Abstract
S-Nitrosothiol (RSNO) formation is one manner by which nitric oxide (•NO) exerts its biological effects. There are several proposed mechanisms of formation of RSNO in vivo: auto-oxidation of •NO, transnitrosation, oxidative nitrosylation, and from dinitrosyliron complexes (DNIC). Both free •NO, generated by •NO donors, and S-nitrosocysteine (CysNO) are widely used to study •NO biology and signaling, including protein S-nitrosation. It is assumed that the cellular effects of both compounds are analogous and indicative of in vivo •NO biology. A quantitative comparison was made of formation of DNIC and RSNO, the major •NO-derived cellular products. In RAW 264.7 cells, both •NO and CysNO were metabolized, leading to rapid intracellular RSNO and DNIC formation. DNIC were the dominant products formed from physiologic •NO concentrations, however, and RSNO were the major product from CysNO treatment. Chelatable iron was necessary for DNIC assembly from either •NO or CysNO, but not for RSNO formation. These profound differences in RSNO and DNIC formation from •NO and CysNO question the use of CysNO as a surrogate for physiologic •NO. Researchers designing experiments intended to elucidate the biological signaling mechanisms of •NO should be aware of these differences and should consider the biological relevance of the use of exogenous CysNO. Antioxid. Redox Signal. 17, 962–968.
Protein S-Nitrosation: Free Nitric Oxide Versus S-Nitrosocysteine
Nitric oxide (•NO) is a free radical and biological signaling molecule that has been assigned an ever-growing list of physiological and pathological functions. Originally, much of the biological activity attributed to •NO was thought to occur via its ability to activate or inhibit enzyme function through reversible binding to heme centers, with the most notable example being activation of soluble guanylyl cyclase. In addition to heme-nitrosyl formation, an important discovery was made demonstrating that S-nitrosation of key cysteine residues in thiol-containing proteins could regulate enzyme function (6). Much like phosphorylation controls protein activity, S-nitrosation was proposed as a new mechanism of •NO signaling through a post-translational modification termed protein S-nitrosylation.
Unlike •NO-heme interactions, however, the dominant biological mechanism by which S-nitrosothiols (RSNO) are formed has not been convincingly elucidated and remains a controversial and active area of research (9). Once •NO is enzymatically synthesized, it is released and randomly diffuses until encountering a suitable target with which to react. These targets are limited under biological conditions, as •NO readily reacts only with metals and other free radicals, including molecular oxygen. •NO does not react readily with free reduced thiols. Therefore, the formation of RSNO requires prior reaction of •NO with other cellular targets to form nitrosating species (e.g., N2O3) (9). Unlike protein phosphorylation, which is tightly controlled by a network of phosphatases and kinases, no such analogous network of enzymes for the specific regulation of S-nitrosation has been discovered. In the absence of specific nitrosating enzymes, nonenzymatic mechanisms must be invoked, including auto-oxidation of •NO, transnitrosation, oxidative nitrosylation, and most recently via dinitrosyliron complexes (DNIC) (1, 9).
Innovation.
Both S-nitrosocysteine (CysNO) and genuine nitric oxide (•NO) are used extensively and often interchangeably in the study of •NO cell signaling and biology. The developing realization that DNIC are major cellular •NO adducts, formed from endogenous •NO, stimulated this study, which is the first to compare formation of cellular dinitrosyliron complexes (DNIC) and S-nitrosothiols (RSNO) from CysNO versus •NO. Simply put, CysNO gave RSNO at very high levels, whereas •NO generated DNIC in large amounts. No evidence for equilibration or interconversion between intracellular RSNO and DNIC was observed. Chelatable iron (Fe) is necessary for DNIC assembly from either •NO or CysNO, but does not significantly influence RSNO formation. Moreover, •NO but not CysNO is capable of liberating Fe from other sources possibly via disrupting Fe-sulfur clusters. It is doubtful from these results that CysNO represents a surrogate for genuine •NO. Although the attraction of using CysNO lies in the substantial formation of cellular RSNO at levels 2–3 orders of magnitude higher than observed for genuine •NO, its relevance in the study of •NO-mediated nitrosation remains controversial.
