Abstract
The rabbit is a classical experimental animal species. A major limitation in using rabbits for biomedical research is the lack of germ-line-competent rabbit embryonic stem cells (rbESCs). We hypothesized that the use of homologous feeder cells and recombinant rabbit leukemia inhibitory factor (rbLIF) might improve the chance in deriving germ-line-competent rbES cells. In the present study, we established rabbit embryonic fibroblast (REF) feeder layers and synthesized recombinant rbLIF. We derived a total of seven putative rbESC lines, of which two lines (M5 and M23) were from culture Condition I using mouse embryonic fibroblasts (MEFs) as feeders supplemented with human LIF (hLIF) (MEF+hLIF). Another five lines (R4, R9, R15, R21, and R31) were derived from Condition II using REFs as feeder cells supplemented with rbLIF (REF+rbLIF). Similar derivation efficiency was observed between these two conditions (8.7% vs. 10.2%). In a separate experiment with 2×3 factorial design, we examined the effects of feeder cells (MEF vs. REF) and LIFs (mLIF, hLIF vs. rbLIF) on rbESC culture. Both Conditions I and II supported satisfactory rbESC culture, with similar or better population doubling time and colony-forming efficiency than other combinations of feeder cells with LIFs. Rabbit ESCs derived and maintained on both conditions displayed typical ESC characteristics, including ESC pluripotency marker expression (AP, Oct4, Sox2, Nanog, and SSEA4) and gene expression (Oct4, Sox2, Nanog, c-Myc, Klf4, and Dppa5), and the capacity to differentiate into three primary germ layers in vitro. The present work is the first attempt to establish rbESC lines using homologous feeder cells and recombinant rbLIF, by which the rbESCs were derived and maintained normally. These cell lines are unique resources and may facilitate the derivation of germ-line-competent rbESCs.
Introduction
Mammalian embryonic stem cells (ESCs) are pluripotent cells derived from the inner cell mass (ICM) of blastocysts (Evans and Kaufman, 1981; Martin, 1981; Thomson et al., 1998). These cells are characterized by unlimited self-renewal and the ability to undergo differentiation into all cell lineages, such as cardiomyocytes (Doss et al., 2008), hepatocytes (Ochiya et al., 2010), adipocytes (Chen et al., 2007), and germ cells (Zhou et al., 2010), making them an attractive resource for the generation of cells that could be used for cell-based transplantation therapies (Intawicha et al., 2009; Volarevic et al., 2011). The ability to maintain ESCs in long-term culture provides a time window to modify the genome of these cells by homologous recombination (HR) (e.g., gene knockout). Genetically modified cells can then be injected into a blastocyst to generate chimeric offspring in animal research (Gama Sosa et al., 2010). If the ESCs are germ-line competent (i.e., contributing to the germ-line cells), transgenic animals can be produced via this approach.
The rabbit is a classical model animal species (Fan and Watanabe, 2003; Intawicha et al., 2009; Lin et al., 2011). It has a short gestation period (30–31 days), large litter size (4–12/litter), and can be housed conveniently in an indoor facility. Compared to mice, rabbits are phylogenetically closer to humans (Fan and Watanabe, 2003). Because of the anatomical, physiological, genetic, and biochemical similarities between the rabbit and the human, this species is preferentially used in pulmonary, cardiovascular, and metabolic studies (Fan and Watanabe, 2003).
ESC technology has developed dramatically in the past decade. Stable ESC lines have been established in many mammalian species, including mouse (Evans and Kaufman, 1981), rat (Buehr et al., 2008; Li et al., 2008), monkey (Takada et al., 2002; Thomson et al., 1995), dog (Vaags et al., 2009), cattle (Wang et al., 2005), and human (Thomson et al., 1998). Despite all of these achievements and efforts, only murine and rat ESCs have successfully transmitted to the germ line (Buehr et al., 2008; Evans and Kaufman, 1981; Li et al., 2008). The lack of germ-line-competent rabbit ESCs (rbESCs) has been recognized as a major limitation in using rabbits for biomedical research and production (Fang et al., 2006; Graves and Moreadith, 1993; Honda et al., 2008; Intawicha et al., 2009; Wang et al., 2007). There is a need to optimize current derivation and culture procedures for rbESCs so that the qualities of the cells can be improved, with an ultimate goal to accomplish germ-line transmission.
The application of feeder cells and leukemia inhibitory factor (LIF), both of which are of mouse origin, is considered to be the technical breakthrough in establishing authentic murine ES cells (Evans and Kaufman, 1981; Martin, 1981; Pease et al., 1990). Besides mouse, homologous feeder cells were reported to support the establishment and culture of human and monkey ESCs, with similar characteristics of those cultured on mouse embryonic fibroblasts (MEFs) (Li et al., 2005; Richards et al., 2002). Recently, a study on cat ESCs (cESC) revealed that homologous feeder cells provided better cell support for the initial attachment of ICMs, generated higher number of cESCs, and maintained these cells in the undifferentiated status longer than MEF cells did (Gomez et al., 2010). Similar findings were reported in buffalo ESC derivation (Kumar et al., 2011). On the other hand, it is known that human leukemia inhibitory factor (hLIF) binds to both human and mouse LIF receptors (LIFRs), whereas mLIF binds only to mouse but not human LIFRs (Owczarek et al., 1997), suggesting differential cross-reactivity and responsiveness of ESCs to LIF of different species origins. However, although mLIF has been utilized on rbESC studies (Intawicha et al., 2009), the effect of rbLIF on rbESC derivation has not been examined.
Pluripotent rbESC lines were established more than 10 years ago (Graves and Moreadith, 1993), but there has been no report on successful derivation and maintenance of rbESC lines using rabbit embryonic fibroblasts (REFs) or rabbit LIF (rbLIF). Previously, most rbESC lines were established on MEFs in the presence of mLIF or hLIF (Fang et al., 2006; Graves and Moreadith, 1993; Honda et al., 2008; Intawicha et al., 2009; Wang et al., 2007). These rbESC lines were derived from fertilized, parthenogenetically activated, as well as cloned embryos. Most of these cell lines were capable of self-renewal, remained undifferentiated in culture, formed embryoid bodies (EBs) containing all three primary germ layers upon induction, and generated positive teratomas after transferring to immunocompromised mice. Following injection into blastocysts and embryo transfer, some of these rbESCs formed coat color chimeras; unfortunately none of them colonized into the germ line.
We hypothesize that the application of rbLIF and REFs will improve the quality of rbESCs and increase our chance in obtaining germ-line-competent rbESC lines. As the first step to test this hypothesis, the objectives of the present work were to: (1) establish REF feeder layer; (2) synthesize recombinant rbLIF; and (3) derive, maintain and characterize rbES cell lines using REF and rbLIF.
Materials and Methods
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless otherwise indicated.
Animal maintenance, hormone administration, and embryo collection
All animal maintenance, care, and use procedures were reviewed and approved by the University Committee on the Use and Care of Animals (UCUCA) of the University of Michigan. Sexually mature (6–18 months old) New Zealand White (NZW) female rabbits were superovulated using our routine regime (Du et al., 2009), consisting of two 0.3-mg, two 0.4-mg, and two 0.5-mg administrations of follicle-stimulating hormone (FSH; Folltropin-V, Bioniche Animal Health Canada, Belleville, Ontario, Canada) at intervals of 12 h, followed by 200 IU of human choriogonadotropin (hCG; Chorulon, Intervet Inc, Millsboro, DE, USA). Superovulated does were mated with fertile males and served as embryo donors.
Embryo collection and culture was described previously (Lin et al., 2011). Dulbecco's phosphate-buffered saline (DPBS; 15240-013, Gibco, Grand Island, NY, USA) containing 0.1% polyvinyl alcohol (PVA; P-8136) was used for flushing embryos from oviducts (PBS-PVP). Medium-199 (M-199) with Earle's salts, l-glutamine, 2.2 g/L sodium bicarbonate, and 25 mM HEPES (12340-014, Gibco) supplemented with 10% fetal bovine serum (FBS; SH0070.03, Hyclone, Logan, UT, USA) was used as the standard manipulation medium. Zygotes were collected at 18–20 h postinsemination (hpi) from embryo donors, and cultured in B2 medium (Laboratories CCD, Paris, France) supplemented with 2.5% FBS at 38.5°C in 5% CO2 and humidified air. Blastocyst-stage embryos were collected for ESC derivation.
