Abstract
Significance: Posttranslational modification of proteins through phosphorylation, glycosylation, and oxidation adds complexity to the proteome by reversibly altering the structure and function of target proteins in a highly controlled fashion. Recent Advances: The study of reversible cysteine oxidation highlights a role for this oxidative modification in complex signal transduction pathways. Nitric oxide (NO), and its respective metabolites (including reactive nitrogen species), participates in a variety of these cellular redox processes, including the reversible oxidation of cysteine to S-nitrosothiols (RSNOs). RSNOs act as endogenous transporters of NO, but also possess beneficial effects independent of NO-related signaling, which suggests a complex and versatile biological role. In this review, we highlight the importance of RSNOs as a required posttranslational modification and summarize the current methods available for detecting S-nitrosation. Critical Issues: Given the limitations of these indirect detection methods, the review covers recent developments toward the direct detection of RSNOs by phosphine-based chemical probes. The intrinsic properties that dictate this phosphine/RSNO reactivity are summarized. In general, RSNOs (both small molecule and protein) react with phosphines to yield reactive S-substituted aza-ylides that undergo further reactions leading to stable RSNO-based adducts. Future Directions: This newly explored chemical reactivity forms the basis of a number of exciting potential chemical methods for protein RSNO detection in biological systems. Antioxid. Redox Signal. 17, 981–991.
Introduction
Nitric oxide (NO) is an important and established signaling molecule involved in a variety of critical biological processes, including vasodilation, blood pressure regulation, neuronal signaling, and the immune response. Much of NO's role as a second messenger in signal transduction occurs through the activation of soluble guanylyl cyclase (sGC), including relaxation of smooth muscle cells, inhibition of platelet aggregation, promotion of angiogenesis, and decreased inflammation (35). NO also modifies protein cysteine residues through reactive nitrogen species (RNS) making RNS-associated biological signaling a subject of intense research interest. RNS arise from endogenously produced NO in oxygenated environments and elicit a variety of effects within cells. At low concentrations, RNS evoke a diverse range of signaling events, but higher concentrations can lead to cytotoxicity (8, 21, 59). Accumulating evidence suggests that many proteins actually require RNS oxidation for their normal catalytic function (5, 11, 43). S-Nitrosothiols (R-S-N=O) represent one of the most studied and described RNS-based modifications of protein cysteine residues. S-Nitrosothiols (RSNOs) may act as endogenous transporters and storage forms of NO, but also appear to have other profound effects on NO-related cell signaling.
The Chemistry and Biology of S-Nitrosothiols
While NO clearly activates sGC, and various S-nitrosothiols act as NO carriers and donors, protein S-nitrosation reveals profound sGC-independent signaling effects, suggesting a role other than NO transport and storage. S-Nitrosation of caspase 3 inhibits apoptosis (33, 63) and poly-S-nitrosation of the ryanodine receptor (RyR 2) reversibly activates calcium channels to enhance cardiac contractility (73). NO-induced S-nitrosation of N-ethylmaleimide-sensitive factor inhibits platelet granule exocytosis and may be one means by which NO regulates thrombosis (41). S-Nitrosation also inhibits the transcription factor nuclear factor kappa B (37, 51) and recent work demonstrates a role for glyceraldehyde-3-phosphate dehydrogenase S-nitrosation in the regulation of cytosolic heme transport to inducible nitric oxide synthase (7). The formation of protein S-nitrosothiols from the reaction of endogenously produced NO and RNS, and subsequent de-nitrosation events defines a new paradigm of protein posttranslational modification/activity relationship analogous to protein phosphorylation and de-phosphorylation. The various roles of S-nitrosated proteins have recently been extensively reviewed (6, 19, 20, 25).
S-nitrosothiol formation and decomposition
Synthetically, the quickest route to generate an S-nitrosothiol is through direct nitrosation by treatment of a thiol with acidic nitrite (which acts as an NO+ donor), N2O3, or an alkyl nitrite (6). In organic solvents, tert-butyl nitrite or nitrosonium tetrafluoroborate act as efficient nitrosating agents (70). Organic and small-molecule S-nitrosothiols are easily quantified based on their characteristic absorbance at roughly 336 and 545 nm and usually exist as bright green, red, or pink solids or oils (24). Several organic and small-molecule RSNOs have been readily isolated and characterized, including S-nitroso-N-acetyl-penicillamine (SNAP), S-nitrosoglutathione (GSNO), tritylthionitrite, and S-nitroso-N-acetyl cysteine (6). The treatment of thiol-containing proteins with acidified nitrite can yield S-nitrosated proteins. However, trans-nitrosation, the transfer of the nitroso group from one thiol to another, remains one of the most common methods for preparing S-nitrosothiol-modified proteins (36).
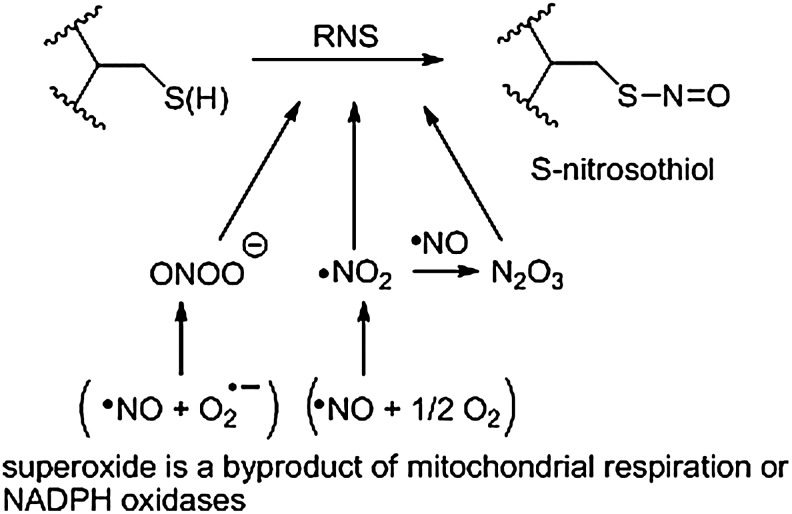
Under physiological conditions, protein cysteine residues react with certain RNS and O2 to form RSNOs and Figure 1 shows the proposed in vivo formation of S-nitrosothiols. These mechanisms could hold for both small-molecule and protein-containing thiols. Interestingly, the reaction of reduced glutathione (GSH) with NO does not produce GSNO directly, but gives glutathione disulfide (or oxidized glutathione [GSSG]) and nitroxyl (HNO) (26). The formation of GSNO, or any RSNO, from NO requires oxygen and only occurs after NO oxidation by O2 to form·NO2 or N2O3 (Fig. 1) (57). The reaction of NO with the superoxide anion (O2−) generates peroxynitrite (ONOO−), a potent oxidant capable of nitrosating various protein targets (9, 28). The most likely RNS capable of nitrosating target thiols in biological systems is N2O3 and its formation is kinetically facilitated in hydrophobic environments such as biological membranes (34). Thiol transnitrosation remains the most common method for thiol nitrosation within the proteome and occurs via the transfer of [NO+] from neighboring RSNOs or from iron nitrosyl species that generate NO+ from the reaction of NO with both ferrous and ferric heme groups (38, 48, 77). These transnitrosation events are not random, and highly selective [NO+] transfer between two protein thiols, based on specific protein–protein interactions, occurs in cells (39).
FIG. 1.
The formation of protein S-nitrosothiols. Several types of reactive nitrogen species (RNS) can form S-nitrosothiols (RSNOs) in vivo, including peroxynitrite (ONOO−), nitrogen dioxide (NO2), or dinitrogen trioxide (N2O3).