In general, it can be assumed that endogenous formation of RSNO arises predominantly from enzymatic synthesis of •NO, with some contribution from dietary nitrite. Nevertheless, a large volume of research on •NO signaling and RSNO biology has been conducted in the absence of a source of genuine •NO. Instead, many researchers use RSNO, like S-nitrosocysteine (CysNO) or S-nitrosoglutathione (GSNO), as a surrogate for •NO. Formation of intracellular RSNO from nitrosthiols occurs via transnitrosation, the transfer of a nitrosonium equivalent (NO+), with no requirement for release of free •NO. As a result, transnitrosation does not influence the total amount of RSNO (4).
The extensive use of CysNO as a surrogate for •NO in studies on protein S-nitrosation demands a comparison of CysNO with a genuine •NO source, in particular because of the recognition that DNIC represent a greater reservoir of intracellular •NO adducts than RSNO. Iron (Fe) is at the heart of DNIC formation and it potentially mediates RSNO formation as well. It is clear, therefore, that an inherent inter-relationship exists between RSNO and DNIC formation through the convergence of reactions involving cellular Fe, thiols, and •NO (Fig. 1). Since it is unknown whether exogenous CysNO and •NO have similar effects on RSNO and DNIC levels (8), we set out simply to contrast the yields of RSNO and DNIC after cell treatment with either physiologic •NO concentrations liberated from the •NO-donor (Z)-1-[N-[3-aminopropyl]-N-[4-(3-aminopropylammonio)butyl]-amino]diazen-1-ium-1,2-diolate (Sper/NO) or comparable concentrations of CysNO.
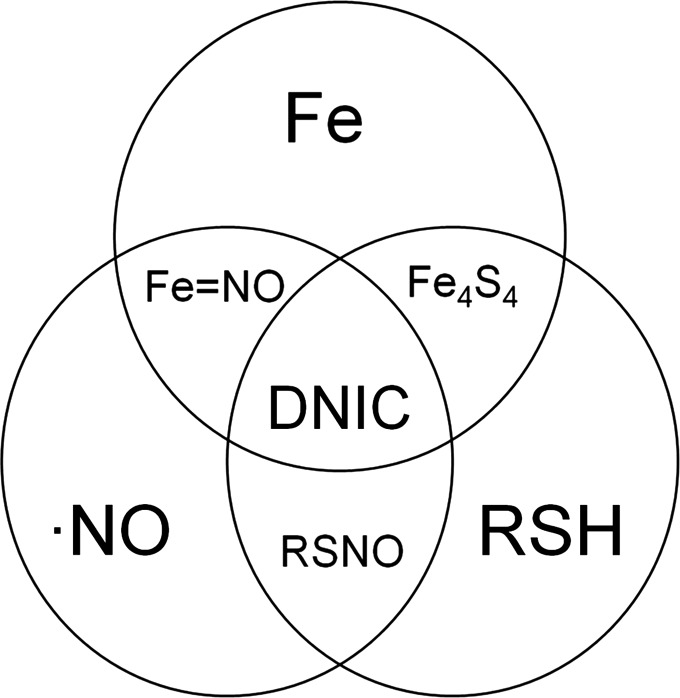
FIG. 1.
DNIC are at the convergence of NO, Fe, and thiols. Fe4S4, iron-sulfur clusters; •NO, nitric oxide; Fe, chelatable iron; RSH, reduced thiols; Fe=NO, iron nitrosyls; DNIC, dinitrosyliron complexes; RSNO, S-nitrosothiols.
Does CysNO Mirror the Effects of •NO Treatment on the Yield of Cellular RSNO and DNIC?