Preparation of REF feeder cells
REF cells were prepared similarly as in the mouse (Roach and McNeish, 2002). Briefly, female NZW rabbits were mated. The fetuses were collected on 15–16 days of gestation. This is about one-half of full gestation length. Tissues were minced, trypsinized, and used to establish cell culture in DMEM supplemented with 10% FBS. Rabbit embryonic fibroblast cell lines were established. Cells at passages 2–3 were used for establishing REF feeder cells. When these cultures were approximately 80% confluent, medium was removed and replaced with culture medium supplemented with mitomycin C (M-0503) at 10 μg/mL and incubated for 3 h. After treatment, the mitomycin C solution was removed and cells were washed three times. To measure DNA synthesis, cells were labeled with 5-bromo-2′-deoxyuridine (BrdU) using a commercial Kit (2750, Millipore, Billerica, MA, USA) as described previously (Gomez et al., 2010). For cytotoxicity, cells were labeled with a dual fluorescent stain (calcein and ethidium homodimer-1) using a commercial kit (L3224, Invitrogen, Carlsbad, CA, USA) to evaluate membrane integrity and esterase activity, as described previously (Gomez et al., 2010). The amounts of DNA-labeled BrdU as well as calcein and ethidium homodimer-1 incorporation into the cells were examined to determine the cell viability and proliferation status of mitomycin C–treated REFs.
Synthesis of recombinant rbLIF
Sequencing rbLIF cDNA for recombinant synthesis
Although the complete rbLIF cDNA sequence was not yet available, the incomplete cDNA sequence of rbLIF was obtained at the Ensembl website, in which 18 bp were known at the 5′ end, followed by 159 unknown base pairs, followed by 429 known base pairs (www.ensembl.org/Oryctolagus_cuniculus/Gene/Summary?g=ENSOCUG00000003768/). To obtain the full-length cDNA sequence of the corresponding functional region (designated as pLIF7.2b, between brackets; Fig. 1A) (Gearing et al., 1987; Gearing et al., 1989; Gough et al., 1988) for recombinant synthesis of rbLIF, we designed the forward (rbLIF-F1) and the reverse primers (rbLIF-R1) as follows: rbLIF-F1, 5′-ATGAAGATCTTGGCGGCA-3′; rbLIF-R1, 5′-TACACAGCCCAAGGGGAGCCGTTG-3′.
FIG. 1.
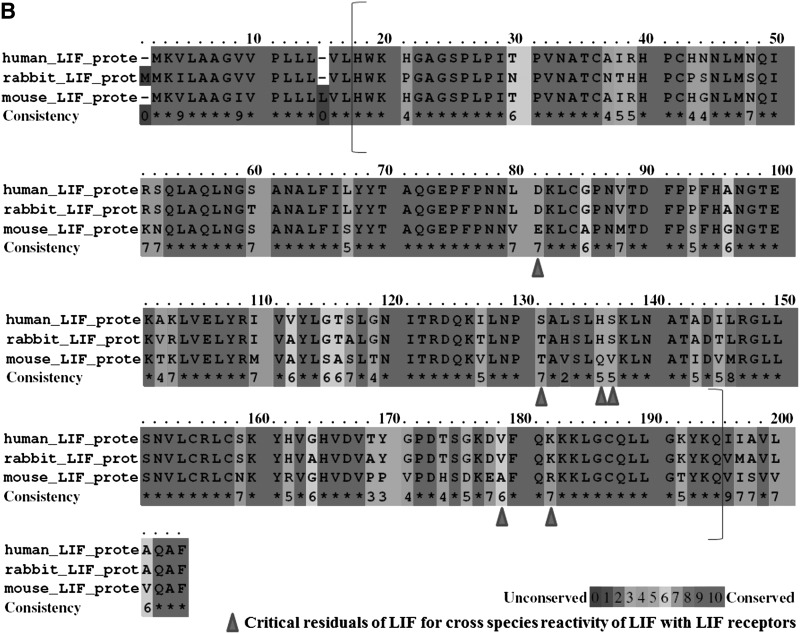
The cDNA and suggested protein sequence of rabbit pLIF7.2b. The complete sequence of rabbit pLIF7.2b cDNA (A, between the brackets) was cloned and sequenced to synthesize functional recombinant rbLIF. The italic and underlined sequences are revealed by our work. The recombinant LIF protein composition suggested by the pLIF7.2b sequence (B, between brackets) was compared with that of hLIF and mLIF, revealing higher homology between rbLIF and hLIF than between mLIF and hLIF. In particular, rbLIF and hLIF are homologous in five (D81, H136, S137, V179, and K182) of the six critical residues (B, indicated by arrowheads) for cross-species reactivity of LIF with LIF receptors. The only exception is at S131, where rbLIF is homologous to mLIF (T), but different from hLIF (S).
The liver tissues from three NZW rabbits were collected and subjected to standard total RNA extraction. The extracted RNAs were stored at −80°C prior to use. Reverse transcriptase PCRs were performed using primers rbLIF-F1 and rbLIF-R1 and rabbit liver cDNA as template. The PCR reaction was carried out for 35 cycles with the annealing temperature at 55°C. The PCR products of the right size were shipped to GENEWIZ, Inc. (South Plainfield, NJ, USA) for sequencing. Finally we compared protein sequence of rbLIF with those of human and mouse LIF using PRALINE (Simossis and Heringa 2005).
Expression and purification of recombinant rbLIF
The functional region of rbLIF cDNA (pLIF7.2b, shown in Fig. 1A) was subcloned into the pGEX-6P-1 vector (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA), following a similar strategy as described previously (Gearing et al., 1989). The recombinant rbLIF (r-rbLIF) was expressed as fusion proteins with the 26-kDa glutathione S-transferase (GST), separated by a thrombin-specific protease cleavage site. The fusion product was purified on a glutathione–agarose affinity matrix, and the r-rbLIF was released from the matrix by cleavage with thrombin, using the GST Purification Modules (GE Healthcare Bio-Sciences Corp.). After purification, LIF concentration was determined by enzyme-linked immunosorbent assay (ELISA) with human LIF antibody (AB-250-NA, R & D Systems, Minneapolis, MN, USA), which cross-reacts with rabbit LIF, as described previously (Schoonjans et al., 2003).
Rabbit ES cell derivation, maintenance, and characterization
ESC derivation and maintenance
Rabbit zygotes or embryos (one to two celled) were cultured in vitro to blastocyst (days 4–5) stage and used for ESC derivation. The base medium (BASE) consisted of 76% knockout DMEM (10829-018, Invitrogen), 20% FBS, 2 mM GlutaMax (35050-61, Invitrogen), 1% nonessential amino acids, 0.1 mM β-mercaptoethanol (444203, Millipore), and 10 ng/mL human recombinant basic fibroblast growth factor (bFGF; 13256-029, Invitrogen). Two culture conditions were compared for rbESC derivation and maintenance: Condition I (MEFs+BASE supplemented with 10 ng/mL hLIF (LIF1005, Millipore) and Condition II (REFs+BASE supplemented with 10 ng/mL rbLIF). Before seeding the embryos onto mitomycin C–treated REFs or MEFs, the zona pellucida (ZP) of each blastocyst was removed mechanically under an inverted microscope. For derivation and maintenance of rbESCs, the cells were cultured in Condition I or Condition II at 38°C in 5% CO2 with humidified air. Five to seven days after plating, the cell outgrowths were picked and dissociated into small clumps before reseeding onto the new feeders in one well of a six-well plate. Passaging was performed by incubating the ESC-like colonies with StemPro Acctutase (A11105-01, Invitrogen) for 3 min followed by plating small cell clumps at the cell density of 1×103 cells/cm2 onto new feeders in a six-well culture dish. Passaging was performed at 3- to 4-day intervals. For cryopreservation, dissociated rbESCs were frozen in cryopreservation medium consisting of 90% FBS and 10% dimethyl sulfoxide (DMSO), and stored in liquid nitrogen tanks.
Measuring the population doubling time and colony-forming efficiency of rbESCs
To evaluate the effects of different combinations of LIF and feeder cells on the maintenance of rbESCs, we measured the population doubling time (PD) and the colony-forming efficiency (CE) of rbESC line R4 (passage 5) in a 2×3 factorial experiment (MEFs vs. REFs, and BASE medium supplemented with 10 ng/mL mLIF, hLIF vs. rbLIF).
