed] [Google Scholar]
- Todorov P. Hristova E. Konakchieva R., et al. Comparative studies of different cryopreservation methods for mesenchymal stem cells derived from human fetal liver. Cell Biol. Int. 2010;34:455–462. doi: 10.1042/CBI20090127. [DOI] [PubMed] [Google Scholar]
- Tong C.K. Vellasamy S. Tan B.C., et al. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol. Int. 2011;35:221–226. doi: 10.1042/CBI20100326. [DOI] [PubMed] [Google Scholar]
- Vourc'h P. Romero-Ramos M. Chivatakarn O., et al. Isolation and characterization of cells with neurogenic potential from adult skeletal muscle. Biochem. Biophys. Res. Commun. 2004;317:893–901. doi: 10.1016/j.bbrc.2004.03.121. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Chu Y. Shen W., et al. Effect of 5-azacytidine induction duration on differentiation of human first-trimester fetal mesenchymal stem cells towards cardiomyocyte-like cells. Interact. Cardiovasc. Thorac. Surg. 2009;9:943–946. doi: 10.1510/icvts.2009.211490. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Shen W. Sun B., et al. Plasticity of marrow mesenchymal stem cells from human first-trimester fetus: From single-cell clone to neuronal differentiation. Cell Reprogram. 2011;13:57–64. doi: 10.1089/cell.2010.0044. [DOI] [PubMed] [Google Scholar]
- Zheng Y.B. Gao Z.L. Xie C., et al. Characterization and hepatogenic differentiation of mesenchymal stem cells from human amniotic fluid and human bone marrow: A comparative study. Cell Biol. Int. 2008;32:1439–1448. doi: 10.1016/j.cellbi.2008.08.015. [DOI] [PubMed] [Google Scholar]