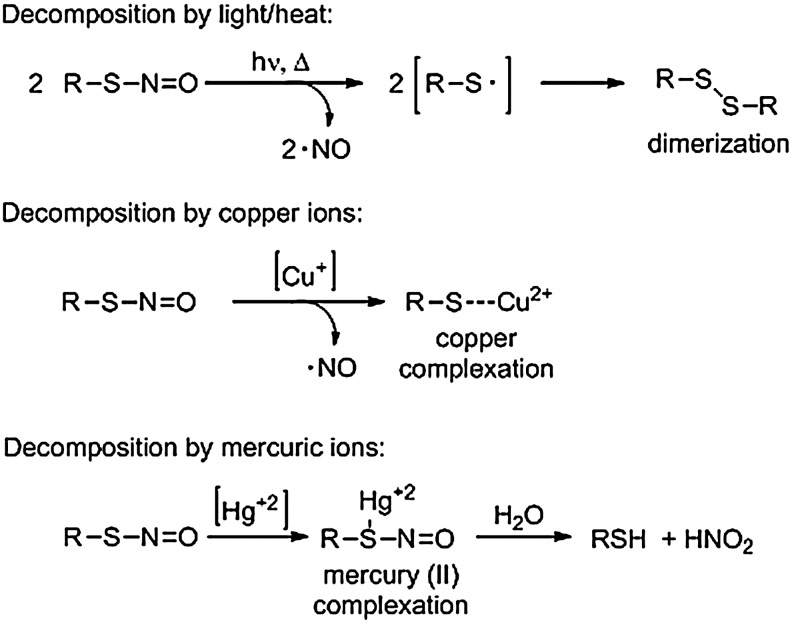
Generally RSNOs decompose via homolytic cleavage of the S-NO bond in the presence of white light, generating NO and a thiyl radical (RS·), which quickly dimerizes (24, 60), suggesting a biological role for RSNOs as NO storage and transport devices (Fig. 2) (70). The decomposition of RSNOs to NO by copper ions in solution is also well established. Cu(I) ions at concentrations as low as 10−6 M react as a catalytic reductant and decompose RSNOs to form NO (6). For many RSNOs, this rate of decomposition is effectively zero order, suggesting that the formation of Cu+ is the rate limiting step (70). Protein-bound copper sources are also capable of generating NO from RSNOs (12). Overall, RSNO decomposition rate does not depend on the rate of thermolysis but on the presence of copper ions in solution, light, and the proximity of other thiolates. In addition to homolytic cleavage, other metals (particularly Hg+2) facilitate heterolytic RSNO cleavage to the thiol and nitrous acid (Fig. 2).
FIG. 2.
Decomposition of RSNOs by heat/light or metal ions. The decomposition of RSNOs releases nitric oxide (NO), highlighting a possible role for this posttranslation modification in NO storage and transport.
Structure and stability of S-nitrosothiols
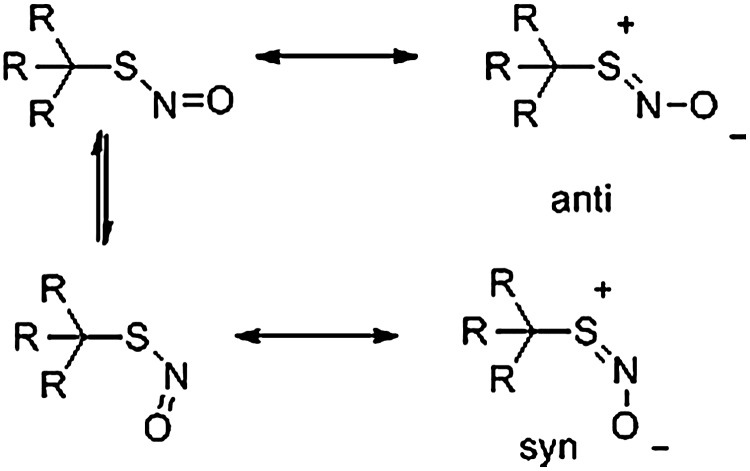
Examination of several small-molecule RSNOs shows that the bond dissociation energy of the RS-NO bond only lies between 23 and 32 kcal/mol (2). RSNO stability depends on the substituents attached to the carbon alpha to the SNO group (2). Primary RSNOs are inherently unstable and have only been characterized by mass spectrometry or through spectroscopic methods, whereas tertiary RSNOs, including SNAP, are easily crystallized, indefinitely stable, and have been characterized by X-ray crystallography (16). The structure of the RSNO (primary vs. tertiary) directly affects the spectroscopic properties of the RSNO (both 15N nuclear magnetic resonance [NMR] and UV-vis spectroscopy) in a predictable manner (68). Through resonance, S-nitrosothiols exhibit S-N double bond character yielding two geometric isomers: syn and anti (Fig. 3) convertible through bond rotation of the S-N single bond resonance form. Hybrid density functional theory calculations and examination of existing crystallographic data show that sterically hindered RSNOs exist in the antiorientation; however, less-sterically hindered compounds show a strong preference for the syn orientation (2). These studies form the basis for predicting the reactivity of small-molecule and protein RSNOs. X-Ray crystallographic studies of the S-nitrosation of human thioredoxidin show that both Cys-62 and Cys-69 form protein SNOs predominantly in the syn form as predicted for primary-substituted thiols (69). Despite these advances in understanding RSNO chemistry, S-nitrosothiols demonstrate a wide range of stability and reactivity and clear structure/reactivity relationships do not exist at this time.
FIG. 3.
Syn- and antiresonance forms of an S-nitrosothiol. Primary-substituted RSNOs favor a syn-orientation, which tertiary RSNOs exist in the anti-isomer.
Current RSNO Detection Methods
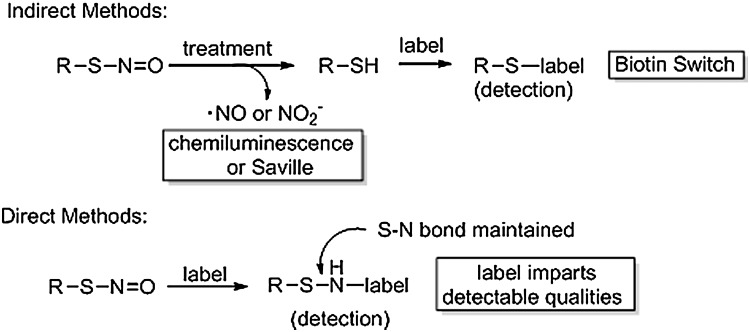
Stable small-molecule S-nitrosothiols are easily quantified because of their characteristic absorbance patterns at roughly 336 and 545 nm (24). Concentrations typically obtained in biological assays (high nM–low μM) remain too low for detection using UV/Vis spectroscopy, which presents a challenge for researchers looking to correlate protein RSNOs to their in vivo function. The inherent instability of RSNOs (weak S-N bond strength and susceptibility to metal-ion-catalyzed decomposition) makes their detection difficult. To date, the most widely used methods rely upon the complete removal of the NO group from the RSNO followed by “tagging” the nascent thiol (via the biotin switch method) or detecting the liberated NO species (chemiluminescence or Saville assay). Thus, each method indirectly detects the RSNO group and, at present, no universal direct method exists to specifically identify protein RSNOs. We will briefly review the current methods for quantifying protein S-nitrosation and discuss the potential for direct covalent labeling methods.
The biotin switch technique
The most widely cited method for the indirect detection of protein S-nitrosothiols is the biotin switch technique (BST) (17, 29, 53). The BST consists of three basic steps: (i) blocking all free cysteine thiols, (ii) reduction of RSNOs with ascorbate, and (iii) in situ labeling of the nascent thiols with a biotin label (Fig. 4). RSNO concentrations are determined based on a biotin pull-down method or through western blot analysis. The advantage of this technique is that it can be performed without specialized equipment and can detect low μM concentrations of biotinylated protein. Given the chemical instability of RSNOs, the numerous processing steps required for this method likely destroy some S-nitrosated proteins and decrease the confidence that this assay accurately accounts for all of the protein S-nitrosothiols in a particular sample. Also, this technique hinges on the assumption that ascorbate specifically reduces RSNO, despite some evidence that ascorbate reduces sulfenic acids (RSOHs) as well as some disulfides (31, 40).
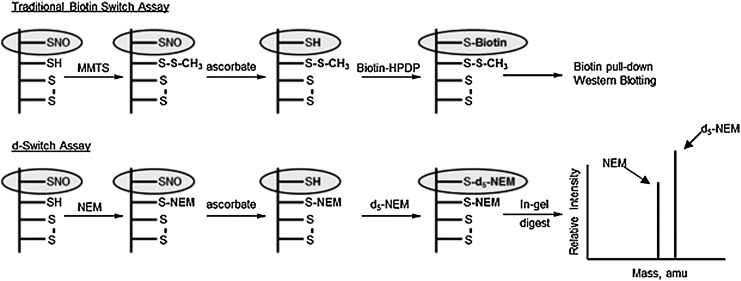
FIG. 4.