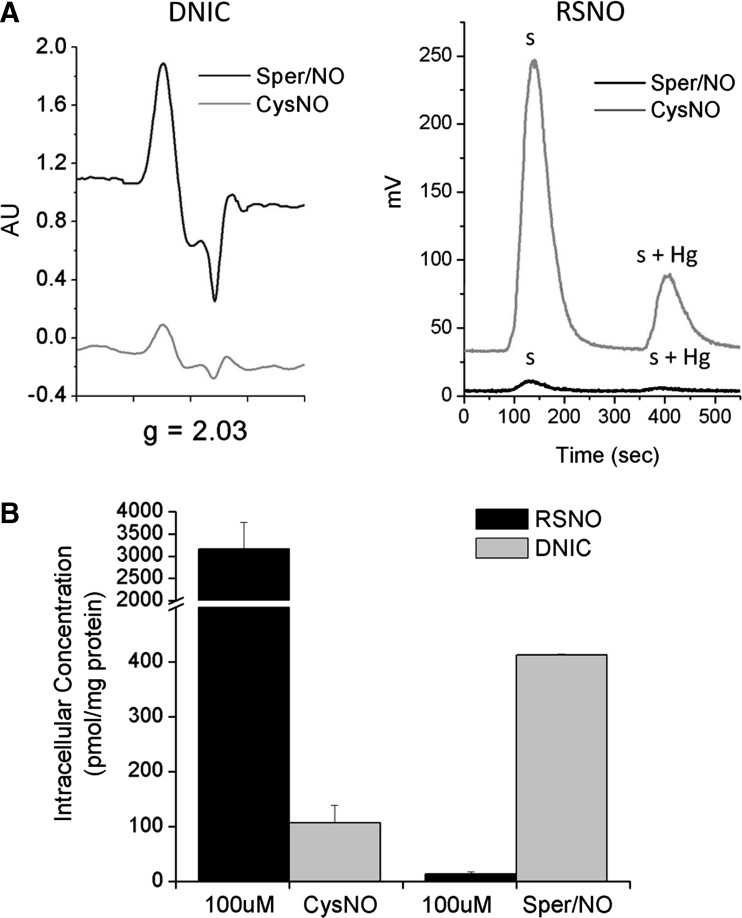
CysNO is frequently used interchangeably for •NO because it is presumed to have the same net effect on intracellular RSNO formation. Comparison of DNIC formation by CysNO and •NO have not been performed. After 1 h, RAW 264.7 cells exposed to physiologic •NO concentrations gave robust DNIC formation in ∼30-fold molar excess compared to the amount of RSNO formed (Fig. 2A, B). Conversely, in cells treated with CysNO, the dominant product was RSNO at levels 2–3 orders of magnitude greater than those achievable from •NO itself. The concentrations of DNIC after CysNO treatment were substantially diminished compared to cells treated with •NO. Both the absolute amounts and the proportions of the two dominant •NO-derived cellular adducts, DNIC and RSNO, are vastly different when cells are treated with CysNO versus •NO.
FIG. 2.
Comparing yields of intracellular RSNO and DNIC formation from exposure to CysNO or NO. RAW 264.7 cells were treated with 100 μM CysNO or 100 μM Sper/NO for 1 h in reaction buffer (Hank's balanced salt solution supplemented with 10 mM HEPES and 100 μM diethylenetriamine-pentaacetic acid). (A) Representative raw data of DNIC measurements in whole cells by EPR (left) and RSNO measurement in cell lysates by chemiluminescence (right). s, sulfanilamide treated; s+Hg, sulfanilamide+Hg treated. (B) Quantification of DNIC and RSNO from (A). Mean±SEM (n=4). EPR, electron paramagnetic resonance; CysNO, S-nitrosocysteine.
Are There Temporal Differences in the Patterns of RSNO Formation from CysNO and •NO?
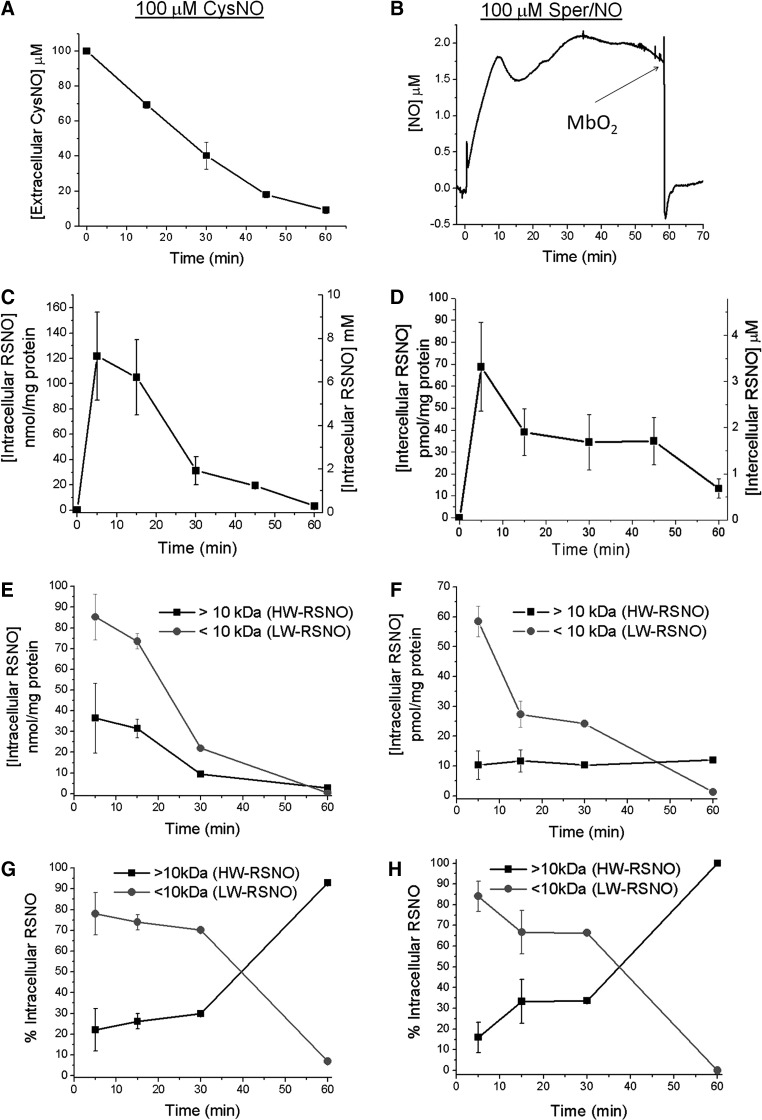
We set out to determine if the dramatic disparity in the yields of intracellular RSNO could be explained by changes in the exposure conditions over time (0–60 min). Despite extracellular CysNO levels changing dramatically and steady-state •NO concentrations remaining stable (Fig. 3A, B), there was a 90% decrease in the amount of RSNO detected after 1 h under both treatment conditions (Fig. 3C, D). Interestingly, both compounds generated intracellular RSNO concentrations that were greatest at early time points (5 min) and decreased over time. These results suggest that a population of intracellular thiols may exist that are susceptible to rapid nitrosation by a variety of mechanisms (i.e., transnitrosation, DNIC, or N2O3), but the RSNO products decompose irreversibly. The net result would be the accumulation of oxidized thiols that are no longer susceptible to nitrosation via any mechanism. This would explain a gradual decrease in the yields of RSNO even in the face of a continual supply of nitrosating agents.