PD time was calculated as PD (h)=total time/Log2 (N2/N1) (Fang et al., 2006), where N1 is the number of seeded cells and N2 is the number of cells after culture. A total number of 1×105 rbESCs from R4 (passage 5) were seeded and cultured under conditions according to specific experimental design.
CE was measured as described previously (Fang et al., 2006). We seeded 1,000 cells of R4 (passage 5) in the 3-cm culture dishes for 5 days under culture conditions according to specific experimental design, and counted the colony number on day 5. CE was calculated as CE=(number of colonies/1000)×100%.
Alkaline phosphatase staining
The rbESC cultures were rinsed with DPBS prior to fixation in 4% paraformaldehyde (P6148) solution. Fifteen minutes after fixation at room temperature, rbESCs were rinsed with DPBS three times and then stained with alkaline phosphatase (AP) solution for 30 min, as previously described (Intawicha et al., 2009). The AP staining buffer (100 mM Tris–HCl, 100 mM NaCl, 50 mM MgCl2, pH 9.5) consisted of 0.25 M Trizma maleate (T3128), 0.008 M MgCl2 (M-8266), 0.17 g/L Fast-Red TR salt (F8764), and 0.4 g/L α-naphthyl phosphate (N7255). The stained cells were observed under an inverted microscope after washing with DPBS.
Immunocytochemical staining for ESC-specific markers
Immunostaining was performed to characterize ESC-specific markers, including SSEA4, Oct4, Nanog, and Sox2. Cells were either stained directly after washing with PBS (for cell-surface antigen) or fixed at room temperature with 4% paraformaldehyde for 10 min and permeated with 0.2% Triton X-100 and 0.1% Tween in PBS for at least 30 min after washing with PBS. Cells were then incubated with 2% goat serum to block nonspecific binding, followed by primary antibody (anti-Sox-2, Chemicon MAB 4343, Millipore; anti-SSEA-4, Chemicon MAB 4304, Millipore; anti-Oct-4, Santa Cruz, CA SC8628, Santa Cruz, CA; anti-Nanog, Abcam, ab21603, Cambridge, MA) for 1 h. All antibodies were diluted in blocking solution (for Oct-4, 1:500; for SSEA-4, 1:15; for Nanog, 1:150; for Sox-2, 1:50, R&D Systems, MAB2018) and incubated with samples at 4°C overnight after an additional three washes (15 min/wash) with 0.05% Tween-20 (Amersham Life Science, Oakville, Ontario, Canada). After incubation with the primary antibody, the rbESCs were washed again with DPBST three times and then incubated with the secondary antibodies [Oct4, fluorescein isothiocyanate (FITC)-conjugated rabbit antigoat immunoglobulin G (IgG), 1:400, F736; Nanog, FITC-conjugated goat antirabbit IgG, 1:300, F9887; SSEA-4, FITC-conjugated rabbit anti-rat IgG+IgM+IgA, 1:300, Abcam ab520; Sox-2, 1:500, Invitrogen A21202] for 40 min.
Karyotyping
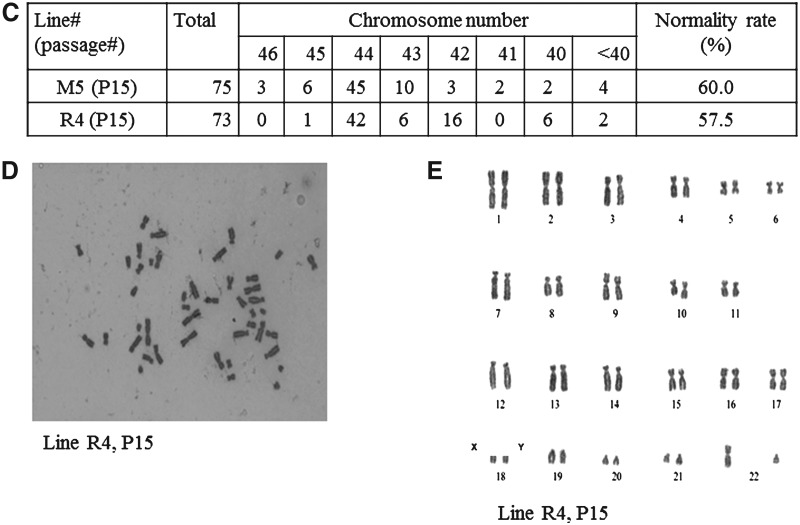
Cells from selected lines (R4 and M5) at passage 15 were subjected to karyotyping. The cells were incubated in growth medium supplemented with 100 ng/mL colcemid (KaryoMax Colcemid Solution, Invitrogen, 15212-012) for 2–3 h at 38°C in 5% CO2. Then, cells were trypsinized and pelleted at 1500 rpm for 5 min, resuspended in 6 mL of 75 mM KCl, and kept in a 37°C water bath for 10 min. The cells were centrifuged and fixed in acetic acid/methanol (vol/vol=1:3) for 10 min. The centrifugation and fixing steps were repeated three times. The cell pellet was resuspended in 0.5 mL of acid/methanol solution, and one drop of cell suspension was dropped onto a prechilled, clean microscope slide. The chromosomes were stained with 5% Giemsa (Invitrogen, 10092013) for 15 min. The chromosomes were examined at 1,000×magnification under oil. We examined 75 metaphase spreads for Line M5 and 73 metaphase spreads for Line R4.
In vitro differentiation of rbESCs
The putative rbESC lines were cultured beyond passage 10 before induction of differentiation. The rbESCs were digested with StemPro Accutase (Invitrogen) for 3 min at 37°C and plated on an extra-low cluster plate (Corning Inc., NY, USA) for 5–7 days to induce formation of EBs. Expression of the cell markers for the three germ layers in EBs was assessed by RT-PCR.
RT-PCR
RT-PCR was used to detect the expression of Oct4, Sox2, Nanog, c-Myc, Klf4, and Dppa5 for rbESCs and Gata4, Desmin, Brachyury, Pax6, and Nestin for derived EBs. GAPDH was used as a control of each RT-PCR. The primers and the annealing temperature are shown in Table 1.
Table 1.
Primer Sequences for RT-PCR Analysis
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) | Product size (bp) |
|---|---|---|---|
| Oct4 | CCTTCGCAGGGGGGCCTA | CATGGGGGAGCCCAGAGCA | 673 |
| Sox2 | CCGCTACGACGTAGCGCG | CGAGCCCATGGAGCCGAGC | 133 |
| Nanog | CCCAGCTGTGTGTGTGCTCAA | CCAGGCTTGGGAGTACCAGG | 382 |
| c-Myc | GACGCGTCCTTCTCCCCCA | CTCTGGTTACCATGTCACCG | 112 |
| Klf4 | CCACAGACCTGGAAAGTGGT | GGAAGACGAGGATGAAGCTG | 187 |
| Dppa5 | GACCTGAAAGATCCCGAGGT | GTAGGAGCCGTAAACCACCA | 165 |
| Desmin | AGCAGGAGATGATGGAATAC | TCCAGCTTCCGGTAGG | 281 |
| Gata4 | CTCAGAAGGCAGAGAGTGTG | CCGCATTGCAAGAGGCCTGG | 321 |
| Nestin | AGGGGGAAGAGGAAGAGGAGGAGA | TGCTGCAGCCCGTTCACCACA | 394 |
| Brachyury | GCTTCCCCGAGACGCAGTTCAT | TGTCGGGGTAGGTTGGAGTGTTT | 360 |
| GAPDH | GGAGCCAAACGGGTCATCATCC | GAGGGGCCATCCACAGTCTTCT | 233 |
Rabbit ESCs and EBs derived from ESCs were collected and subjected to RNA isolation immediately or stored in −20°C for a few days and then subjected to RNA isolation. Total RNA was extracted using the Qiagen RNeasy Mini Kit (Qiagen, CA). The RNA preparations were treated with RNase-free DNase I during preparation to remove possible contaminating DNA. The isolated RNA was eluted with RNase-free water and used for RT-PCR immediately.