Schematic of the Biotin Switch (above) and d-Switch assays (below). In the traditional biotin switch technique (BST), S-nitrosated proteins are biotinylated and detected using western blotting techniques. The d-Switch method simultaneously quantifies free thiol/RSNO ratios for each protein by comparing ratios of d0/d5-N-ethyl maleimide (NEM) adducts, respectively.
Since its original development, many improvements to the BST have been reported.
In the “fluorescence switch,” the substitution of fluorescein-5-maleimide for biotin-HPDP in the final alkylation step of the BST enhances the overall sensitivity of the assay (64). The powerful advantage of this method is that it easily couples to 2-dimensional gel electrophoresis (2-DE) that allows for the individual identification of S-nitrosated proteins. Another useful modification to the BST was the development of a microarray-based BST (18). By using an antibiotin antibody, this assay achieves greater sensitivity as well as applications for high throughput analysis of the S-nitroso proteome. The utility of these techniques was recently reviewed (65).
Recently, Thatcher et al. reported on the d-Switch method for the quantification of RSNOs in cell lysates (58). This method, similar to the BST, allows for the simultaneous quantification of both S-nitrosated and non-nitrosated cysteines. Figure 4 outlines the basic steps of both the BST and the d-Switch methods. In the d-Switch assay, the initial blocking step uses N-ethyl maleimide (NEM) as opposed to iodoacetamide (IAA). Following the RSNO reduction, the nascent thiol groups are alkylated with D5-NEM, allowing for the direct comparison of thiol/RSNO ratios by mass spectrometry (MS). This method provides powerful advantages over the traditional BST. The direct examination of individual cysteine residues is valuable in determining the cysteine microenvironment, propensity for oxidation, and relative cysteine reactivity on a protein-by-protein basis. In the standard BST, S-nitrosated proteins are biotinylated and quantified by western blot. In the d-Switch method, ratios of free thiol/S-nitrosothiol are determined by liquid chromatography MS (LC-MS) on individual cysteine-containing peptides of interest. This new technique provides a powerful alternative to quickly discern changes in S-nitrosation of reactive cysteine residues on a protein-by-protein basis.
Chemiluminescence-based quantification
A complementary technique for S-nitrosothiol detection is reductive chemiluminescence using reagents designed specifically to promote stoichiometric NO release from RSNOs. Chemiluminescence-based assays correlate NO concentration to the generated luminescence (NO2*→NO2) when NO reacts with ozone, producing NO2 in the excited state (NO2*). We summarize the major strategies for detecting RSNOs below; a more detailed review of all RSNO-based chemiluminescence techniques was recently published (3). In the tri-iodide method (I3−), helium gas is bubbled through a chamber containing potassium iodide (KI), iodine, and acidic acid. This method simultaneously detects RSNOs, iron nitrosyls, and nitrite (15, 52). To detect SNOs specifically, nitrite can be removed from these samples by pretreating with 5%–10% acidified sulfanilamide (10, 47).
In the 3C method, which contains copper chloride (CuCl), carbon monoxide (CO), and cysteine, excess cysteine in the assay serves to drive the trans-nitrosation equilibrium in favor of cysteine-SNO (Cys-SNO) and maintains the Cu+ redox state of the metal, required to generate NO from Cys-SNO (22). The CO gas prevents auto-capture of NO by heme in solution. This 3C assay has been modified to circumvent the need for CO gas in the reaction chamber (CuCl/Cys only, 2C method) by treating samples with a nitrite stabilization solution (K3FeCN6 and NEM) prior to analysis (14). At neutral pH, both the 3C and modified 2C assays are not compatible with plasma or red blood cell samples because of excess foaming, requiring the experiment to be run in glacial acetic acid to reduce foaming-associated detection issues (42). However, care must be taken if working under these conditions as nitrite will act as a nitrosating agent at low pH. Free thiols must be blocked with NEM or an equivalent reagent to ensure accuracy. In both the 3C and modified 2C assays, samples must also be treated with mercury chloride (HgCl2) as a control to ensure the accurate detection of RSNOs (3). While chemiluminescence provides a useful and accurate alternative to the BST, its biggest drawback is the failure to indicate which cysteine residue was modified, and proper care must be taken during sample preparation to prevent spurious RSNO formation.
The Saville assay
Another method for indirect S-nitrosothiol detection is the Saville assay (54). This method relies on the mercuric (Hg+2)-ion-facilitated hydrolysis of an RSNO to nitrite followed by the classic Griess assay for nitrite detection (46, 71). The difference in nitrite concentrations of complex mixtures±HgCl2 is taken as the total [RSNO] content for the sample (22). Unfortunately, this assay has a limit of detection of ∼500 nM making it less useful for detection of many biological SNOs and the mercuric ions may complex with protein thiols yielding intractable mixtures.
Recently in relatively similar chemistry, gold nanoparticles (AuNPs) were used to detect S-nitrosated peptide residues (13). AuNPs react with protein SNOs to yield AuNP-protein conjugates and release NO (30). In this assay, free thiols are first blocked with IAA and the sample is digested. Following digest, AuNPs are introduced and the AuNP-alkylated peptides are harvested by centrifugation and exchanged from the AuNP surface by incubation with excess dithiothreitol (DTT) (13). The peptides are then analyzed by LC-MS, where ratios of free thiol/S-nitrosothiol are obtained. This method was highly specific in a pure protein system and has yet to be applied to complicated biological mixtures.
Direct MS-based detection
A recent kinetic analysis of thioredoxin (Trx) nitrosation by GSNO illustrates the power of direct RSNO detection by MS-based techniques. Marletta et al. demonstrated that coupling the RapidFire DS Module™ (Agilent Technologies), a highly sophisticated dual-valve desalting system, to an eletrospray ionization time-of-flight mass spectrometry allowed for the accurate time-dependent detection of Trx S-nitrosation using physiologically relevant concentrations of GSNO and Trx (1). This technique removed salts, GSH, GSSG, and GSNO from the samples in a time-dependent fashion, allowing for the rapid detection of Trx-SNO formation at short time intervals while simultaneously monitoring the redox status of the protein (1). This new method provides a powerful tool for directly studying low-level S-nitrosation in a physiologically relevant context by capturing and visualizing protein RSNO adducts in the MS prior to trans-nitrosation or disulfide-bond-formation events.
Chemical Reactions of RSNOs
As described, the weak S-N bond strength (23–32 kcal/mol) makes RSNOs susceptible to homolytic S-N bond cleavage upon heating and exposure to light and to various reducing agents (especially Cu+ ions and ascorbate; Fig. 2). Other metals, such as the mercuric ion, facilitate heterolytic S-N bond cleavage to a thiolate and the nitrosonium ion (Fig. 2). This chemistry forms the basis of the most widely utilized RSNO detection strategies by removing the −NO group to yield a free thiol that can be labeled (BST) or by identifying the liberated nitrogen oxide (chemiluminescence and Saville assays; Fig. 5). While these methods and their modifications provide most of the current information regarding the role of RSNOs in biology, none of these methods directly detect S-nitrosated species. These indirect approaches suffer from multiple treatment steps that may degrade RSNOs and reagent selectivity issues that may lead to false positive RSNO detection if other oxidized thiol derivatives [disulfides, RSOH, and sulfenyl chlorides (RSCls)] cross-react. In addition, methods that rely only upon the detection of the liberated nitrogen oxide (chemiluminescence and Saville assays) cannot identify the specific S-nitrosated protein or which cysteine residue was modified. To overcome these problems, direct methods of labeling RSNO-containing proteins are being explored and developed. Ideally, the reagent would rapidly and selectively react with a protein RSNO to give a stable adduct amenable to chromatography or electrophoresis with properties suitable for immunological, spectroscopic, or mass spectrometric detection. Further, the ability of the reagent to maintain the S-N bond linkage would provide valuable confirmatory chemical information as to the initial identity and location of the modification (Fig. 5).
