Abstract
Objectives
No longitudinal studies have examined how iron measures change over menopause. Our objectives were to examine iron measures in individual women at premenopause and at postmenopause and, secondarily, to determine if any changes contributed to insulin resistance.
Methods
In a subset of participants (n=70) in a longitudinal study of menopause, we measured ferritin, transferrin, and soluble transferrin receptor (sTfR) once in the premenopause and once in the postmenopause. We also examined associations between menopausal status and change in iron markers after adjustment for age at menopause, race/ethnicity, and waist circumference. In linear regression models, we examined associations between premenopause iron measures and changes in iron markers over menopause with homeostasis model assessment of insulin resistance (HOMA-IR) changes over menopause, before and after adjustment for age at menopause, race/ethnicity, changes in waist circumference, C-reactive protein (CRP), and sex hormone-binding globulin (SHBG) levels.
Results
Women had lower ferritin (p<0.01), higher sTfR:ferritin levels (p<0.01), lower HOMA-IR (p=0.022), and lower glucose (p=0.05) in premenopause compared to postmenopause. After adjustment, lower premenopausal iron levels (sTfR:ferritin levels β=11.0, 95% confidence interval [CI] 0.017-22.0) and larger increases in iron over menopause (changes in sTfR:ferritin β=13.6, 95% CI 0.93-26.3) were associated with larger increases in HOMA-IR.
Conclusions
From premenopause to postmenopause, women on average have increases in measures of iron stores. Women who had the greatest changes in iron over menopause (lower measures of premenopausal iron and greater increases in iron measures over the menopause) had the strongest associations between changes in iron and changes in insulin resistance.
Introduction
Menopause, traditionally defined as cessation of menstrual bleeding, is assumed to lead to an increase in iron stores over the menopausal transition. However, studies examining iron measures by menopausal status or age have been cross-sectional,1–3 and to date, no longitudinal studies have examined whether menopause is actually associated with an increase in iron measures.
As women undergo the menopausal transition, they can experience increases in insulin resistance and other cardiovascular risk factors that are not fully explained by changes in adiposity or sex hormones.4 Iron, a dietary micronutrient and strong pro-oxidant, catalyzes reactions that cause increased oxidative stress, a risk factor for insulin resistance.5 Therefore, an increase in measures of iron over menopause could potentially contribute to women's increasing insulin resistance over menopause.
Several markers can represent iron stores. Ferritin, the primary cellular storage protein for iron, is also an acute-phase reactant and, thus, may overestimate iron stores in inflammatory states.6 Transferrin levels are typically used for diagnosis of iron overload rather than iron deficiency.7 Serum levels of soluble transferrin receptor (sTfR) reflect transferrin receptor not bound to transferrin, with low levels of sTfR reflecting higher levels of receptor saturation with iron and lower erythropoiesis.6 As sTfR is increased in iron deficiency anemia, and ferritin is low in iron deficiency anemia and elevated in anemia of chronic disease, the sTfR:ferritin ratio can be useful for further distinguishing women with elevated ferritin levels due to lack of iron as opposed to the inflammation associated with chronic disease.6
The Study of Women's Health Across the Nation (SWAN) was designed to characterize biologic and sociologic changes that occur during and after menopause.4 We measured several iron markers premenopause and postmenopause in order to ascertain (1) if measures of iron increase from premenopause to postmenopause within women longitudinally, (2) if insulin resistance and glucose increase from premenopause to postmenopause within women longitudinally, and (3) if premenopausal measures of iron and changes in measures of iron over menopause are associated with changes in insulin resistance and glucose over menopause, apart from other factors associated with insulin resistance, including changes in adiposity, inflammation,8 and sex hormone-binding globulin (SHBG).9
Materials and Methods
Participants
SWAN is a multisite, longitudinal cohort study of community-based groups of women.4,10,11 Eligibility criteria for entry into the SWAN longitudinal cohort were age of 42–52 years, intact uterus and at least one ovary, no current use of estrogens or other medications known to affect ovarian function, and at least one menstrual period in the 3 months before screening. Participants were enrolled at one of the seven clinic sites, one of which was located in the Detroit area. This report is based on data from Michigan SWAN site women.4 SWAN is approved by each local Institutional Review Board (IRB), and the reported study was approved by the University of Michigan IRB.