FIG. 3.
Contrasting temporal patterns of cellular RSNO formation from CysNO and NO treatments. For all experiments RAW 264.7 cells were treated with either 100 μM CysNO or Sper/NO in reaction buffer. At the indicated time points (5, 15, 30, 45, or 60 min) RSNO were quantified by chemiluminescence. (A) Measurements of extracellular CysNO concentrations. (B) Real-time electrochemical measurement of steady-state •NO concentrations in the medium (•NO-selective electrode ∼1 mm above monolayer) of Sper/NO-treated cells. The experiment was terminated by addition of the •NO scavenger MbO2 to verify electrode response and presence of •NO. (C) Chemiluminescent measurements of intracellular RSNO after treatment with CysNO (5–60 min). (D) Chemiluminescent measurements of intracellular RSNO after treatment with Sper/NO (5–60 min). (E) Chemiluminescent measurements of intracellular RSNO after treatment with CysNO followed by passage of cell lysate through a 10-kDa cutoff filter. (F) Chemiluminescent measurements of intracellular RSNO after treatment with Sper/NO followed by passage of cell lysate through a 10-kDa cutoff filter. (G) Data from panel (E) expressed as a percentage of total RSNO. (H) Data from panel (F) expressed as a percentage of total RSNO. LW-RSNO, low-molecular-weight RSNO; HW-RSNO, higher-molecular-weight RSNO; MbO2, oxymyoglobin.
It has been shown previously that CysNO is actively taken up by RAW 264.7 cells resulting in the transnitrosation of intracellular thiols. The total measured RSNO would incorporate CysNO itself and a combination of other low-molecular-weight RSNO (LW-RSNO), in particular GSNO, and higher-molecular-weight RSNO (HW-RSNO). We measured HW-RSNO (>10 kDa) and LW-RSNO (<10 kDa) at 5–60 min subsequent to CysNO treatment. LW-RSNO represented >80% of the total at times ≤30 min, whereas by 60 min, HW-RSNO were the most abundant population due to their increased stability (Fig. 3E, G). Interestingly, similar patterns were observed after •NO treatment. The total amount of RSNO was much lower, but the decay of LW-RSNO and the emergence of a basal pool of HW-RSNO was comparable (Fig. 3F, H). This stable population of HW- RSNO appears persistent regardless of the source.