FIG. 5.
Strategies of indirect and direct RSNO detection. Indirect RSNO detection and quantification involves the dissociation of the RS-NO bond followed by alkylation of the nascent thiol. Direct labeling methods would maintain the RS-NO bond character and directly alkylate the −SNO group.
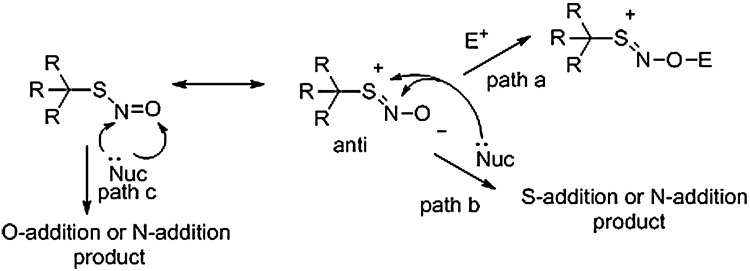
Besides homo- and heterolytic S-N bond cleavage reactions, RSNOs participate in other unique chemistry. Figure 6 depicts the RSNO structure and its major resonance forms, which supports a negative charge on the oxygen atom and a positive charge on the sulfur atom. These resonance structures indicate possible reactions of the oxygen atom as a nucleophile (with electron-poor reagents, path a) and reactions of the S or N atom with nucleophiles (as an electrophile, path b) as depicted in Figure 6. In addition, nucleophiles could react with either the N or O atom of the noncharged resonance form to yield addition products (path c, Fig. 6). The addition of a nucleophile at the sulfur atom with the displacement of nitroxyl (HNO/−NO) has been suggested as a possible reaction mechanism (72). Each of these hypothetical pathways may yield stable adducts or unstable products prone to further reaction generating secondary adducts. The exploration and development of these pathways suggest possible chemical methods for RSNO labeling.
FIG. 6.
Reaction of nucleophiles/electrophiles with RSNOs. The different resonance forms of the RSNO allow for both nucleophilic and electrophilic addition reactions. In both cases, there would be a direct alkylation of the −SNO.
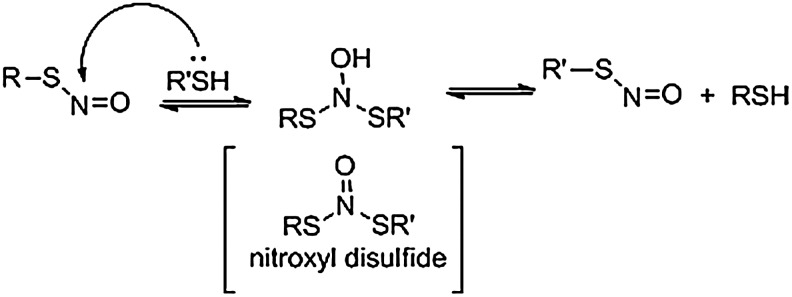
The reactivity of RSNOs with traditional organic electrophiles (path a) is not well documented. However, the reaction of various nucleophiles, including thiolates, amines, hydrazines, hydroxylamine, azide, bisulfite (S2−2), hydrogen peroxide, and ascorbate, with RSNOs has been described and reviewed (70). Other work shows that the reaction of sulfinic acids with RSNOs generates the corresponding sulfonyl compound (24). The reaction of RSNOs with other thiols/thiolates through nucleophilic attack at the nitroso group (through either path b or c) provides a mechanism of trans-nitrosation (transfer of the NO group from one thiol to another). This chemistry helps in explaining the biology of NO transfer of the nitroso group unit from small-molecular-weight thiols to proteins (and vice versa). Figure 7 shows a relatively simple organic-based mechanism for trans-nitrosation from one S-nitrosothiol to a thiol. Addition of the thiol (or thiolate) to the nitroso group of an RSNO generates an addition product intermediate that collapses to give the new S-nitrosothiol and thiol. 15N NMR studies provide equilibrium constants for this process for a number of small thiols (68). Despite this simple model, theoretical studies suggest that a more complicated nitroxyl disulfide intermediate may be involved in thiol trans-nitrosation (27). Similar mechanisms would explain nitroso group transfer from an S-nitrosothiol to a secondary amine or hydrogen peroxide to yield N-nitroso compounds or peroxynitrite. The kinetic profile of these reactions plays an important role in determining the biological importance of these reactions. Clearly, RSNOs react with nucleophiles to produce a variety of products that permit the exploration of labeling methods through this chemistry. Two recent excellent reviews also cover chemical methods for protein RSNO labeling, as well as other oxidative thiol modifications (32, 65).
FIG. 7.
Mechanism of a basic trans-S-nitrosation reaction. The mechanism of trans-nitrosation may involve the direct addition of a thiolate to an RSNO.
Reactions of RSNOs with Phosphines
Reactions of S-nitrosothiols with phosphines in organic systems
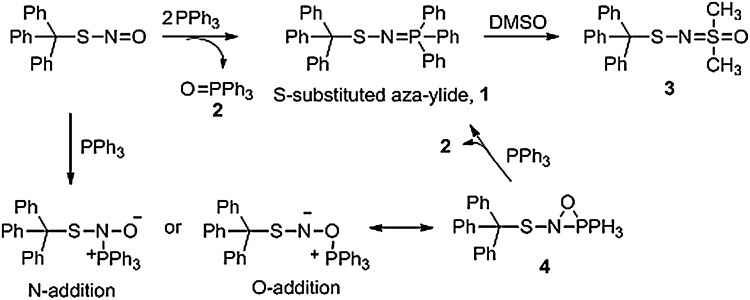
Early work by Haake shows that the reaction of trityl S-nitrosothiol with two equivalents of triphenylphosphine in benzene yields 1 equivalent of an N-tritylthio-triphenylphosphonium compound (1) and 1 equivalent of triphenylphosphine oxide (2, Fig. 8) (23). Mechanistically, the reaction appears to occur from the nucleophilic addition of triphenyl phosphine to the organic RSNO (Fig. 8). Addition of the phosphine may occur at either the N or O atom to generate an addition product that can exist as a neutral three-membered ring (4). Reaction of this species with an additional equivalent of triphenylphosphine gives the N-tritylthio-triphenylphosphonium (1, an S-substituted aza-ylide) compound, which contains the S-N-P linkage and has recently been characterized by X-ray crystallography and 31P NMR spectroscopy (4), and 2. Reaction of the S-substituted aza-ylide (1) with dimethyl sulfoxide (DMSO) yields the observed DMSO-based product (3) (23). While a completely organic example, this work demonstrates that phosphines react as nucleophiles with RSNOs to give unique aza-ylide-type products and has inspired the development of several phosphine-based chemical probes for the detection of protein S-nitrosothiols.
FIG. 8.
The reaction of triphenylphosphine with trityl S-nitrosothiol. The treatment of trityl S-nitrosothiol with two equivalents of triphenylphosphine results in the formation of phosphine oxide and an S-substituted aza-ylide, the first example of the direct labeling of an RSNO using phosphines.
Detecting S-nitrosothiols with phosphines in aqueous systems
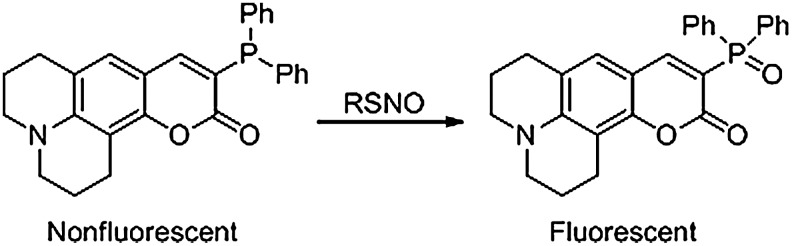
A coumarin-based phosphine, which fluoresces upon phosphine oxidation, has been developed as an indirect means to detect S-nitrosation (Fig. 9) (44). This dye exploits the fact that phosphine oxide is always generated during the reaction of phosphines with RSNOs (Fig. 8). In an aqueous buffered system, this coumarin-phosphine quantified glutathione S-nitrosation levels at low mM concentrations (44). This coumarin-phosphine shows no affinity for disulfide bonds, further validating reports that triarylphosphines do not react with disulfides (4, 44, 56). While the specificity and utility of this dye in an in vivo system has not been investigated, it could provide a useful means to quantify RSNO concentration in vitro.