For the purposes of this report, inclusion criteria were natural menopause as opposed to menopause due to hysterectomy or oophorectomy and known date of final menstrual period. The sample size of 70 participants was based on estimates of the differences in ferritin observed in cross-sectional studies between premenopausal and postmenopausal women.2,3 Women were defined as postmenopausal if they had no menses for ≥12 months. We excluded women who were using estrogen or progestin therapy at the premenopausal or postmenopausal visit and who had diabetes by self-report at any examination, as changes in insulin resistance and glucose in this population might be affected by subsequent medication use and behavior modification. Women were selected at random and were of similar age, race/ethnicity, and anthropometric size compared to Michigan SWAN women without diabetes who had undergone natural menopause.
Data collection
SWAN annually collects survey data regarding menstrual status and medical and surgical history from questionnaires, as well as examination data for anthropometrics. Phlebotomy was performed in the morning after an overnight fast and blood was refrigerated 1–2 hours after phlebotomy; after centrifugation, the serum was aliquoted and frozen. Serum was stored at −70°C. Insulin was measured in serum by solid phase radioimmunoassay (Coat-A-Count, Diagnostics Product Corp., Los Angeles, CA), and glucose was measured using a hexokinase-coupled reaction (Roche Molecular Biochemicals Diagnostics, Indianapolis, IN). Insulin resistance was estimated using homeostasis model assessment of insulin resistance (HOMA-IR), defined as:
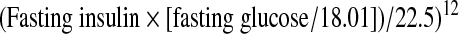 |
High-sensitivity C-reactive protein (CRP) was quantitated using an ultrasensitive rate immunonephrelometric method (hs-CRP, Dade-Behring, Marburg, Germany). SHBG was a de novo two-site chemiluminescent assay, with intra-assay coefficients of 9.9% and 6.1%.
Michigan-specific archival specimens were used for measurement of iron markers in the Rifai Laboratory (Boston, MA).13 Ferritin was measured by a particle-enhanced immunoturbidometric assay (Hitachi 917 analyzer (Roche Diagnostics) and Kamiya Biomedical reagents, Seattle, WA). The day-to-day variability of ferritin at the concentrations of 37.9 ng/mL and 261 ng/mL were 2.5% and 2.6%, respectively. sTfR was measured by a particle-enhanced immunoturbidometric assay using the Hitachi 917 analyzer and Roche Diagnostics reagents. The day-to-day variability of sTfR at concentrations of 2.25 mg/L and 7.00 mg/L were 2.2% and 1.4%, respectively. Transferrin, measured by an immunoturbidometric assay (Hitachi 917 analyzer and Roche Diagnostics reagents) had a day-to-day variability of 2.6% and 2.4% at concentrations of 167 mg/dL and 249 mg/dL, respectively.
Statistical analyses
First, we compared iron measures premenopause and postmenopause using Wilcoxon nonparametric tests and McNemar's tests for categorical variables. Next, we created stepwise linear regression models that examined change in iron measures with menopause, adjusting for age of menopause, time between premenopausal measurement and postmenopausal measurement, race/ethnicity, and change in waist circumference over the menopausal transition. Separate models were created for each iron measure. Stepwise linear regression models examined the association between changes in iron with changes in HOMA-IR.
In additional analyses, models were constructed using log-transformed and nontransformed measures of iron and HOMA-IR, but the findings were similar, and we report the findings from nontransformed models for ease of interpretation. We also created models where women were categorized as being iron sufficient or insufficient, and we observed a similar pattern in results (not shown). We examined time from menopause, which was highly correlated with age at menopause, and observed a similar pattern of results (not shown). Finally, we conducted analyses where change in waist circumference was forced into the model regardless of whether or not it met stepwise criteria for significance. The SAS analysis system was used for all analyses (SAS Institute, Cary, NC).
Results
Participant characteristics are illustrated in Table 1. On average, 5 years had elapsed between the time of each woman's premenopausal iron measurement and postmenopausal iron measurement. The mean age at natural menopause was 50.9 (±2.9) years, and follicle-stimulating hormone (FSH) levels were 13.1 (±11.8) mIU/mL in premenopause and 74.9 (±50.1) mIU/mL in postmenopause. Forty-one percent of women were Caucasian and 59% were African Americans. Women gained a mean 2.3 (±6.7) kg between their premenopausal and postmenopausal assessment and also had a significant increase in body mass index (BMI) and waist circumference. Women had worsened insulin resistance, as evidenced by the increases in their HOMA-IR from premenopause to postmenopause (p=0.02).
Table 1.