Reverse transcription (RT) was carried out using 2.5 units of reverse transcriptase in the presence of 1 unit of RNase inhibitor, and 5 μM oligo(dT) or random hexamers with approximately 0.5 μg of total RNA in a final volume of 20 μL. The solution was incubated at 42°C for 15 min to generate single-stranded cDNA. Four microliters of the RT products from rbESCs or EBs were used in the subsequent PCR to amplify transcripts of six pluripotent marker genes for ESCs (Oct4, Sox2, Nanog, c-Myc, Klf4, and Dppa5) or five germ layer marker genes for EBs (Desmin, Gata4, Nestin, Pax6, and Brachyury). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was amplified by RT-PCR in all RNA samples as an internal positive control. The PCR reaction was carried out in a total volume of 20 μL containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 0.2 mM dNTPs, 0.2 mM oligonucleotide primers, and 0.4 unit Taq DNA polymerase (Invitrogen). The PCR was initiated at 95°C for 2 min, followed by 35 cycles of 30 sec at 95°C, 30 sec at 57°C, and 30 sec at 72°C. All RT-PCR reactions, including the appropriate internal positive and negative controls, were repeated twice. A volume of 10 μL PCR products was analyzed on a 1.5% agarose gel. All PCR reactions were performed in a Hybaid Px2 Thermal Cycler.
Statistical analysis
Population doubling time and colony-forming efficiency were presented as means±standard error of the means (SEM). Means were compared using the General Linear Model (SPSS 11.0, Chicago, US). Statistical significance was considered when a p value was less than 0.05.
Results
Synthesis of recombinant rabbit LIF protein
We first obtained the cDNA sequence necessary for the synthesis of functional rbLIF. This region was reportedly used for the synthesis of functional recombinant human and murine LIF (Gearing et al., 1987; Gough et al., 1988). The sequence is shown in Fig. 1A and designated as pLIF7.2b (between brackets). The italic and underlined sequences were revealed by our work and were validated by three biological repeats.
A comparison of the recombinant LIF protein composition suggested by the pLIF7.2b sequence with those of the hLIF and mLIF revealed that rbLIF is 83.2% homologous with hLIF, which is higher than that of hLIF with mLIF (74.2%) and that of rbLIF with mLIF (75.3%).
There are six residues (D81, S131, H136, S137, V179, K182; Fig. 1B, indicated by arrows) that are reportedly critical for the cross species binding of hLIF and mLIF receptor (Layton et al., 1994). We compared these six residues among hLIF, rbLIF, and mLIF. Interestingly, rbLIF and hLIF share the same code in 5 (D81, H136, S137, V179, and K182) out of these six residues (83.3%). The only exception is at S131 residue of hLIF: the corresponding residue of rbLIF and mLIF is T.
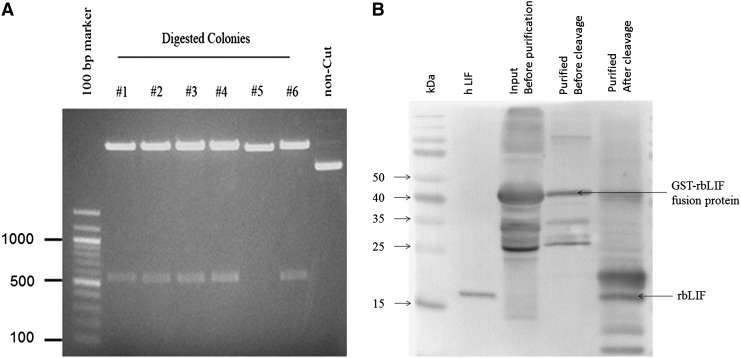
We introduced the functional region rbLIF (pLIF7.2b ) into pGEX-6P-1 vector between restriction sites BamHI and NotI and selected colony #3 for the expression after confirming the size (542 bp) (Fig. 2A) and sequence. Both the total input protein (before purification) and the purified fusion protein (before thrombin cleavage) were found to be positive after western blotting by anti-human LIF antibody at a molecular weight of 46 kDa, but not at 19 kDa (Fig. 2B). After thrombin cleavage, the rbLIF was identified by western blotting with the anti-human LIF antibody, at a similar molecular weight (19 kDa) to human LIF (Fig. 2B).
FIG. 2.
Production of recombinant rbLIF. (A) The functional region of rbLIF was introduced into pGEX-6P-1 vector. Colony #3 was selected for the expression of recombinant protein after confirming the size and sequence. (B) The GST–rbLIF fusion protein was identified after western blotting by anti-human LIF antibody at a molecular weight of 46 kDa (upper arrow) in both the total input protein (before purification) and the purified fusion protein (before thrombin cleavage). After thrombin cleavage, the r-rbLIF was found at the similar molecular weight (19 kDa, lower arrow) with human LIF (positive control). Input and fusion proteins did not show signals at 19-kDa size.
Validation of REF feeder cell preparation
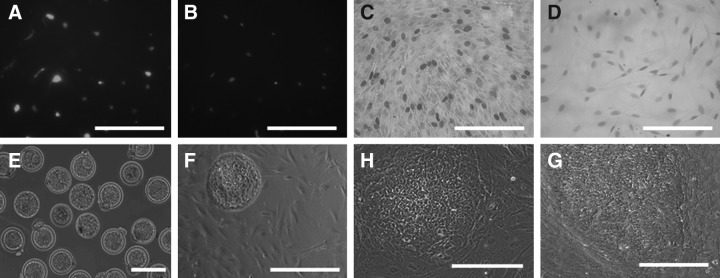
After mitomycin C treatment (3 h), REF cell viability was evaluated by the Calcein AM assay, and the proliferation status was evaluated by the BrdU assay. Approximately 95% of cells were of green fluorescent (Fig. 3A,B) after the calcein AM assay, indicating satisfactory viability of REF cells. Ninety-four percent of the cells stopped dividing, as indicated by the BrdU assay (Fig. 3C,D). These results clearly demonstrated that our feeder cell preparation protocol was working well on REF cells. MEF cells were prepared and validated similarly.
FIG. 3.
Evaluation of REF cells and derivation of rbES cells on REF cells. REF cells were treated with mitomycin-C, followed by calcein AM assay to evaluate their viability and BrdU assay to evaluate their proliferation status. In the calcein AM assay, the presence of light fluorescence indicated viable cells (A), which correlated well with the 4′,6-diamidino-2-phenylindole (DAPI) staining of the same population (B), indicating that almost all cells were still viable. In the BrdU assay, non-mitomycin C–treated REF cells consisted of a large population of dividing cells (dark staining) (C), whereas most mitomycin C–treated cells stopped proliferating, showing no dark staining (D). After seeding blastocysts (E) on the mitomycin C–treated REF cells (F), several putative cell lines were successfully derived and maintained (line R4, passage 10, H), displaying a similar ESC morphology with rbESCs derived and maintained on MEF cells (line M5, passage 10, G). Scale bars, 200 μm.
Establishment of rbES cell lines with Conditions I and II
A total of 123 blastocysts (representative images shown in Fig. 3E) were used for rbESC derivation (Table 2). Because our original hypothesis was that the LIF and feeder cells from the same origin (REF+rbLIF, Condition II) would improve the germ-line transmission efficiency of the derived rbES cell lines, we assigned 37 blastocysts (30%) to Condition I, and 86 blastocysts (70%) to Condition II.
Table 2.
Derivation Efficiencies of rbESC Lines with Blastocysts Developed from Different Culture Conditions
| Treatment | Total no. of embryos | No. ICM attached (%) | No. putative ESC lines (%) |
|---|---|---|---|
| Condition I (MEFs+hLIF) | 37 | 23 (62.2) | 2 (8.7) |
| Condition II (REFs+rbLIF) | 86 | 49 (57.0) | 5 (10.2) |
| Sum | 123 | 72 (58.5) | 7 (9.7) |
rbESC, rabbit embryonic stem cells; ICM, inner cell mass; MEF, mouse embryonic fibroblasts; hLIF, human leukemia inhibitory factor; REF, rabbit embryonic fibroblasts.
A total of 72 ICMs were picked (Table 2). The ICM-pick efficiency was similar between Condition I and Condition II: 23 ICMs (M1-M23) for Condition I (23/37, 62.2%) and 49 ICMs (R1-R49) for Condition II (49/86, 57.0%) (Table 2).