Is There a Requirement for Chelatable Fe in the Formation of DNIC from CysNO?
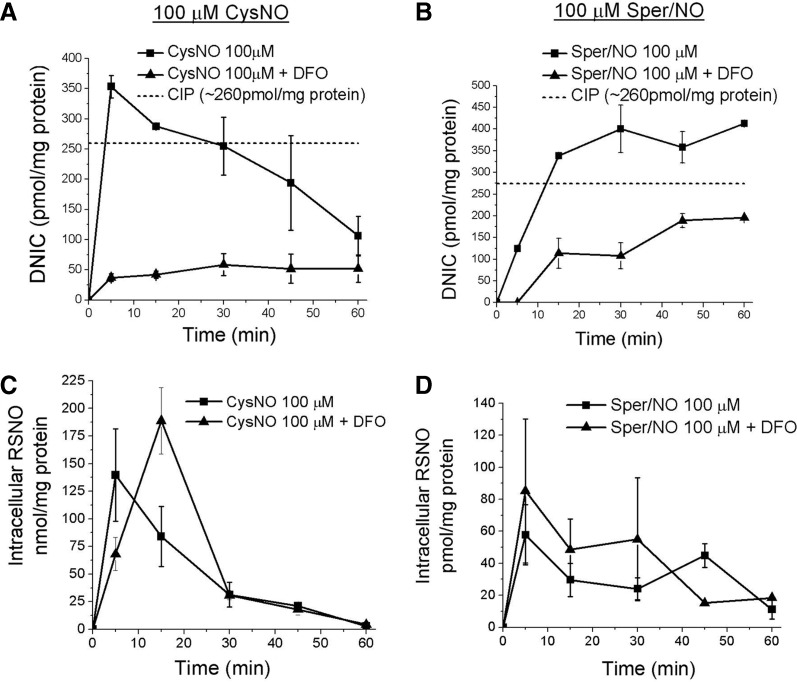
We and others have shown that chelatable Fe is required for the formation of cellular DNIC from physiologic •NO concentrations (3, 7). We set out to compare the effects of desferrioxamine (DFO), a cell-permeable Fe chelator, on DNIC formation from CysNO versus •NO. The chelatable iron pool (CIP), the major source of Fe for DNIC assembly, was observed to be insensitive to cell treatment conditions (∼260 pmol/mg protein). Unsurprisingly, Fe chelation with DFO inhibited the formation of DNIC in cells treated with either CysNO or Sper/NO. In the two treatment paradigms, however, the variations in DNIC with time were quite different, and the percent inhibition was much greater (85%) in the CysNO-treated samples (Fig. 4A, B). It can be postulated that during CysNO exposure, and early •NO exposure, the majority of Fe for DNIC assembly originates from the CIP. Continuous exposure to •NO liberates Fe from other sources, whereas CysNO does not. It is likely that under these conditions •NO, and not CysNO, is capable of disrupting Fe-sulfur clusters to release Fe, and thus CysNO does not mimic the impact of free •NO on Fe homeostasis. The significant and disproportionate effect of DFO on DNIC formation from CysNO may result from a requirement for Fe-induced reduction of CysNO by the CIP—potentially ruling out CysNO transnitrosation as a mechanism for DNIC formation. More importantly, these results further highlight the striking phenotypic, and possibly mechanistic, differences resulting from the use of CysNO instead of •NO.
FIG. 4.
The effect of Fe chelation on intracellular DNIC and RSNO formation from CysNO and Sper/NO. RAW 264.7 cells in reaction buffer were pretreated +/− desferrioxamine (DFO) for 4 h followed by exposure to either 100 μM CysNO or 100 μM Sper/NO for (5–60 min). (A) EPR measurements (g=2.03) of cellular DNIC after CysNO treatment. (B) EPR measurements (g=2.03) of cellular DNIC after Sper/NO treatment. The chelatable iron pool (CIP) was quantified in both (A) and (B) by measuring the g=4.3 signal by EPR in the DFO-treated samples. (C) Chemiluminescent measurements of intracellular RSNO after treatment with CysNO +/− DFO. (D) Chemiluminescent measurements of intracellular RSNO after treatment with Sper/NO +/− DFO. Data represents mean±SEM (n=4).