Characteristics of Women at Premenopause and Postmenopause Time Points (n=70)
| Characteristic | Premenopause | Postmenopause | |
|---|---|---|---|
| Age (years) | 47.7 (3.0) | 53.3 (2.9) | <0.01 |
| Age at natural menopause (years) | 50.9 (2.9) | ||
| Waist circumference (cm) | 99.8 (19.1) | 101.5 (18.6) | <0.01 |
| Body mass index (kg/m2) | 34.3 (9.2) | 34.5 (9.6) | <0.01 |
| Weight (kg) | 89.6 (23.4) | 90.0 (22.2) | <0.01 |
| Ferritin (ng/mL) | 69.5 (81.7) | 128.8 (125.7) | <0.01 |
| Change in ferritin (ng/mL) | 58.2 (104.1) | ||
| Ferritin<100 ng/mL (%) (iron insufficient) | 78.6% | 55.7% | <0.01 |
| Ferritin<15 ng/mL (%) (iron insufficient) | 12.9% | 0 | |
| sTfR (mg/L) | 3.95 (2.69) | 3.57 (1.32) | 0.95 |
| sTfR:ferritin (mean) (SD) | 0.197 (0.452) | 0.0598 (0.079) | <0.01 |
| Change in sTfR:ferritin | −0.14 (0.44) | ||
| sTfr:ferritin>1 (%) (iron insufficient) | 95.7% | 92.9% | 0.038 |
| sTfr:ferritin>2 (%) (iron insufficient) | 91.4% | 77.1% | |
| Transferrin (mg/dL) | 278.5 (44.4) | 268.3 (36.7) | 0.055 |
| Change in transferrin (mg/dL) | −10.2 (37.8) | ||
| Fasting glucose (mg/dL) | 106.5 (48.3) | 109.0 (51.1) | 0.05 |
| Change in fasting glucose (mg/dL) | 1.2 (50.8) | ||
| HOMA-IR | 4.14 (4.01) | 5.21 (4.56) | 0.022 |
| Change in HOMA-IR | 0.92 (3.68) | ||
| Sex hormone-binding globulin (SHBG) (nmol/L) | 42.8 (21.1) | 43.1 (21.6) | 0.87 |
| C-reactive protein (mg/L) | 7.0 (10.9) | 7.2 (7.6) | 0.18 |
Mean (standard deviation [SD]) or percentages shown.
HOMA-IR, homeostasis model assessment of insulin resistance; sTfR, soluble transferrin receptor.
In unadjusted comparisons, women had significantly lower ferritin and higher sTfR:ferritin levels at premenopause than at postmenopause, consistent with lower iron measures in the premenopause. In contrast, transferrin levels were slightly lower in postmenopause, but this result was not statistically significant (p=0.055) (Table 1). In adjusted analyses, no variables except for menopause status were significantly associated with iron status; postmenopause was associated with an increase in ferritin (p=0.049) and a decrease in sTfR:ferritin (p<0.01) but not a significant change in transferrin (p=0.13).
In adjusted analyses, lower premenopausal iron measures (as indicated by higher levels of premenopausal sTfR:ferritin) and greater increases in iron measures (as indicated by greater decreases in sTfR:ferritin) over the menopausal transition were significantly associated with increases in HOMA-IR from premenopause to postmenopause (Table 2). In other words, larger increases in iron status were associated with larger increases in HOMA-IR. Similar models substituting transferrin and ferritin for sTfR:ferritin did not show significant associations between these iron measures and change in HOMA-IR (Table 2).
Table 2.