FIG. 9.
A fluorogenic method to detect S-nitrosation. This coumarin-based phosphine fluoresces upon oxidation, providing a useful means to indirectly quantify RSNOs.
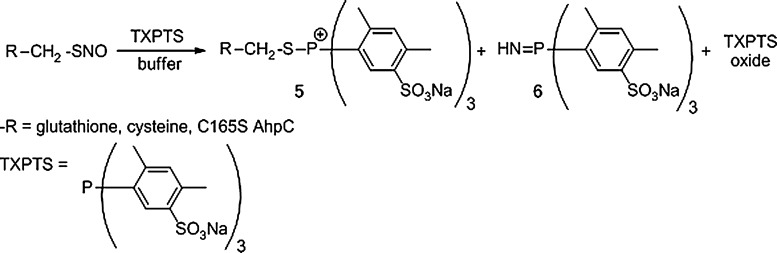
Inspired by the work of M. Haake, we explored the ability of the water-soluble phosphine tris(4,6-dimethyl-3-sulfonatophenyl) phosphine trisodium salt hydrate (TXPTS; Fig. 10) to generate stable adducts of RSNOs (4). Figure 10 summarizes the unique reactivity of TXPTS with RSNOs in aqueous media. This water-soluble triaryl phosphine reacts rapidly and specifically with S-nitrosocysteine (Cys-SNO), GSNO, and a protein-SNO to yield S-alkylphosphonium adducts (5), as determined by 31P-NMR and MS (4). These results highlight the differences between the aqueous and organic-based reactions of phosphines and RSNOs. Time-course NMR and MS experiments fail to detect an S-substituted aza-ylide (1, Fig. 8), as predicted for the organic-based reactions. These experiments instead show a unique set of products including the S-alkylphosphonium adduct (5), an N-substituted aza-ylide (6), and the phosphine oxide (4).
FIG. 10.
Formation of a stable S-alkylphosphonium adduct from the reaction of tris(4,6-dimethyl-3-sulfonatophenyl) phosphine trisodium salt hydrate (TXPTS) and RSNOs. The water-soluble phosphine TXPTS reacted with peptide and protein RSNOs to generate stable adducts that were detected by nuclear magnetic resonance (NMR) and mass spectrometry (MS).
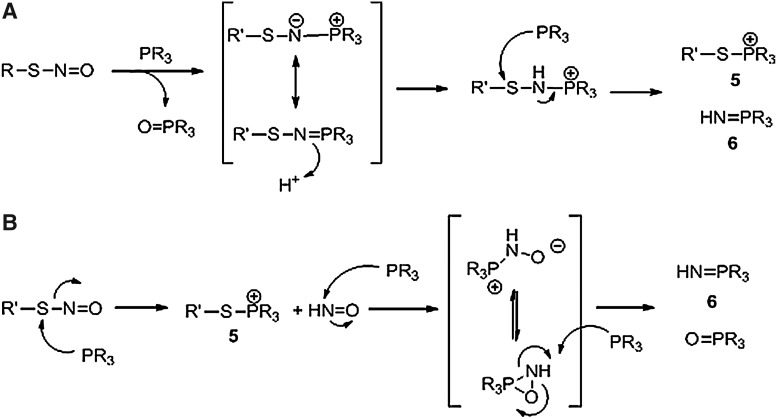
Presently, the mechanism for the formation of these products remains unclear and Figure 11 presents two possibilities. Path A shows the formation of a similar S-substituted aza-ylide (1) intermediate from the reaction of TXPTS and an RSNO. Protonation of this ylide gives a new species that reacts with another equivalent of phosphine to give the S-alkylphosphonium product (5) and the aza-ylide (6, Fig. 11). Alternatively, the direct nucleophilic addition of TXPTS on the sulfur atom directly generates 5 with the displacement of nitroxyl (HNO) that reacts with TXPTS to yield the aza-ylide (6) and phosphine oxide (Fig. 11, path B) (49, 50). Treatment of the S-nitrosated mutant of C165S alkyl hydroperoxide reductase subunit C (AhpC), a 2-cys peroxiredoxin, generates the S-alkylphosphonium adduct of this protein as determined by mass spectrometry. This adduct demonstrates good stability and provides one of the first examples of a covalently labeled protein S-nitrosothiol and highlights the potential for these types of phosphines as covalent labels of protein RSNOs. At present, detection of these adducts relies on 31P NMR spectroscopy and mass spectrometry and, while the −SNO nitrogen atom is accounted for the aza-ylide (6), the reaction destroys the S-N bond of the S-nitrosothiol group (losing some evidence of the initial existence of the RSNO and making this final adduct akin to BST). The aza-ylide (6) is not indefinitely stable in water and decomposes over several hours, presumably releasing ammonia in this process. Nonetheless, these findings encourage the development of new and superior reagents that, containing fluorescent or immunologically detectable groups, could evaluate a wide range of S-nitrosated proteins under different conditions.
FIG. 11.
Proposed mechanisms for the formation of the TXPTS S-alkyl phosphonium adduct. The SNO-derived adduct (5) is formed either by an S-substituted aza-ylide intermediate that quickly reacts in the presence of excess phosphine (path A) or through direct attack of the phosphine at the sulfur atom, releasing nitroxyl (HNO), which reacts with excess phosphine (path B). At this time, the exact labeling mechanism is unknown.
Phosphine and RSNO ligation reactions
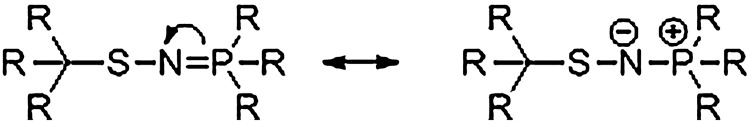
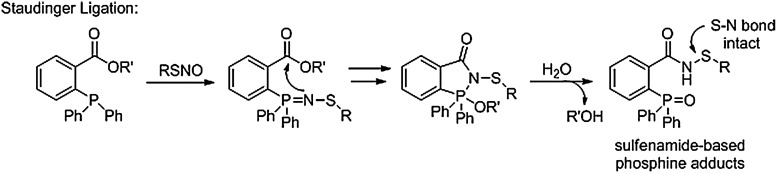
Figure 12 shows the resonance forms of S-substituted aza-ylides that highlight the unique chemical properties of these intermediates. The central nitrogen atom exhibits nucleophilic character amenable to reactions with various electrophiles, including Staudinger-type ligations similar to those developed by Raines (61, 62) and Bertozzi (55, 56). Recent work by Xian and his group demonstrates that small-molecule S-nitrosothiols react with modified triarylphosphines in a similar fashion to initially produce an S-substituted aza-ylide that undergoes further reactions to give ligated products (66, 75). Structural changes to the phosphine dictate the observed ligated products. When an electrophilic group, typically an ester, is strategically placed on a phenyl ring of the triphenyl phosphine, the S-substituted aza-ylide intermediate reacts to form stable sulfenamide products (66). Figure 13 depicts this Staudinger ligation-type mechanism. Such a reaction sequence maintains the S-N bond of the initial RSNO and provides chemical opportunities for the direct labeling of protein RSNOs although the stability of protein sulfenamides in the presence of excess phoshphine or thiolate remains to be thoroughly determined.
FIG. 12.
The resonance forms of S-substituted aza-ylides. Due to the resonance character of the aza-ylide, the nitrogen atom possesses nucleophilic behavior.
FIG. 13.
Phosphines in Staudinger-type ligation reactions with RSNOs. In the presence of a properly situated electrophile, RSNOs can undergo Staudinger-type ligation reactions with phosphines to generate stable sulfenamide products.