Is There a Requirement for Chelatable Fe in the Formation of RSNO from CysNO and •NO?
Recent evidence suggests that there is an inter-relationship between the presence of DNIC or chelatable Fe and RSNO formation from •NO (3, 7). The role of Fe in RSNO formation from CysNO, however, has not been extensively studied in cells. For these reasons, we examined the role of DFO on the yields of RSNO formation from both CysNO and •NO exposure. Although the dramatic differences persisted between the overall magnitude of RSNO formation between CysNO and •NO-treated cells, the effect of DFO pretreatment in both paradigms was not significant (Fig. 4C, D). This result was not unexpected for CysNO, where metal-independent transnitrosation mechanisms of RSNO formation predominate. However, the inability of DFO to inhibit intracellular RSNO formation in the presence of •NO was surprising. This implies that the bulk of RSNO formation by •NO, in these cells and under these experimental conditions, is independent of DNIC formation or chelatable Fe.
Is Intracellular RSNO Formation a Result of Extracellular CysNO and •NO Chemistry?
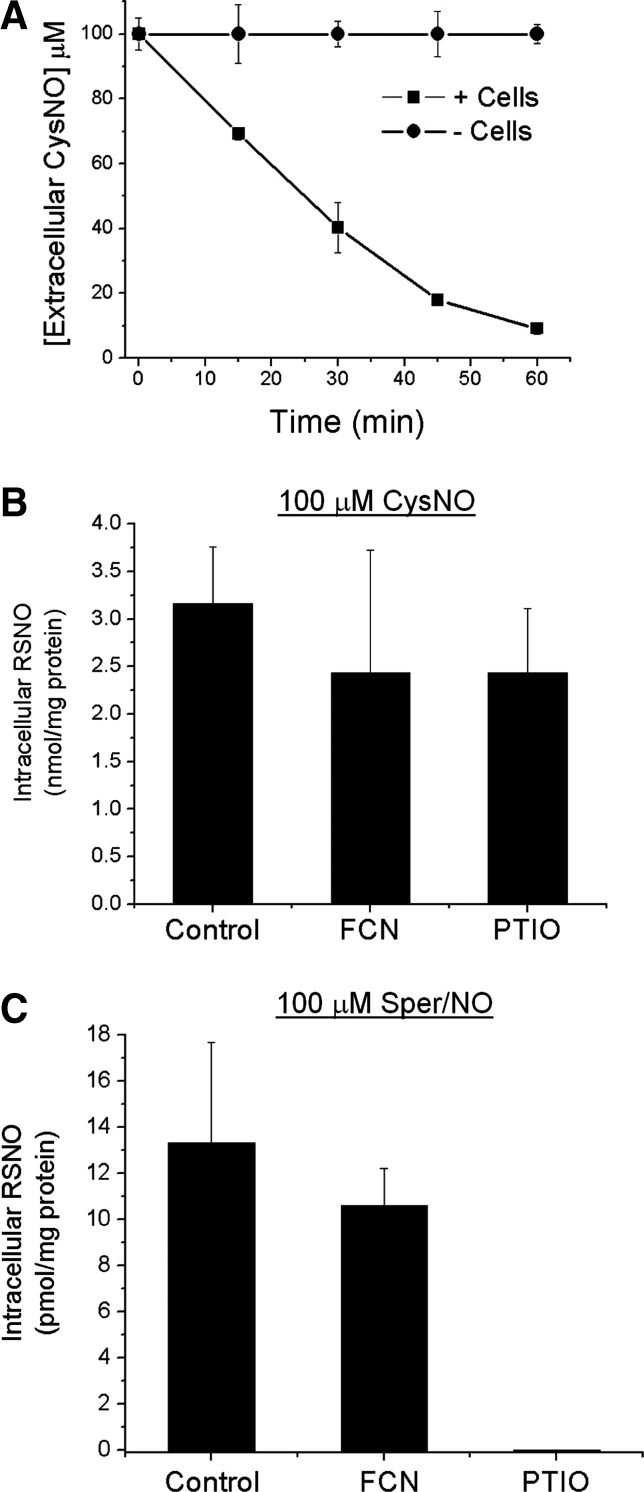
Under cell culture conditions, the volume of media compared to the volume of cells in monolayer is large. Although CysNO does not break down appreciably in the media in the absence of cells (Fig. 5A), it was important to ascertain the contribution of nitrosating species that formed in the media from CysNO and Sper/NO. Extracellularly generated N2O3 that results from the reaction of •NO with •NO2 is a potent nitrosating agent that could potentially be a confounding factor when studying cellular nitrosation mechanisms by •NO. For this reason extracellular scavengers of •NO and •NO2 were studied. Figure 5 demonstrates that scavenging •NO2 did not diminish the yield of intracellular RSNO formation from •NO or CysNO, whereas scavenging of •NO with 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO) completely prevented intracellular S-nitrosation in the presence of Sper/NO but did not diminish the amount of RSNO formed during CysNO treatment (Fig. 5B, C). The mechanism of RSNO formation from CysNO is not via the liberation of extracellular •NO, and the mechanism of RSNO formation from •NO is not via extracellular auto-oxidation to a nitrosating species.