Regression Coefficients and 95% Confidence Intervals in Stepwise Models Examining Associations Between Iron (Independent Variable) and Changes in Insulin Resistance and Glucose (Dependent Variables)
| sTfR:ferritin | |
| Model 1a | Association with change in HOMA-IR |
| Premenopausal sTfR:ferritin | 11.0 (0.017-22.0) |
| Change in sTfR:ferritin over menopause | 13.6 (0.93-26.3) |
| Premenopausal sTfR:ferritin * change in sTfR:ferritin | −1.08 (−3.12-0.95) |
| Race/ethnicity | −1.81 (−3.70-0.079) |
| Intercept (menopausal status) | 0.47 (−0.15-3.09) |
| Model 1b | Association with change in glucose |
| Premenopausal sTfR:ferritin | 14.1 (−137.1-165.3) |
| Change in sTfR:ferritin over menopause | 132.1 (−42.6-306.7) |
| Premenopausal sTfR:ferritin * change in sTfR:ferritin | −34.5 (−62.6-−6.4) |
| Race/ethnicity | −14.7 (−40.3-11.0) |
| Intercept (menopausal status) | 17.06 (−4.75-38.88) |
| Ferritin | |
| Model 2a | Association with change in HOMA-IR |
| Premenopausal ferritin | −0.007 (−0.018-0.004) |
| Change in ferritin over menopause | 0.004 (−0.008-0.016) |
| Premenopausal ferritin * change in ferritin | 0.00001 (−0.00006-0.00008) |
| Race/ethnicity | −2.21 (−4.16-−0.26) |
| Intercept (menopausal status) | 2.48 (0.87-4.09) |
| Model 2b | Association with change in glucose |
| Premenopausal ferritin | 0.033 (−0.12-0.19) |
| Change in ferritin over menopause | −0.022 (−0.20-0.16) |
| Premenopausal ferritin * change in ferritin | 0.0003 (−0.0007-0.0013) |
| Race/ethnicity | −22.28 (−50.3-5.7) |
| Intercept (menopausal status) | 12.80 (−10.3-35.9) |
| Transferrin | |
| Model 3a | Association with change in HOMA-IR |
| Premenopausal transferrin | 0.018 (−0.010-0.047) |
| Change in transferrin over menopause | −0.0004 (−0.13-0.13) |
| Premenopausal transferrin* change in transferrin | 0.000001 (−0.0004-0.0004) |
| Race/ethnicity | −2.29 (−4.18-−0.40) |
| Intercept (menopausal status) | −2.78 (−10.8-5.19) |
| Model 3b | Association with change in glucose |
| Premenopausal transferrin | 0.053 (−0.35-0.45) |
| Change in transferrin over menopause | −0.84 (−2.66-0.99) |
| Premenopausal transferrin * change in transferrin | 0.003 (−0.003-0.009) |
| Race/ethnicity | −23.21 (−49.94-3.52) |
| Intercept (menopausal status) | 3.73 (−106.4-113.8) |
Significant associations are shown in bold type.
In sensitivity analyses, menopause was still associated with a significant increase in ferritin when waist circumference was forced into the model (p=0.046), but there was not a significant change in sTfR:ferritin (p=0.15) or transferrin (p=0.70), suggesting changes in iron with menopause were independent of changes in adiposity. Similarly, the associations between lower premenopausal sTfR:ferritin levels (13.0, p=0.03) and changes in sTFR:ferritin (15.1, p=0.02) were still significant when waist circumference was forced into the models. Addition of waist circumference to the models did not change the association between ferritin and transferrin (premenopausal levels or change in levels) with HOMA-IR (results not shown). We also examined time since menopause and did not find significant associations with iron measures, although only a few years had elapsed since menopause in our sample.
Discussion
We found that from premenopause to postmenopause, ferritin increased and sTfR:ferritin decreased, consistent with an increase in iron stores. We also found that insulin resistance and glucose increased over the menopausal transition. Women who had the largest changes in their iron (lower measures of iron when they were premenopausal and larger increases in iron measures over the menopausal transition) demonstrated the strongest associations between iron and changes in HOMA-IR. To our knowledge, ours is the only longitudinal study examining changes in iron measures over the menopause and, further, the only longitudinal study examining associations with insulin resistance in a community-based population. These findings suggest yet another pathway through which women undergoing the menopausal transition may be at risk for insulin resistance, in addition to unfavorable lifestyle changes14 and the increasing ratio of androgen:estrogen characterizing menopause.15
Previous cross-sectional studies have shown that reproductive-aged women have lower iron markers than middle-aged and elderly women.1–3 In one population-based study, the prevalence of iron deficiency was 11% (95% confidence interval [CI] 10%-13%) in women aged 20–49 years and 5% (95% CI 4%-7%) in women aged 50–69 years.3 Such increases in iron are presumably due in part to cessation of menstrual bleeding; among premenopausal Danish women, the primary determinant of iron measures was self-reported duration of menstrual bleeding.2 Another factor contributing to an increase in iron stores included adiposity.16 In our study, adjustment for the increase in waist circumference diminished the association between menopause and change in sTfR:ferritin, although not between menopause and ferritin levels alone.