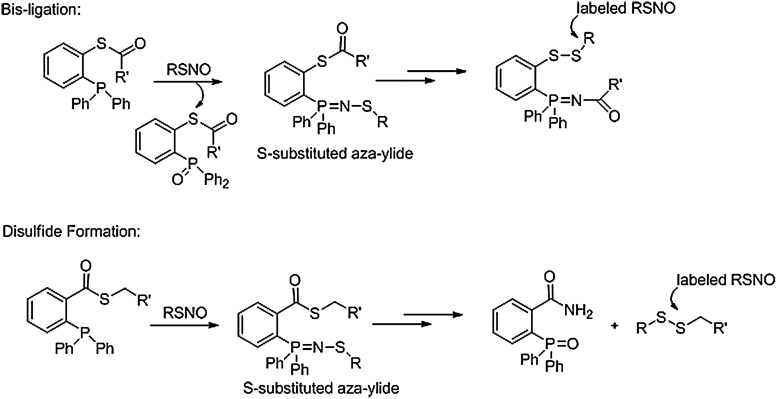
The Xian group has further developed this chemistry to explore alternative methods of chemically labeling protein RSNOs. Using thioester substrates, these reactions include a bis-ligation pathway (Fig. 14) that initially generates an S-substituted aza-ylide that internally reacts to yield a labeled disulfide product and an iminophosphorane ylide. This method captures the RSNO as a relatively stable disulfide with the nitrogen atom being maintained in the label; however, the S-N bond is destroyed during this process. Reversal of the thioester linkage gives phosphines that react with RSNOs to initially yield S-substituted aza-ylides that further react to give reactive sulfenamides that intramolecularly condense to generate a new disulfide that contains the original RSNO (45). Using a biotin-derived phosphine, this method was used to the identify S-nitrosated proteins from RSNO-treated COS-7 cells (74). Treatment of RSNOs with amine-containing phosphines resulted in an elimination that converted cysteine-bearing SNOs to dehydroalanine products, chemistry that may allow specific labeling (67).
FIG. 14.
Other phosphine-based labeling strategies of RSNOs. Several variations in the phosphine structure of the Staudinger-type ligation with RSNOs yield both bis-ligation and disulfide-containing products.
In general, the reactions of triaryl phosphines with S-nitrosothiols give unique reaction products making this process specific for S-nitrosothiol detection. Unlike trialkyl phosphines, triaryl phosphines react slowly with disulfides and would be expected to yield phosphine oxides. Preliminary evidence suggests that TXPTS may reduce protein RSOH to give the protein thiol and phosphine oxide. Triaryl phosphines react with nitroxyl (HNO) to yield aza-ylide products and in some cases difficulty could exist in distinguishing S-nitrosothiols from HNO using phosphine reagents (49, 50). While not thoroughly investigated, N-nitroso, O-nitroso, and C-nitroso species should react with phosphines to give aza-ylide products that could complicate S-nitrosothiol detection, and reactions with these species must be considered as these methods develop.
These methods exploit the unique chemical reactivity of the S-substituted aza-ylide intermediate and provide a useful means for creating novel phosphine adducts of RSNOs. To date, most of this chemistry has been applied in organic/aqueous buffer systems with small-molecule RSNOs but the recent labeling of protein RSNOs in cells highlights the potential and importance of water-soluble Staudinger-based phosphines as useful tools for studying the S-nitroso proteome (65). Each of these phosphine-based methods initially labels the S-nitrosothiol directly as an S-aza ylide (RS-N=PR3) preserving the original S-N bond linkage of the S-nitrosothiol. Except for the Staudinger ligation sequence (Fig. 13), subsequent chemistry destroys this S-N bond converting these to indirect labels. However, this work clearly shows the ability of these reagents to directly react with S-nitrosothiols and form unique adducts for the detection of S-nitrosated proteins. Additionally, these phosphines do not react with free thiols, and therefore, unlike the Biotin Switch assay, require the −SNO functionality to form detectable S-adducts. While nearly all of this chemistry focuses on the reactions of S-nitrosothiols with phosphines, these studies highlight a need to investigate the reaction between RSNOs and other nucleophiles to possibly generate more stable/detectable markers.
Conclusions
Protein-derived S-nitrosothiols play important roles in NO-based signaling in both normal biology and disease. Given their importance, methods for the direct and specific detection of S-nitrosated proteins are required to fully understanding the biology of this posttranslational modification. Currently, the biotin switch and chemiluminesence-based assays are the most commonly used methods for the detection of S-nitrosated proteins. Many refinements and improvements to these assays incorporate mass spectrometry to study proteome-level S-nitrosation. Despite these advances, these methods remain an indirect means of detection and suffer from lengthy preparative protocols, questions regarding reagent selectivity, and the actual loss of the functional group of interest.
Until a few years ago, the possible direct chemical reaction of RSNOs to give distinct adducts remained merely a topic of discussion. Early synthetic organic chemical work shows that triarylphosphines react with organic RSNOs to give S-substituted aza-ylides that demonstrate reactivity similar to other aza-ylides. Based on this early work, a number of intriguing direct methods for the chemical labeling of RSNOs have emerged within the last 5 years. The exact reaction product depends on the structure of both the phosphine and the RSNO. Triaryl phosphines appear necessary to prevent nonspecific reactivity with disulfide bonds (4, 44, 55). TXPTS, a triaryl, highly substituted phosphine (Fig. 10), reacts with RSNOs to form S-alkyl phosphonium adducts (4). RSNOs also undergo cyclization reactions with phosphines containing properly situated electrophiles (typically an ortho-substituted ester or thiosester on one of the phenyl rings) and result in both “traditional” and “traceless” Staudinger ligation products (Figs. 13 and 4) (75, 76). Examples of protein RSNO labeling using these methods now exist, further demonstrating the potential of this unique reactivity between RSNOs and phosphines that could pave the way for new chemical probes for the direct detection of protein S-nitrosation in biological contexts.
Abbreviations Used
- AhpC
alkyl hydroperoxide reductase subunit C
- AuNP
gold nanoparticle
- BST
biotin switch technique
- CO
carbon monoxide
- CuCl
copper chloride
- DMSO
dimethyl sulfoxide
- GSH
glutathione
- GSNO
S-nitrosoglutathione
- GSSG
glutathione disulfide (or oxidized glutathione)
- HgCl2
mercury chloride
- HNO
nitroxyl
- IAA
iodoacetamide
- LC-MS
liquid chromatography mass spectrometry
- MS
mass spectrometry
- N2O3
dinitrogen trioxide
- NEM
N-ethyl maleimide
- NMR
nuclear magnetic resonance
- NO
nitric oxide
- NO2
nitrogen dioxide
- ONOO−
peroxynitrite
- RNS
reactive nitrogen species
- RSCl
sulfenyl chlorides
- RSNO
S-nitrosothiol
- RSOHs
sulfenic acids
- RyR
ryanodine receptor
- sGC
soluble guanylate cyclase
- SNAP
S-nitroso-N-acetyl-pencillamine
- Trx
thioredoxin
- TXPTS
tris(4,6-dimethyl-3-sulfonatophenyl) phosphine trisodium salt hydrate
Acknowledgments
Financial support for work accomplished in SBK's laboratory was provided by the National Institutes of Health (HL62198), the American Heart Association (963031N, 0140020N), and Wake Forest University.