A similar efficiency of putative rbESC derivation was obtained between Condition I and Condition II (Table 2). Two putative rbESC lines (M5 and M23) were derived from Condition I (2/23, 8.7%). Five putative rbESC lines (R4, R9, R15, R21, and R31) were derived from Condition II (5/49, 10.2%). The overall efficiency was 9.7% (7/72). The morphology of putative rbES cell lines derived on Condition II (e.g., R4, Fig. 3H) appeared similar to that of the ones derived on Condition I (e.g., M5, Fig. 3G).
Effects of LIF and feeder cell origins on the maintenance of rbESCs
We examined the effects of LIF origin and feeder cell origin in a 2×3 factorial experiment (REF vs. MEF and BASE medium supplemented with 10 ng/mL hLIF, mLIF vs. rbLIF; Tables 3 and 4). We examined the PD time and CE of the putative rbESC line R4 (passage 5) under these conditions.
Table 3.
Population Doubling (PD) Time of Putative Rabbit ESC Line R4 (Passage 5) with Different Feeders and LIF Supplementation
| hLIF (h) | mLIF (h) | rbLIF (h) | |
|---|---|---|---|
| REFs | 12.72±0.76a | 14.02±1.74a | 14.00±0.72a |
| MEFs | 15.28±2.95a | 15.06±2.81a | 27.04±1.87b |
Numbers with different superscripts differ significantly. The PD time was in normal range under both conditions I (hLIF+MEFs, 15.28 h) and II (rbLIF+REFs, 14.00 h); however, the PD time was abnormally long under the rbLIF+MEFs condition (27.04 h).
ESC, embryonic stem cells; LIF, leukemia inhibitory factor; h, human; m, mouse; rb, rabbit; REFs, rabbit embryonic fibroblasts; MEFs, mouse embryonic fibroblasts.
Table 4.
Colony-Forming Efficiency (CE%) of Putative Rabbit ESC Line R4 (Passage 5) with Different Feeders and LIF Supplementation
Numbers with different superscripts differ significantly. The CE% was in a normal range under both conditions I (hLIF+MEFs, 10.80%) and II (rbLIF+REFs, 12.48%); however, the CE% was abnormally low under the rbLIF+MEFs condition (3.84 h).
ESC, embryonic stem cells; LIF, leukemia inhibitory factor; h, human; m, mouse; rb, rabbit; REFs, rabbit embryonic fibroblasts; MEFs, mouse embryonic fibroblasts.
Our results demonstrated that the origin of LIF and feeder cells affected the rbESC maintenance, as measured by PD and CE. When mouse feeder cells were used, line R4 grew slower with rbLIF (PD, 27.04 h; CE, 3.84%) than with hLIF (PD, 15.28 h; CE, 10.80%) or mLIF (PD, 15.06 h; CE, 14.80%) (Tables 3 and 4). When rabbit feeder cells were used, the PD and CE of line R4 were normal, regardless of the origin of LIF (PD, 12.72–14.02 h; CE, 7.20–12.48%) (Tables 3 and 4).
The two conditions we used to derive rbESC lines (Condition I, MEF+hLIF; Condition II, REF+rbLIF) appeared to support satisfactory rbESC growth in vitro, with similar PD and CE values to those previously reported in rabbits (Fang et al., 2006). In Condition I, the PD time was 15.28 h and the CE was 10.80%. In Condition II, the PD time was 14.00 h and the CE was 12.48%. The rbESCs appeared to grow faster than human ESC and induced pluripotential cells (iPSCs), as the PDs are shorter and the CEs are higher of our rbESCs comparing to those reported for human ESC or iPSCs (PD, 43.2–47.8 h; CE, 0.24–0.83%) (Amit et al., 2000; Fang et al., 2006; Takahashi et al., 2007).
Typical pluripotency marker expressions of rbESCs derived and cultured in Conditions I and II
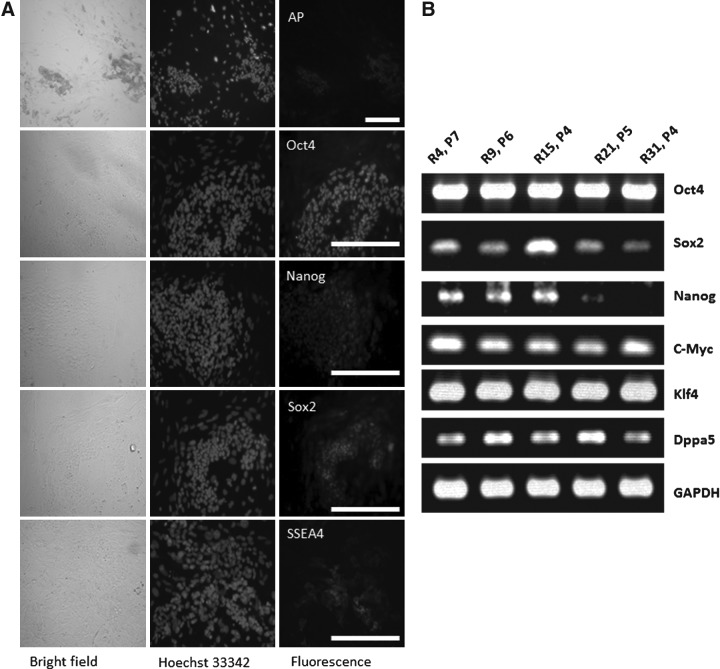
All five putative rbESC lines (R4, passage 7; R9, passage 6; R15, passage 4; R21, passage 5; R31, passage 4) derived on Condition II expressed AP (representative image shown in Fig. 4A, top panel). They expressed Oct-4, Nanog, Sox-2, and SSEA-4 as determined by immunochemistry staining (representative images shown in Fig. 4A, lower panels). These rbESC lines also expressed pluoripotent genes, such as Oct-4, Sox-2, Nanog, C-Myc, Klf4, and Dppa5 (Fig. 4B), as determined by RT-PCR assays. More variance in the expression levels of Nanog and Sox-2 among different cell lines was observed comparing to other genes examined (Fig. 4B). Lines R21 and R31 showed lower expression levels of Nanog than lines R4, R9, and R15, whereas lines R4 and R15 expressed higher levels of Sox2 than the other three lines (R9, R21, and R31). The expression levels of other genes examined (Oct-4, C-Myc, Klf4, Dppa5, and GAPDH) appeared to be similar among different lines.
FIG. 4.
Specific ESC markers, gene expression, and karyotyping results of putative rbESCs derived and maintained under Condition II. Putative rbESC lines (R4, R9, R15, R21, and R31) derived and maintained under Condition II (REF+rbLIF) are positive for AP (A, top panel) and other pluripotency markers, including Oct-4, Nanog, Sox2, and SSEA4 (B, lower panels). (A) Representative staining images of R4 (passage 7). RT-PCR assays revealed that these cell lines (R4, passage 7; R9, passage 6; R15, passage 4; R21, passage 5; and R31, passage 4) expressed pluripotency genes such as Oct-4, Sox-2, Nanog, c-Myc, Klf4, and Dppa5 (B). GAPDH was used as the internal control. Karyotyping analysis on line R4 (passage 15) and line M5 (passage 15) revealed 57.5% and 60.0% normal ploidy rates, respectively (C). Both R4 (D and E) and M5 (images not shown) were identified as male cell lines. Scale bars, 200 μm.
Both putative rbESC lines derived on Condition I (M5, P6 and M23, P4) were also positive for AP, Oct-4, Nanog, Sox-2, and SSEA-4 staining, and expressed pluoripotent genes, including Oct-4, Sox-2, Nanog, C-Myc, Klf4, and Dppa5 (images not shown).
Rabbit ESCs derived and cultured under Conditions I and II maintained normal karyotypes
Selected rbESC lines (M5 representing Condition I, and R4 representing Condition II) were subjected to karyotyping analysis. Seventy-three metaphase spreads of R4 cells (passage 15) and 75 metaphase spreads of M5 cells (passage 15) were counted. There were 60% of M5 and 57.5% of R4 spreads showing normal rabbit karyotype with 44 chromosomes (Fig. 4C). Both R4 (Fig. 4D,E) and M5 were found to be male cell lines.