Previous observations have suggested that changes in iron measures over the menopausal transition contribute to increased insulin resistance over the transition. First, among cross-sectional1,17,18 and prospective19 studies of healthy postmenopausal women, postmenopausal ferritin levels are associated with increased odds of the metabolic syndrome. The same association is not consistently observed in premenopausal women,1,19 presumably because of the lower ferritin levels among premenopausal women. Second, greater levels of iron have been associated with increased risk of diabetes in nested case-control studies of healthy women without iron overload disorders or metabolic disorders, such as polycystic ovarian syndrome (PCOS).13,20,21 Third, among premenopausal women with PCOS, a hyperandrogenic and hyperinsulinemic syndrome affecting approximately 3% of the U.S. female population,22 measures of ferritin are elevated compared to healthy women.23 Finally, the elevated measures of iron observed in iron overload disorders are associated with greater diabetes risk.5
Although explanations for the associations between insulin resistance and iron are speculative, inflammation as represented by ferritin may increase insulin resistance24; however, we found that measures of CRP, another inflammatory marker, did not increase over menopause. Hepcidin, a peptide hormone produced by the liver, inhibits intestinal iron uptake and is decreased among premenopausal women with PCOS.23 Thus, insulin resistance may have contributed to increased iron stores among postmenopausal women rather than vice-versa. In our healthy postmenopausal population, however, menopause was strongly associated with increases in iron stores, and among healthy postmenopausal women,25 the relationships between hepcidin and iron absorption may differ from those reported in premenopausal women. Finally, ferritin and other iron markers have also been associated with elevated liver function tests, particularly alanine aminotransferase, which represents nonalcoholic fatty liver disease and increased risk of insulin resistance.17 Therefore, the relationship between iron and insulin resistance may also be confounded or mediated by fatty liver disease.
We found that the association between iron measures and HOMA-IR was significant when iron was represented by sTfR:ferritin but not by ferritin alone or transferrin alone. Two of the nested case-control studies13,26 examined a single measure of sTfR:ferritin and found associations with future diabetes. Of note, we did not find large changes in the serum sTfR levels between premenopause and postmenopause, suggesting that the aspect of iron metabolism that may have changed from premenopause to postmenopause was not erythropoiesis alone.
Strengths of our report include its random selection from a longitudinal community-based study. Limitations include its small sample size. We used proxy measures for iron and did not have bone marrow biopsy confirmation of iron stores. Similarly, SWAN used proxy measures for insulin resistance, such as HOMA-IR, as is common in large epidemiologic studies, rather than insulin clamp measurements. The cohort was relatively young; and it is possible that as the cohort ages and their time since menopause increases, their iron stores will increase even more and that iron associations with HOMA-IR may become more pronounced. We did not measure additional markers that may have helped us delineate the mechanisms through which iron might increase insulin resistance, specifically hepcidin and markers of nonalcoholic fatty liver disease. Finally, this study is observational and cannot demonstrate cause and effect between iron and insulin resistance.
In conclusion, we observed changes in iron markers consistent with increased iron stores over the menopause. In addition, we observed small increases in insulin resistance over the menopause and associations between iron measures and insulin resistance. Women who had the largest changes in iron measures, that is, lower iron in the premenopause and larger increases over the transition, had the strongest associations between changes in iron and changes in HOMA-IR. Given the increasing number of menopausal women and the fact that iron is a modifiable risk factor, the contribution of iron to insulin resistance should be replicated in larger studies before and after consideration of additional confounders, particularly liver metabolism, adipocytokines, and oxidative stress.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women's Health (ORWH) (grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). This work was also supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K23DK071552, Michigan Institute for Clinical Health Research (MICHR), and the University of Michigan Center for Integrated Approaches to Complex Diseases, School of Public Health.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Cho G. Shin J. Yi K, et al. Serum ferritin levels are associated with metabolic syndrome in postmenopausal women but not in premenopausal women. Menopause. 2011;18:1120–1124. doi: 10.1097/gme.0b013e318217e172. [DOI] [PubMed] [Google Scholar]