References
- 1.Barglow KT. Knutson CG. Wishnok JS. Tannenbaum SR. Marletta MA. Site-specific and redox-controlled S-nitrosation of thioredoxin. Proc Natl Acad Sci U S A. 2011;108:E600–E606. doi: 10.1073/pnas.1110736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartberger MD. Houk KN. Powell SC. Mannion JD. Lo KY. Stamler JS. Toone EJ. Theory, spectroscopy, and crystallographic analysis of S-nitrosothiols: conformational distribution dictates spectroscopic behavior. J Am Chem Soc. 2000;122:5889–5890. [Google Scholar]
- 3.Basu S. Wang X. Gladwin MT. Kim-Shapiro DB. Chemiluminescent detection of S-nitrosated proteins: comparison of tri-iodide, copper/CO/cysteine, and modified copper/cysteine methods. Methods Enzymol. 2008;440:137–156. doi: 10.1016/S0076-6879(07)00808-7. [DOI] [PubMed] [Google Scholar]
- 4.Bechtold E. Reisz JA. Klomsiri C. Tsang AW. Wright MW. Poole LB. Furdui CM. King SB. Water-soluble triarylphosphines as biomarkers for protein S-nitrosation. ACS Chem Biol. 2010;5:405–414. doi: 10.1021/cb900302u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bindoli A. Fukuto JM. Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler AR. Rhodes P. Chemistry, analysis, and biological roles of S-nitrosothiols. Anal Biochem. 1997;249:1–9. doi: 10.1006/abio.1997.2129. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarti R. Aulak KS. Fox PL. Stuehr DJ. GAPDH regulates cellular heme insertion into inducible nitric oxide synthase. Proc Natl Acad Sci U S A. 2010;107:18004–18009. doi: 10.1073/pnas.1008133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claiborne A. Yeh JI. Mallett TC. Luba J. Crane EJ., 3rd Charrier V. Parsonage D. Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry. 1999;38:15407–15416. doi: 10.1021/bi992025k. [DOI] [PubMed] [Google Scholar]
- 9.Deem S. Kim SS. Min JH. Eveland R. Moulding J. Martyr S. Wang X. Swenson ER. Gladwin MT. Pulmonary vascular effects of red blood cells containing S-nitrosated hemoglobin. Am J Physiol Heart Circ Physiol. 2004;287:H2561–H2568. doi: 10.1152/ajpheart.00310.2004. [DOI] [PubMed] [Google Scholar]
- 10.Dejam A. Hunter CJ. Pelletier MM. Hsu LL. Machado RF. Shiva S. Power GG. Kelm M. Gladwin MT. Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Hertog J. Groen A. van der Wijk T. Redox regulation of protein-tyrosine phosphatases. Arch Biochem Biophys. 2005;434:11–15. doi: 10.1016/j.abb.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Dicks AP. Williams DL. Generation of nitric oxide from S-nitrosothiols using protein-bound Cu2+ sources. Chem Biol. 1996;3:655–659. doi: 10.1016/s1074-5521(96)90133-7. [DOI] [PubMed] [Google Scholar]
- 13.Faccenda A. Bonham CA. Vacratsis PO. Zhang X. Mutus B. Gold nanoparticle enrichment method for identifying S-nitrosylation and S-glutathionylation sites in proteins. J Am Chem Soc. 2010;132:11392–11394. doi: 10.1021/ja103591v. [DOI] [PubMed] [Google Scholar]
- 14.Fang K. Ragsdale NV. Carey RM. MacDonald T. Gaston B. Reductive assays for S-nitrosothiols: implications for measurements in biological systems. Biochem Biophys Res Commun. 1998;252:535–540. doi: 10.1006/bbrc.1998.9688. [DOI] [PubMed] [Google Scholar]
- 15.Feelisch M. Rassaf T. Mnaimneh S. Singh N. Bryan NS. Jourd'Heuil D. Kelm M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 16.Field L. Dilts RV. Ravichandran R. Lenhert PG. Carnahan GE. Unusually stable thionitrite from N-acetyl-D,L-penicillamine—X-ray crystal and molecular-structure of 2-(acetylamino)-2-carboxy-1,1-dimethylethyl thionitrite. J Chem Soc Chem Commun. 1978:249–250. [Google Scholar]
- 17.Forrester MT. Foster MW. Stamler JS. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J Biol Chem. 2007;282:13977–13983. doi: 10.1074/jbc.M609684200. [DOI] [PubMed] [Google Scholar]
- 18.Foster MW. Forrester MT. Stamler JS. A protein microarray-based analysis of S-nitrosylation. Proc Natl Acad Sci U S A. 2009;106:18948–18953. doi: 10.1073/pnas.0900729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster MW. McMahon TJ. Stamler JS. S-nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 20.Gaston BM. Carver J. Doctor A. Palmer LA. S-nitrosylation signaling in cell biology. Mol Interv. 2003;3:253–263. doi: 10.1124/mi.3.5.253. [DOI] [PubMed] [Google Scholar]
- 21.Giles GI. Jacob C. Reactive sulfur species: an emerging concept in oxidative stress. Biol Chem. 2002;383:375–388. doi: 10.1515/BC.2002.042. [DOI] [PubMed] [Google Scholar]
- 22.Gow A. Doctor A. Mannick J. Gaston B. S-nitrosothiol measurements in biological systems. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:140–151. doi: 10.1016/j.jchromb.2007.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haake M. Zur desoxygenierung von tritylthionitrit. Tet Lett. 1972;33:3405–3408. [Google Scholar]
- 24.Hart TW. Some observations concerning the S-nitroso and S-phenylsulphonyl derivatives of L-cysteine and glutathione. Tet Lett. 1985;26:2013–2016. [Google Scholar]
- 25.Hogg N. The biochemistry and physiology of S-nitrosothiols. Annu Rev Pharmacol Toxicol. 2002;42:585–600. doi: 10.1146/annurev.pharmtox.42.092501.104328. [DOI] [PubMed] [Google Scholar]
- 26.Hogg N. Singh RJ. Kalyanaraman B. The role of glutathione in the transport and catabolism of nitric oxide. FEBS Lett. 1996;382:223–228. doi: 10.1016/0014-5793(96)00086-5. [DOI] [PubMed] [Google Scholar]
- 27.Houk KN. Hietbrink BN. Bartberger MD. McCarren PR. Choi BY. Voyksner RD. Stamler JS. Toone EJ. Nitroxyl disulfides, novel intermediates in transnitrosation reactions. J Am Chem Soc. 2003;125:6972–6976. doi: 10.1021/ja029655l. [DOI] [PubMed] [Google Scholar]
- 28.Howard CM. Sexton DJ. Mutus B. S-nitrosoglutathione/glutathione disulphide/Cu2+-dependent stimulation of L-arginine transport in human platelets. Thromb Res. 1998;91:113–120. doi: 10.1016/s0049-3848(98)00060-7. [DOI] [PubMed] [Google Scholar]
- 29.Jaffrey SR. Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001:PL1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 30.Jia HY. Liu Y. Zhang XJ. Han L. Du LB. Tian Q. Xu YC. Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. J Am Chem Soc. 2009;131:40–41. doi: 10.1021/ja808033w. [DOI] [PubMed] [Google Scholar]
- 31.Landino LM. Koumas MT. Mason CE. Alston JA. Ascorbic acid reduction of microtubule protein disulfides and its relevance to protein S-nitrosylation assays. Biochem Biophys Res Commun. 2006;340:347–352. doi: 10.1016/j.bbrc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Leonard SE. Carroll KS. Chemical ‘omics' approaches for understanding protein cysteine oxidation in biology. Curr Opin Chem Biol. 2011;15:88–102. doi: 10.1016/j.cbpa.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Lima B. Forrester MT. Hess DT. Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Sanchez LM. Muntane J. de la Mata M. Rodriguez-Ariza A. Unraveling the S-nitrosoproteome: tools and strategies. Proteomics. 2009;9:808–818. doi: 10.1002/pmic.200800546. [DOI] [PubMed] [Google Scholar]