Rabbit ESCs derived on Conditions I and II were capable of differentiating into three germ layers in vitro
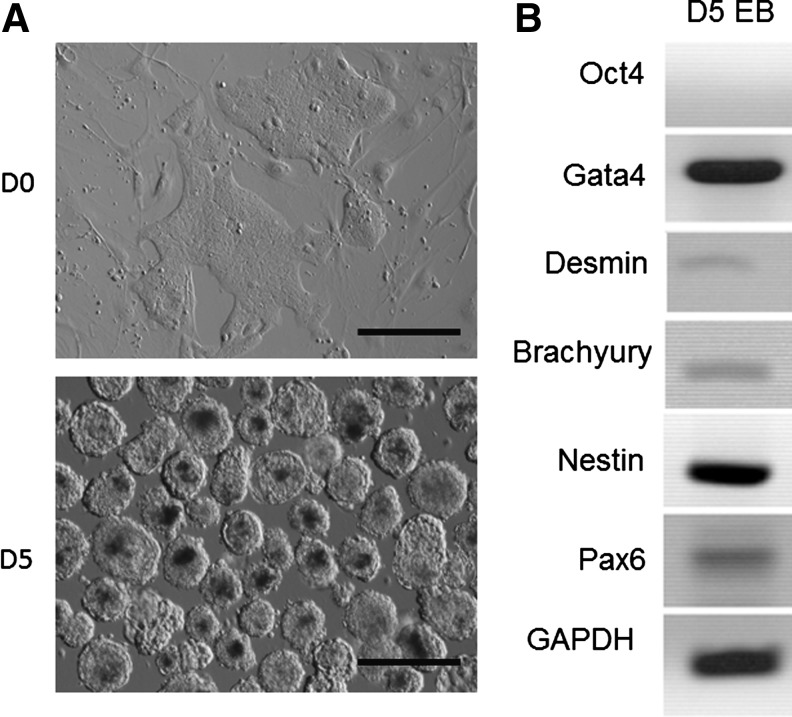
Putative rbESC lines derived on Condition I (M5, P12 and M23, P11) or Condition II (R4, P10; R9, P12; R15, P11; R21, P10 and R31, P10) all formed EBs in the presence of serum in suspension culture (representative image shown in Fig. 5A). RT-PCR results showed that EBs derived from these cell lines expressed the marker genes for all three germ layers (Gata4 for endoderm, Desmin and Brachyury for mesoderm, Nestin and Pax6 for ectoderm), but not the pluripotent gene Oct-4 (representative images shown in Fig. 5B). Higher gene expression levels were found for endoderm-specific genes (e.g., Gata4) and ectoderm genes (e.g., Nestin and Pax6) than for mesoderm-specific genes (e.g., Desmin and Brachyury).
FIG. 5.
In vitro differentiation of rbESC line R4. Rabbit ESC line R4 (passage 10) was used for EB formation (A, upper panel, D0). When these cells were cultured in specialized nonadherent dishes, we observed EB formation starting at D1. The number and the size of the EBs continued to increase throughout EB culture (A, lower panel, D5). The EBs expressed all three primary germ layer markers (Gata4 for endoderm, Desmin and Brachyury for mesoderm, Nestin and Pax6 for ectoderm), but not the pluripotency gene Oct4 (B). GAPDH was used as the internal control. Scale bars, 200 μm.
Discussion
In the present work, we derived rbESC lines using homologous LIF and feeder cells. The use of REFs as feeder cells has been attempted by Graves and Moreadith (Graves and Moreadith, 1993). They harvested day-16 rabbit fetuses and established REF feeders. However, they found that REFs promoted differentiation of putative rbESCs into a spindle-shaped cell type that did not passage. Hamster embryonic fibroblasts also promoted differentiation of putative hamster ESCs in an early study (Doetschman et al., 1988). Recently, homologous feeder cells were reported to support the derivation and culture of human, monkey, buffalo, and cat ESCs, in addition to mESCs (Anand et al., 2011; Gomez et al., 2010; Li et al., 2005; Richards et al., 2002). For example, hESCs can now also be established and maintained on human embryonic fibroblasts, adult fallopian tube epithelia, or foreskin fibroblasts (Hovatta et al., 2003; Richards et al., 2002). The use of homologous feeder cells is particularly important for hESCs to avoid animal contaminations. On animal ESCs, the application of homologous feeder cells would similarly avoid cross-species contamination. More importantly, it may provide an environment to improve the quality of the ESCs, which may consequently enhance the chance of germ-line transmission, a major goal of animal ESC research and application. It is suggested that cell-surface and soluble factors required to support ESC self-renewal in an undifferentiated status are species-specific (Gomez et al., 2010), and LIF has been shown to have different cross-species reactivity (Owczarek et al., 1997).
In the present study, we found that rbESC lines derived and maintained on REFs displayed similar morphologies and characteristics as the ones derived and maintained on MEF feeders. We did not observe a higher percentage of cells differentiating on the REFs as compared to those on the MEFs. This improvement over the earlier study (Graves and Moreadith, 1993) may come from the overall quality improvement on media and reagents used for processing the feeder cells and for the ESC derivation and culture, as well as the optimized protocols. For example, GlutaMax was used in the present study instead of L-glutamine. Knockout DMEM, a medium optimized for growth of undifferentiated ESCs, is now available and was used in the present study. Notably, we used higher concentrations of FBS (20% vs. 15%) and nonessential amino acids (1% vs. 0.5%) in the ESC culture medium as compared to the Graves et al. report. We also note that there is a need to establish drug resistant REF feeders, which will be essential for gene targeting in rbESCs.
Recombinant mouse and human LIF have been synthesized in the Escherichia coli system (Gearing et al., 1987; Gearing et al., 1989; Gough et al., 1988). We used a similar strategy and synthesized recombinant rbLIF in E. coli. LIF derived from E. coli exhibited identical biological potency as mammalian LIF or LIF derived from yeast (Gough et al., 1988; Hilton et al., 1988). At the same concentration (10 ng/mL), our homemade recombinant rbLIF and commercially purchased hLIF supported the derivation and maintenance of rbESC lines with similar efficiencies. This is not surprising considering the fact that hLIF and rbLIF are almost identical in the binding sites that are critical for cross-species reactivity. Previous studies in mLIF and hLIF found that mLIF cannot bind to hLIF receptor (hLIF-R), whereas hLIF binds to mLIF receptor (mLIF-R) with a much higher affinity. However, replacing six residues in mLIF with those from hLIF would enable mLIF to bind hLIF-R, confirming the critical roles of these six amino acids for the binding specificity across species (Layton et al., 1994). The present work revealed that rbLIF and hLIF are identical in five of these six sites, suggesting hLIF and rbLIF may be interchangeable in in vitro experiments. Interestingly, rbLIF was found beneficial in deriving mESCs in certain strains such as C57BL/6N (Schoonjans et al., 2003). Similar efficiency of ESC derivation (34% vs. 38%) was reported between basic ESC culture medium supplemented with mLIF (1000 IU/mL) or rbLIF (10 ng/mL), whereas much higher efficiency (61%) was achieved when the basic culture medium was conditioned by a rabbit fibroblast cell line transduced with genomic rbLIF (15 ng/mL) (Schoonjans et al., 2003). Moreover, this approach of using culture medium conditioned with genomic rbLIF (instead of mLIF) resulted in successful establishment of germ-line-competent ESC lines from several presumed “nonpermissive” inbred mouse strains (Schoonjans et al., 2003). We also found that the recombinant rbLIF supported the maintenance of mESC lines (data not shown). These results suggested that the recombinant rbLIF may be also useful for ESC studies in species other than rabbits.
LIF signaling pathway is central in maintaining mESCs (Smith et al., 1988), but not hESCs (Thomson et al., 1998). The LIF-dependence of rbESCs is not clear yet. In the present work, supplementation hLIF (in Condition I) or rbLIF (in Condition II) in the medium is necessary for rbESC derivation and maintenance, consistent with some previous reports (Hsieh et al., 2011; Intawicha et al., 2009), but not several others (Fang et al., 2006; Honda et al., 2009; Wang et al., 2005). In our work, upon withdrawing hLIF or rbLIF from the culture medium, rbESCs quickly undergo spontaneous differentiation (data not shown). The requirement for exogenous LIF may indicate the low expression levels of LIF by the feeder cells in our work. If the feeder cells can express sufficient amount of LIF, the requirement for exogenous LIF may become unnecessary, which could explain why LIF was not supplemented in several rbESC reports (Fang et al., 2006; Honda et al., 2009; Wang et al., 2005). In mouse ESC studies, it is reported that when feeder cells expressed high levels of LIF, exogenous LIF becomes unnecessary (Feng et al., 2009). The optimal concentration of rbLIF for promoting rbESC derivation and further passage to maintain pluripotency is under investigation.