- 2.Milman N. Byg K. Ovesen L. Kirchhoff M. Jurgensen K. Iron status in Danish women, 1984–1994: A cohort comparison of changes in iron stores and the prevalence of iron deficiency and iron overload. Eur J Haematol. 2003;71:51–61. doi: 10.1034/j.1600-0609.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 3.Looker A. Dallman P. Carroll M. Gunter E. Johnson C. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 4.Sowers M. Derby C. Jannausch M. Torrens J. Pasternak R. Insulin resistance, hemostatic factors, and hormone interactions in pre- and perimenopausal women: SWAN. J Clin Endocrinol Metab. 2003;88:4904–4910. doi: 10.1210/jc.2003-030350. [DOI] [PubMed] [Google Scholar]
- 5.Phelps G. Chapman I. Hall P. Braund W. Mackinnon M. Prevalence of genetic haemochromatosis among diabetic patients. Lancet. 1989;2:233–234. doi: 10.1016/s0140-6736(89)90426-1. [DOI] [PubMed] [Google Scholar]
- 6.Punnonen K. Irjala K. Rajamaki A. Serum trasnferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052. [PubMed] [Google Scholar]
- 7.Guyatt G. Oxman A. Ali M. Willan A. McIlroy W. Patterson C. Laboratory diagnosis of iron-deficiency anemia: An overview. J Gen Intern Med. 1992;7:145–153. doi: 10.1007/BF02598003. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan A. Manson J. Rifai N. Buring J. Ridker P. C-reactive protein, interleukin-6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 9.Ding E. Song Y. Manson J, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton-Tyrrell K. Wildman R. Matthews K, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 11.Sowers M. McConnell D. Jannausch M, et al. Oestrogen metabolites in relation to isoprostanes as a measure of oxidative stress. Clin Endocrinol (Oxf) 2008;68:806–813. doi: 10.1111/j.1365-2265.2007.03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson R. Pratley R. Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 13.Jiang R. Manson J. Meigs J. Ma J. Rifai N. Hu F. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA. 2004;291:711–717. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- 14.Sternfeld B. Wang H. Quesenberry C, Jr, et al. Physical activity and changes in weight and waist circumference in midlife women: Findings from the Study of Women's Health Across the Nation. Am J Epidemiol. 2004;160:912–922. doi: 10.1093/aje/kwh299. [DOI] [PubMed] [Google Scholar]
- 15.Torrens J. Sutton-Tyrrell K. Zhao X, et al. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: Study of Women's Health Across the Nation. Menopause. 2009;16:257–264. doi: 10.1097/gme.0b013e318185e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J. Hankinson S. Stampfer M. Rifai N. Willett W. Ma J. Body iron stores and their determinants in healthy postmenopausal U.S. women. Am J Clin Nutr. 2003;78:1160–1167. doi: 10.1093/ajcn/78.6.1160. [DOI] [PubMed] [Google Scholar]
- 17.Choi K. Lee K. Kim H, et al. Association among serum ferritin, alanine aminotransferase levels, and metabolic syndrome in Korean postmenopausal women. Metabolism. 2005;54:1510–1514. doi: 10.1016/j.metabol.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Sun L. Franco O. Hu F, et al. Ferritin concentrations, metabolic syndrome, and type 2 diabetes in middle-aged and elderly Chinese. J Clin Endocrinol Metab. 2008;93:4690–4696. doi: 10.1210/jc.2008-1159. [DOI] [PubMed] [Google Scholar]
- 19.Vari I. Balkau B. Kettaneh A, et al. Ferritin and transferrin are associated with metabolic syndrome abnormalities and their change over time in a general population: Data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2007;30:1795–1801. doi: 10.2337/dc06-2312. [DOI] [PubMed] [Google Scholar]
- 20.Forouhi N. Harding A. Allison M, et al. Elevated serum ferritin levels predict new-onset type 2 diabetes: Results from the EPIC-Norfolk prospective study. Diabetologia. 2007;50:949–956. doi: 10.1007/s00125-007-0604-5. [DOI] [PubMed] [Google Scholar]
- 21.Salonen J. Tuomainen T. Nyyssonnen K. Lakka H. Punnonen K. Relation between iron stores and non-insulin-dependent diabetes in men: Case-control study. BMJ. 1998;317:727. doi: 10.1136/bmj.317.7160.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo J. Feigenbaum S. Yang J. Pressman A. Selby J. Go A. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1357–1363. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 23.Luque-Ramirez M. Alvarez-Blasco F. Alpanes M. Escobar-Morreale H. Role of decreased circulating hepcidin concentrations in the iron excess of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96:846–852. doi: 10.1210/jc.2010-2211. [DOI] [PubMed] [Google Scholar]
- 24.Chen X. Scholl T. Stein T. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women. Diabetes Care. 2006;29:1077–1082. doi: 10.2337/diacare.2951077. [DOI] [PubMed] [Google Scholar]
- 25.Huang X. Fung E. Yip C. Zeleniuch-Jacquotte A. Serum prohepcidin is associated with soluble transferrin receptor-1 but not ferritin in healthy postmenopausal women. Blood Cell Mol Dis. 2008;41:265–269. doi: 10.1016/j.bcmd.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajpathak S. Wylie-Rosett J. Gunter M, et al. Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes Metab. 2009;11:472–479. doi: 10.1111/j.1463-1326.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