- 35.Lowenstein CJ. Nitric oxide regulation of protein trafficking in the cardiovascular system. Cardiovasc Res. 2007;75:240–246. doi: 10.1016/j.cardiores.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marino SM. Gladyshev VN. Structural analysis of cysteine S-nitrosylation: a modified acid-based motif and the emerging role of trans-nitrosylation. J Mol Biol. 2010;395:844–859. doi: 10.1016/j.jmb.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall HE. Hess DT. Stamler JS. S-nitrosylation: physiological regulation of NF-kappaB. Proc Natl Acad Sci U S A. 2004;101:8841–8842. doi: 10.1073/pnas.0403034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miranda KM. Nims RW. Thomas DD. Espey MG. Citrin D. Bartberger MD. Paolocci N. Fukuto JM. Feelisch M. Wink DA. Comparison of the reactivity of nitric oxide and nitroxyl with heme proteins. A chemical discussion of the differential biological effects of these redox related products of NOS. J Inorg Biochem. 2003;93:52–60. doi: 10.1016/s0162-0134(02)00498-1. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell DA. Morton SU. Fernhoff NB. Marletta MA. Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc Natl Acad Sci U S A. 2007;104:11609–11614. doi: 10.1073/pnas.0704898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteiro G. Horta BB. Pimenta DC. Augusto O. Netto LE. Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc Natl Acad Sci U S A. 2007;104:4886–4891. doi: 10.1073/pnas.0700481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrell CN. Matsushita K. Chiles K. Scharpf RB. Yamakuchi M. Mason RJ. Bergmeier W. Mankowski JL. Baldwin WM., 3rd Faraday N. Lowenstein CJ. Regulation of platelet granule exocytosis by S-nitrosylation. Proc Natl Acad Sci U S A. 2005;102:3782–3787. doi: 10.1073/pnas.0408310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagababu E. Rifkind JM. Determination of S-nitrosothiols in biological fluids by chemiluminescence. Methods Mol Biol. 2011;704:27–37. doi: 10.1007/978-1-61737-964-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paget MS. Buttner MJ. Thiol-based regulatory switches. Annu Rev Genet. 2003;37:91–121. doi: 10.1146/annurev.genet.37.110801.142538. [DOI] [PubMed] [Google Scholar]
- 44.Pan J. Downing JA. McHale JL. Xian M. A fluorogenic dye activated by S-nitrosothiols. Mol Biosyst. 2009;5:918–920. doi: 10.1039/b822283e. [DOI] [PubMed] [Google Scholar]
- 45.Pan J. Xian M. Disulfide formation via sulfenamides. Chem Commun (Camb) 2011;47:352–354. doi: 10.1039/c0cc02076a. [DOI] [PubMed] [Google Scholar]
- 46.Park JK. Kostka P. Fluorometric detection of biological S-nitrosothiols. Anal Biochem. 1997;249:61–66. doi: 10.1006/abio.1997.2159. [DOI] [PubMed] [Google Scholar]
- 47.Pelletier MM. Kleinbongard P. Ringwood L. Hito R. Hunter CJ. Schechter AN. Gladwin MT. Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006;41:541–548. doi: 10.1016/j.freeradbiomed.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Reisz JA. Bechtold E. King SB. Oxidative heme protein-mediated nitroxyl (HNO) generation. Dalton Trans. 2010;39:5203–5212. doi: 10.1039/c000980f. [DOI] [PubMed] [Google Scholar]
- 49.Reisz JA. Klorig EB. Wright MW. King SB. Reductive phosphine-mediated ligation of nitroxyl (HNO) Org Lett. 2009;11:2719–2721. doi: 10.1021/ol900914s. [DOI] [PubMed] [Google Scholar]
- 50.Reisz JA. Zink CN. King SB. Rapid and selective nitroxyl (HNO) trapping by phosphines: kinetics and new aqueous ligations for HNO detection and quantitation. J Am Chem Soc. 2011;133:11675–11685. doi: 10.1021/ja203652z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynaert NL. Ckless K. Korn SH. Vos N. Guala AS. Wouters EF. van der Vliet A. Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samouilov A. Zweier JL. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal Biochem. 1998;258:322–330. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 53.Saurin AT. Neubert H. Brennan JP. Eaton P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc Natl Acad Sci U S A. 2004;101:17982–17987. doi: 10.1073/pnas.0404762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saville B. A scheme for the colorimetric determination of microgram amounts of thiols. Analyst. 1958;83:670–672. [Google Scholar]
- 55.Saxon E. Armstrong JI. Bertozzi CR. A “traceless” Staudinger ligation for the chemoselective synthesis of amide bonds. Org Lett. 2000;2:2141–2143. doi: 10.1021/ol006054v. [DOI] [PubMed] [Google Scholar]
- 56.Saxon E. Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 57.Singh RJ. Hogg N. Joseph J. Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J Biol Chem. 1996;271:18596–18603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 58.Sinha V. Wijewickrama GT. Chandrasena RE. Xu H. Edirisinghe PD. Schiefer IT. Thatcher GR. Proteomic and mass spectroscopic quantitation of protein S-nitrosation differentiates NO-donors. ACS Chem Biol. 2010;5:667–680. doi: 10.1021/cb100054m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stamler JS. Hausladen A. Oxidative modifications in nitrosative stress. Nat Struct Biol. 1998;5:247–249. doi: 10.1038/nsb0498-247. [DOI] [PubMed] [Google Scholar]
- 60.Sun J. Steenbergen C. Murphy E. S-nitrosylation: NO-related redox signaling to protect against oxidative stress. Antioxid Redox Signal. 2006;8:1693–1705. doi: 10.1089/ars.2006.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tam A. Raines RT. Protein engineering with the traceless Staudinger ligation. Methods Enzymol. 2009;462:25–44. doi: 10.1016/S0076-6879(09)62002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tam A. Soellner MB. Raines RT. Water-soluble phosphinothiols for traceless Staudinger ligation and integration with expressed protein ligation. J Am Chem Soc. 2007;129:11421–11430. doi: 10.1021/ja073204p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tannenbaum SR. White FM. Regulation and specificity of S-nitrosylation and denitrosylation. ACS Chem Biol. 2006;1:615–618. doi: 10.1021/cb600439h. [DOI] [PubMed] [Google Scholar]
- 64.Tello D. Tarin C. Ahicart P. Breton-Romero R. Lamas S. Martinez-Ruiz A. A “fluorescence switch” technique increases the sensitivity of proteomic detection and identification of S-nitrosylated proteins. Proteomics. 2009;9:5359–5370. doi: 10.1002/pmic.200900070. [DOI] [PubMed] [Google Scholar]
- 65.Wang H. Xian M. Chemical methods to detect S-nitrosation. Curr Opin Chem Biol. 2010;15:32–37. doi: 10.1016/j.cbpa.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H. Xian M. Fast reductive ligation of S-nitrosothiols. Angew Chem Int Ed Engl. 2008;47:6598–6601. doi: 10.1002/anie.200801654. [DOI] [PubMed] [Google Scholar]
- 67.Wang H. Zhang J. Xian M. Facile formation of dehydroalanine from S-nitrosocysteines. J Am Chem Soc. 2009;131:13238–13239. doi: 10.1021/ja905558w. [DOI] [PubMed] [Google Scholar]
- 68.Wang K. Hou Y. Zhang W. Ksebati MB. Xian M. Cheng JP. Wang PG. 15N NMR and electronic properties of S-nitrosothiols. Bioorg Med Chem Lett. 1999;9:2897–2902. doi: 10.1016/s0960-894x(99)00499-0. [DOI] [PubMed] [Google Scholar]
- 69.Weichsel A. Brailey JL. Montfort WR. Buried S-nitrosocysteine revealed in crystal structures of human thioredoxin. Biochemistry. 2007;46:1219–1227. doi: 10.1021/bi061878r. [DOI] [PubMed] [Google Scholar]
- 70.Williams DLH. The chemistry of S-nitrosothiols. Acc Chem Res. 1999;32:869–876. [Google Scholar]
- 71.Wink DA. Kim S. Coffin D. Cook JC. Vodovotz Y. Chistodoulou D. Jourd'heuil D. Grisham MB. Detection of S-nitrosothiols by fluorometric and colorimetric methods. Methods Enzymol. 1999;301:201–211. doi: 10.1016/s0076-6879(99)01083-6. [DOI] [PubMed] [Google Scholar]
- 72.Wong PS. Hyun J. Fukuto JM. Shirota FN. DeMaster EG. Shoeman DW. Nagasawa HT. Reaction between S-nitrosothiols and thiols: generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 73.Xu L. Eu JP. Meissner G. Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J. Li S. Zhang D. Wang H. Whorton AR. Xian M. Reductive ligation mediated one-step disulfide formation of S-nitrosothiols. Org Lett. 2010;12:4208–4211. doi: 10.1021/ol101863s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J. Wang H. Xian M. Exploration of the “traceless” reductive ligation of S-nitrosothiols. Org Lett. 2009;11:477–480. doi: 10.1021/ol802663q. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J. Wang H. Xian M. An unexpected Bis-ligation of S-nitrosothiols. J Am Chem Soc. 2009;131:3854–3855. doi: 10.1021/ja900370y. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y. Hogg N. S-nitrosohemoglobin: a biochemical perspective. Free Radic Biol Med. 2004;36:947–958. doi: 10.1016/j.freeradbiomed.2004.01.008. [DOI] [PubMed] [Google Scholar]