The present work demonstrated that the combination of REF and rbLIF is able to support the derivation and maintenance of rbESC lines in a similar manner as MEFs and hLIF did. Interestingly, the combination of rbLIF and MEFs did not support the culture of rbESC line R4 well, demonstrated by long PD time and low CE, whereas other combinations of feeder cells and LIF were able to maintain this cell line in normal growth. We do not know the molecular mechanisms leading to such interaction effects between rbLIF and MEF on the rbESC culture yet, but careful attention should be paid when using this combination for rbESC culture.
In the present work, bFGF was supplemented to both Condition I and Condition II. In fact, we were not successful in deriving putative rbESC lines without the supplementation of bFGF (data not shown). The FGF pathway plays important role for derivation and maintenance of rbESCs: it promotes rbESC self-renewal and prevents differentiation. Absence of FGF results in slow growth and quick differentiation (Fang et al., 2006; Hsieh et al., 2011; Wang et al., 2008; Wang et al., 2007). Mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and phosphatidylinositol-3-kinase (PI3K)/AKT pathways, both of which are downstream of the FGF pathway, are required for maintaining rbESCs in an undifferentiated state. Such a requirement is different from that in mice and rats. In these two species, the activation of FGF pathway promotes differentiation (Buehr et al., 2008; Li et al., 2008), whereas the use of the 3i medium, two of the three inhibiting the FGF pathway, resulted in germ-line-transmitting ESCs. Recently, it was proposed that FGF-dependent ESCs, including most human ESC lines, are actually “primed”-state ESCs and have little chance transmitting to the germ cells (Blair et al., 2011).
Therefore, it is possible that the rbESCs derived in the present study, as well as all other reported rbESC lines, are also primed-state stem cells. To capture the naïve state rather than primed-state rbESCs, it may be worth exploring the use of earlier-stage preimplantation embryos. We used day-4 to day-5 blastocysts in the present work. Many embryos were already at the expanded or even the hatched stage. Because the naïve-state and primed-state ESCs may very likely come from cells at two different embryo stages—the pre- and the postimplantation epiblast (Blair et al., 2011)—we speculate that the use of earlier-stage embryos (e.g., days 3–4) may eliminate the dependency of rbESCs on bFGF and derive more naïve-state ESCs.
The long-term goal of the present study is to derive germ-line-competent rbESCs for the production of gene-targeted transgenic rabbits. To date, only mouse and rat ESCs have been proven to be germ-line transmissible and are used to produce gene-targeted transgenic animals via the ESC injection approach. Nuclear transfer, in combination with gene targeting in somatic cells, has been used as an alternative to produce gene-targeted pigs, cattle, sheep, and goats. Unfortunately, the rabbit has been proven to be an extremely difficult species to clone (Du et al., 2009). Since the first report of successful rabbit cloning a decade ago (Chesne et al., 2002), no gene-targeted transgenic rabbits were produced via this approach. Using REFs and rbLIF in the present study, we obtained satisfactory efficiency in rbESC derivation and are satisfied with the quality of these cell lines, as confirmed by immunochemistry staining, gene expression assays, and in vitro differentiation assays. The PD and CE of rbESCs derived in Condition II are similar to those reported previously (Fang et al., 2006). One cell line (R4) is now at over 25 passages and still displays typical rbESC morphology and maintains normal ploidy (data not shown). These results clearly demonstrate that homologous feeder cells and LIF are able to support the derivation and culture of rbESCs; however, we cannot conclude whether or not they are better than feeder cells and LIFs that are of foreign species origin in improving the totipotency of these cells. Future work regarding ESC injection and embryo transfer is required to test whether or not the ESCs derived on homologous feeder cells and LIF are more competent for germ-line transmission.
Acknowledgments
This study was supported by the National Institutes of Health, USA (Grant Number 5R44RR023774 to Dr. Jie Xu).
Disclosure Statement
The authors declare that no conflicts of interest exist.
References
- Amit M. Carpenter M.K. Inokuma M.S. Chiu C.P. Harris C.P. Waknitz M.A. Itskovitz-Eldor J. Thomson J.A. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Anand T. Kumar D. Singh M.K. Shah R.A. Chauhan M.S. Manik R.S. Singla S.K. Palta P. Buffalo (Bubalus bubalis) embryonic stem cell-like cells and preimplantation embryos exhibit comparable expression of pluripotency-related antigens. Reprod. Domest. Anim. 2011;46:50–58. doi: 10.1111/j.1439-0531.2009.01564.x. [DOI] [PubMed] [Google Scholar]
- Blair K. Wray J. Smith A. The liberation of embryonic stem cells. PLoS Genet. 2011;7:e1002019. doi: 10.1371/journal.pgen.1002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M. Meek S. Blair K. Yang J. Ure J. Silva J. McLay R. Hall J. Ying Q.L. Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Chen T.L. Shen W.J. Qiu X.W. Li T. Hoffman A.R. Kraemer F.B. Generation of novel adipocyte monolayer cultures from embryonic stem cells. Stem Cells Dev. 2007;16:371–380. doi: 10.1089/scd.2006.0037. [DOI] [PubMed] [Google Scholar]
- Chesne P. Adenot P.G. Viglietta C. Baratte M. Boulanger L. Renard J.P. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat. Biotechnol. 2002;20:366–369. doi: 10.1038/nbt0402-366. [DOI] [PubMed] [Google Scholar]
- Doetschman T. Williams P. Maeda N. Establishment of hamster blastocyst-derived embryonic stem (ES) cells. Dev. Biol. 1988;127:224–227. doi: 10.1016/0012-1606(88)90204-7. [DOI] [PubMed] [Google Scholar]
- Doss M.X. Sachinidis A. Hescheler J. Human ES cell derived cardiomyocytes for cell replacement therapy: A current update. Chin. J. Physiol. 2008;51:226–229. [PubMed] [Google Scholar]
- Du F. Xu J. Zhang J. Gao S. Carter M.G. He C. Sung L.Y. Chaubal S. Fissore R.A. Tian X.C., et al. Beneficial effect of young oocytes for rabbit somatic cell nuclear transfer. Cloning Stem Cells. 2009;11:131–140. doi: 10.1089/clo.2008.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.J. Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fan J. Watanabe T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol. Ther. 2003;99:261–282. doi: 10.1016/s0163-7258(03)00069-x. [DOI] [PubMed] [Google Scholar]
- Fang Z.F. Gai H. Huang Y.Z. Li S.G. Chen X.J. Shi J.J. Wu L. Liu A. Xu P. Sheng H.Z. Rabbit embryonic stem cell lines derived from fertilized, parthenogenetic or somatic cell nuclear transfer embryos. Exp. Cell Res. 2006;312:3669–3682. doi: 10.1016/j.yexcr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Feng S. Mo L. Wu R. Chen X. Zhang M. Establishment of an exogenous LIF-free culture system for mouse embryonic stem cells. Cloning Stem Cells. 2009;11:437–443. doi: 10.1089/clo.2009.0008. [DOI] [PubMed] [Google Scholar]
- Gama Sosa M.A. De Gasperi R. Elder G.A. Animal transgenesis: An overview. Brain Struct. Funct. 2010;214:91–109. doi: 10.1007/s00429-009-0230-8. [DOI] [PubMed] [Google Scholar]
- Gearing D.P. Gough N.M. King J.A. Hilton D.J. Nicola N.A. Simpson R.J. Nice E.C. Kelso A. Metcalf D. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF) EMBO J. 1987;6:3995–4002. doi: 10.1002/j.1460-2075.1987.tb02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing D.P. Nicola N.A. Metcalf D. Foote S. Willson T.A. Gough N.M. Williams R.L. Production of leukemia inhibitory factor in Escherichia coli by a novel procedure and its use in maintaining embryonic stem cells in culture. Bio/Technology. 1989;7:1157–1161. [Google Scholar]