FIG. 5.
Excluding extracellular chemistry of CysNO and Sper/NO on intracellular RSNO formation. (A) CysNO is metabolized by cells and does not decay in reaction buffer in the absence of cells. 100 μM CysNO was added to 100 mm2 culture dishes +/− RAW 264.7 cells (30×106). CysNO concentrations were measured in the buffer by chemiluminescence (0–60 min). RAW 264.7 cells were treated with either 100 μM CysNO (B) or 100 μM Sper/NO (C) for 1 h in reaction buffer +/− 1 mM FCN to scavenge extracellular •NO2 or +/− 1 mM PTIO to scavenge extracellular •NO. The RSNO content in the cell lysate was quantified by chemiluminescence. Data represent mean±SEM (n=3). PTIO, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide; FCN, ferrocyanide.
Concluding Remarks
The message from this study of RAW 264.7 cells is starkly demonstrated in Figure 2 and in subsequent time-course studies: there are vast differences in the overall magnitude, type, and stability of intracellular •NO adducts formed upon exposure of cells to CysNO versus genuine •NO. The corollary is that the phenotypic effects observed subsequent to CysNO exposure should not be assumed to be indicative of, or synonymous with, the cellular effects of physiologic •NO. The mechanism of intracellular RSNO formation from CysNO, via transnitrosation, does not require release of free •NO. Protein S-nitrosation in response to •NO can only involve transnitrosation subsequent to oxidation or oxidative nitrosylation. Therefore, inferences drawn on the effects of •NO are problematic when studies solely use CysNO; careful comparisons and parallel studies are needed with both CysNO and •NO.
Notes
Materials and Methods
Chemicals
Spermine nonoate (Sper/NO) was a generous gift from Dr. Joseph Hrabie (NIH/NCI). Diethylenetriamine-pentaacetic acid (DTPAC), DFO mesylate, ferrocyanide (FCN), PTIO, sulfanilamide, and N-ethylmaleimide were purchased from Sigma-Aldrich Corporation.
Cell culture
RAW 264.7 were grown to 80% confluence in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 1% penicillin/streptomycin. Immediately before treatments, cells were washed 3×with phosphate-buffered saline and placed in Hank's balanced salt solution (HBSS) containing 10 mM HEPES and 100 μM DTPAC.
Treatment conditions
•NO treatment
We chose a concentration of 100 μM Sper/NO for two reasons. First, this amount gives approximate physiologic steady-state •NO concentrations, although at the higher limit (1–2 μM, Fig. 3B). Second, this concentration of •NO was the minimum that could be used to produce detectable and accurately quantifiable intracellular RSNO.
CysNO treatment
Although the physiologically relevant concentration CysNO has not been fully defined, the concentrations of CysNO used commonly by researchers for in vitro RSNO experiments range from low μM to high mM. We used 100 μM CysNO because this concentration would deliver approximately an equimolar amount of •NO equivalents over the course of the experiment. Ninety percent of CysNO and 75% of Sper/NO decay by 1 h (Fig. 3A, B) such that the total amount of •NO or equivalents would be similar (1.7±0.1 moles of •NO are released per mole of decomposed Sper/NO).
Quantification
RSNO
RSNO were measured by chemiluminescence with a Sievers nitric oxide analyzer 280i according to the manufacturer's protocols and as previously described (5).