- Gomez M.C. Serrano M.A. Pope C.E. Jenkins J.A. Biancardi M.N. Lopez M. Dumas C. Galiguis J. Dresser B.L. Derivation of cat embryonic stem-like cells from in vitro-produced blastocysts on homologous and heterologous feeder cells. Theriogenology. 2010;74:498–515. doi: 10.1016/j.theriogenology.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Gough N.M. Gearing D.P. King J.A. Willson T.A. Hilton D.J. Nicola N.A. Metcalf D. Molecular cloning and expression of the human homologue of the murine gene encoding myeloid leukemia-inhibitory factor. Proc. Natl. Acad. Sci. USA. 1988;85:2623–2627. doi: 10.1073/pnas.85.8.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves K.H. Moreadith R.W. Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol Reprod Dev. 1993;36:424–433. doi: 10.1002/mrd.1080360404. [DOI] [PubMed] [Google Scholar]
- Hilton D.J. Nicola N.A. Metcalf D. Specific binding of murine leukemia inhibitory factor to normal and leukemic monocytic cells. Proc. Natl. Acad. Sci. USA. 1988;85:5971–5975. doi: 10.1073/pnas.85.16.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A. Hirose M. Inoue K. Ogonuki N. Miki H. Shimozawa N. Hatori M. Shimizu N. Murata T. Hirose M., et al. Stable embryonic stem cell lines in rabbits: potential small animal models for human research. Reprod. Biomed. Online. 2008;17:706–715. doi: 10.1016/s1472-6483(10)60320-3. [DOI] [PubMed] [Google Scholar]
- Honda A. Hirose M. Ogura A. Basic FGF and Activin/Nodal but not LIF signaling sustain undifferentiated status of rabbit embryonic stem cells. Exp. Cell Res. 2009;315:2033–2042. doi: 10.1016/j.yexcr.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Hovatta O. Mikkola M. Gertow K. Stromberg A.M. Inzunza J. Hreinsson J. Rozell B. Blennow E. Andang M. Ahrlund-Richter L. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum. Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- Hsieh Y.C. Intawicha P. Lee K.H. Chiu Y.T. Lo N.W. Ju J.C. LIF and FGF cooperatively support stemness of rabbit embryonic stem cells derived from parthenogenetically activated embryos. Cell Reprogram. 2011;13:241–255. doi: 10.1089/cell.2010.0097. [DOI] [PubMed] [Google Scholar]
- Intawicha P. Ou Y.W. Lo N.W. Zhang S.C. Chen Y.Z. Lin T.A. Su H.L. Guu H.F. Chen M.J. Lee K.H., et al. Characterization of embryonic stem cell lines derived from New Zealand white rabbit embryos. Cloning Stem Cells. 2009;11:27–38. doi: 10.1089/clo.2008.0040. [DOI] [PubMed] [Google Scholar]
- Kumar D. Anand T. Singh K.P. Singh M.K. Shah R.A. Chauhan M.S. Palta P. Singla S.K. Manik R.S. Derivation of buffalo embryonic stem-like cells from in vitro-produced blastocysts on homologous and heterologous feeder cells. J. Assist. Reprod. Genet. 2011;28:679–688. doi: 10.1007/s10815-011-9572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton M.J. Owczarek C.M. Metcalf D. Clark R.L. Smith D.K. Treutlein H.R. Nicola N.A. Conversion of the biological specificity of murine to human leukemia inhibitory factor by replacing 6 amino acid residues. J. Biol. Chem. 1994;269:29891–29896. [PubMed] [Google Scholar]
- Li P. Tong C. Mehrian-Shai R. Jia L. Wu N. Yan Y. Maxson R.E. Schulze E.N. Song H. Hsieh C.L., et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. Wang S. Xie Y. Lu Y. Zhang X. Wang L. Yang S. Wolf D. Zhou Q. Ji W. Homologous feeder cells support undifferentiated growth and pluripotency in monkey embryonic stem cells. Stem Cells. 2005;23:1192–1199. doi: 10.1634/stemcells.2004-0286. [DOI] [PubMed] [Google Scholar]
- Lin T.A. Chen C.H. Sung L.Y. Carter M.G. Chen Y.E. Du F. Ju J.C. Xu J. Open-pulled straw vitrification differentiates cryotolerance of in vitro cultured rabbit embryos at the eight-cell stage. Theriogenology. 2011;75:760–768. doi: 10.1016/j.theriogenology.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiya T. Yamamoto Y. Banas A. Commitment of stem cells into functional hepatocytes. Differentiation. 2010;79:65–73. doi: 10.1016/j.diff.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Owczarek C.M. Zhang Y. Layton M.J. Metcalf D. Roberts B. Nicola N.A. The unusual species cross-reactivity of the leukemia inhibitory factor receptor alpha-chain is determined primarily by the immunoglobulin-like domain. The Journal of biological chemistry. 1997;272:23976–23985. doi: 10.1074/jbc.272.38.23976. [DOI] [PubMed] [Google Scholar]
- Pease S. Braghetta P. Gearing D. Grail D. Williams R.L. Isolation of embryonic stem (ES) cells in media supplemented with recombinant leukemia inhibitory factor (LIF) Dev. Biol. 1990;141:344–352. doi: 10.1016/0012-1606(90)90390-5. [DOI] [PubMed] [Google Scholar]
- Richards M. Fong C.Y. Chan W.K. Wong P.C. Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- Roach M.L. McNeish J.D. Methods for the isolation and maintenance of murine embryonic stem cells. Methods Mol. Biol. 2002;185:1–16. doi: 10.1385/1-59259-241-4:1. [DOI] [PubMed] [Google Scholar]
- Schoonjans L. Kreemers V. Danloy S. Moreadith R.W. Laroche Y. Collen D. Improved generation of germline-competent embryonic stem cell lines from inbred mouse strains. Stem Cells. 2003;21:90–97. doi: 10.1634/stemcells.21-1-90. [DOI] [PubMed] [Google Scholar]
- Simossis V.A. Heringa J. PRALINE: A multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Nucleic Acids Res. 2005;33:W289–W294. doi: 10.1093/nar/gki390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.G. Heath J.K. Donaldson D.D. Wong G.G. Moreau J. Stahl M. Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Takada T. Suzuki Y. Kondo Y. Kadota N. Kobayashi K. Nito S. Kimura H. Torii R. Monkey embryonic stem cell lines expressing green fluorescent protein. Cell transplantation. 2002;11:631–635. doi: 10.3727/000000002783985350. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson J.A. Kalishman J. Golos T.G. Durning M. Harris C.P. Becker R.A. Hearn J.P. Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Vaags A.K. Rosic-Kablar S. Gartley C.J. Zheng Y.Z. Chesney A. Villagomez D.A. Kruth S.A. Hough M.R. Derivation and characterization of canine embryonic stem cell lines with in vitro and in vivo differentiation potential. Stem Cells. 2009;27:329–340. doi: 10.1634/stemcells.2008-0433. [DOI] [PubMed] [Google Scholar]
- Volarevic V. Ljujic B. Stojkovic P. Lukic A. Arsenijevic N. Stojkovic M. Human stem cell research and regenerative medicine—present and future. Br. Med. Bull. 2011;99:155–168. doi: 10.1093/bmb/ldr027. [DOI] [PubMed] [Google Scholar]
- Wang L. Duan E. Sung L.Y. Jeong B.S. Yang X. Tian X.C. Generation and characterization of pluripotent stem cells from cloned bovine embryos. Biol. Reprod. 2005;73:149–155. doi: 10.1095/biolreprod.104.037150. [DOI] [PubMed] [Google Scholar]
- Wang S. Shen Y. Yuan X. Chen K. Guo X. Chen Y. Niu Y. Li J. Xu R.H. Yan X., et al. Dissecting signaling pathways that govern self-renewal of rabbit embryonic stem cells. J. Biol. Chem. 2008;283:35929–35940. doi: 10.1074/jbc.M804091200. [DOI] [PubMed] [Google Scholar]
- Wang S. Tang X. Niu Y. Chen H. Li B. Li T. Zhang X. Hu Z. Zhou Q. Ji W. Generation and characterization of rabbit embryonic stem cells. Stem Cells. 2007;25:481–489. doi: 10.1634/stemcells.2006-0226. [DOI] [PubMed] [Google Scholar]
- Zhou G.B. Meng Q.G. Li N. In vitro derivation of germ cells from embryonic stem cells in mammals. Mol. Reprod. Dev. 2010;77:586–594. doi: 10.1002/mrd.21187. [DOI] [PubMed] [Google Scholar]