Real-time •NO measurements
Cells were grown in 10-cm culture plates. A •NO-selective electrode (amiNO-2000, innovative instruments) connected to an Apollo 4000 free radical analyzer (World Precision Instruments) was positioned ∼1 mm above, and perpendicular to the cell. The electrode was allowed to equilibrate at 37°C in HBSS for 2 h followed by addition of the •NO-donor Sper/NO (n=3).
Electron paramagnetic resonance
Electron paramagnetic resonance (EPR) was performed on a Bruker X-band EMX Plus EPR spectrometer. Samples were frozen and read in liquid N2. DNIC were detected at g=2.03, modulation amplitude 10G, 200G scan range, 90 s scan time, one scan. For quantification, the double integral of the first derivative spectra was compared to a standard curve generated with synthetic diglutathion DNIC as previously described (2). The CIP was estimated by treating cells with 1 mM DFO for 4 h. The resulting Fe3+-DFO g=4.3 signal was read with a modulation amplitude of 10G, 200G scan range, 30 s scan time, four scans. For quantification, the double integral of the first derivative spectra was compared to that of a standard curve generated with Fe3+-DFO as previously described (2).
Abbreviations Used
- CIP
chelatable iron pool
- CysNO
S-nitrosocysteine
- DFO
desferrioxamine
- DNIC
dinitrosyliron complexes
- DTPAC
diethylenetriamine-pentaacetic acid
- EPR
electron paramagnetic resonance
- FCN
ferrocyanide
- Fe
iron
- GSNO
S-nitrosoglutathione
- HBSS
Hank's balanced salt solution
- HW-RSNO
higher-molecular-weight S-nitrosothiol
- LW-RSNO
low-molecular-weight S-nitrosothiol
- MbO2
oxymyoglobin
- •NO
nitric oxide
- PTIO
2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide
- RSNO
S-nitrosothiol
- Sper/NO
(Z)-1-[N-[3-aminopropyl]-N-[4-(3-aminopropylammonio)butyl]-amino]diazen-1-ium-1,2-diolate
Acknowledgments
The project described was supported in part by Award Number 1R01GM094175-01A1 from the National Institute of General Medical Science and a grant from the American Cancer Society. We also acknowledge ongoing support from the UIC Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Science or the National Institutes of Health.
References
- 1.Bosworth CA. Toledo JC., Jr. Zmijewski JW. Li Q. Lancaster JR., Jr. Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc Natl Acad Sci U S A. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickok JR. Sahni S. Mikhed Y. Bonini MG. Thomas DD. Nitric oxide suppresses tumor cell migration through N-Myc downstream-regulated gene-1 (NDRG1) expression: role of chelatable iron. J Biol Chem. 2011;286:41413–41424. doi: 10.1074/jbc.M111.287052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickok JR. Sahni S. Shen H. Arvind A. Antoniou C. Fung LW. Thomas DD. Dinitrosyliron complexes are the most abundant nitric oxide-derived cellular adduct: biological parameters of assembly and disappearance. Free Radic Biol Med. 2011;51:1558–1566. doi: 10.1016/j.freeradbiomed.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keszler A. Zhang Y. Hogg N. Reaction between nitric oxide, glutathione, and oxygen in the presence and absence of protein: how are S-nitrosothiols formed? Free Radic Biol Med. 2010;48:55–64. doi: 10.1016/j.freeradbiomed.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacArthur PH. Shiva S. Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Stamler JS. Jaraki O. Osborne J. Simon DI. Keaney J. Vita J. Singel D. Valeri CR. Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toledo JC., Jr. Bosworth CA. Hennon SW. Mahtani HA. Bergonia HA. Lancaster JR., Jr. Nitric oxide-induced conversion of cellular chelatable iron into macromolecule-bound paramagnetic dinitrosyliron complexes. J Biol Chem. 2008;283:28926–28933. doi: 10.1074/jbc.M707862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanin AF. Dinitrosyl iron complexes with thiolate ligands: physico-chemistry, biochemistry and physiology. Nitric Oxide. 2009;21:1–13. doi: 10.1016/j.niox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y. Hogg N. S-nitrosohemoglobin: a biochemical perspective. Free Radic Biol Med. 2004;36:947–958. doi: 10.1016/j.freeradbiomed.2004.01.008. [DOI] [PubMed] [Google Scholar]