Abstract
Introduction
This study analyzed associations between plasma vitamin D3 (25OHD3) and bone mineral density (BMD) and whether the effects of conjugated equine estrogens (CEE) on BMD are modulated by 25OHD3.
Methods
Fifty cynomolgus monkeys were fed a diet containing 25OHD3 (providing a woman's equivalent of 1000 IU/day of 25OHD3). The monkeys underwent bilateral oophorectomy and were randomized to either CEE (equivalent of 0.45 mg/day) (n=25) or placebo (n=25) and continued receiving the same diet. 25OHD3 and BMD were measured at randomization and after 6 months. BMD also was measured after 20 months (equivalent to 6 human years). Associations between 25OHD3 and BMD were subsequently analyzed.
Results
Baseline 25OHD3 plasma concentrations varied from 26 to 95 ng/mL (mean±standard deviation [SD] 54 ± 15 ng/mL). Higher plasma concentrations of 25OHD3 were associated with a significantly increased BMD. Monkeys on both CEE and placebo had increased BMD over 20 months; however, the increase was not significantly different (0.034 g/cm2 vs. 0.020 g/cm2, respectively; p=0.064). The 20-month BMD increased significantly with CEE treatment in those with higher vs. lower 25OHD3 concentrations (p=0.027). The percent change in BMD over 20 months also increased significantly with CEE treatment in those with higher vs. lower 25OHD3 concentrations (p=0.018). A higher 25OHD3 concentration had no significant effect on BMD in those receiving placebo.
Conclusions
Monkeys fed a diet containing 1000 IU/day equivalent of 25OHD3 have a wide range of plasma 25OHD3 concentrations. Those receiving CEE with higher 25OHD3 concentrations had higher BMDs, suggesting 25OHD3 and CEE have synergistic effects on BMD.
Introduction
Aside from the well-accepted role of vitamin D in skeletal health, epidemiologic and cross-sectional studies have suggested associations between vitamin D status and diabetes,1,2 energy metabolism,3 heart disease,4–6 cancer (including colorectal,7 prostate,8 and breast9), and compromised immune function.10–12 However, some clinicians and researchers are suggesting caution regarding widespread measurement of 25-hydroxyvitamin D3 (25OHD3) or recommendation of robust vitamin D repletion in the absence of clear evidence demonstrating a benefit.
A recent report by the Institute of Medicine (IOM) suggested that most women receive adequate amounts of 25OHD3 and that the prevalence of 25OHD3 deficiency has been overestimated.10,11 According to this report, the current recommended dietary allowance (RDA) of 25OHD3 is 600 IU/day in women up to age 70 and 800 IU/day in those >70 years of age.11 The IOM committee suggested that evidence supporting a role for calcium and vitamin D on non-skeletal health outcomes is lacking.11 It should be noted, however, that the IOM's recommendations are somewhat controversial, and some believe the dietary allowances should be higher. The International Osteoporosis Foundation (IOF), for instance, suggests that 800–1000 IU/day is the average supplemental dose to reach an appropriate serum 25OHD concentration.13 Furthermore, they state that those at higher risk may require doses up to 2000 IU/day to achieve an adequate concentration.13 The National Osteoporosis Foundation (NOF) recommends 400–800 IU/day of 25OHD3 for adults less than age of 50 and 800–1000 IU/day for those 50 years of age or older.14 Similar to the IOF, they suggest some people will need more oral 25OHD3, with an upper limit of safety being 4000 IU/day.14 Other guidelines, including those of the Endocrine Society,15 are available and provide an additional perspective, highlighting the lack of clear and well-designed evidence-based data.
The role of vitamin D in bone health is well established,11,16 but less is known about the correlation of serum 25OHD3 concentrations, the effect of higher supplementation, and bone mineral density (BMD). Much of the current evidence related to vitamin D in health outcomes is from retrospective and cohort studies, research that is hampered by the effects of multiple confounding variables (such as diet, sun exposure, compliance, and chronic medical conditions). Similarly, there is significant evidence that race and heritability play a major role in plasma 25OHD3 concentrations.17–21 The results of one study, for instance, demonstrated that individual differences were predominantly the result of genetics.19 The genetic differences were able to be demonstrated in the winter, when sun exposure was minimal, but not in the summer, suggesting environmental factors (predominantly sun exposure) may be able to compensate for vitamin D deficiency related to genetics.19 Little is known about these individual differences and whether supplementation resulting in higher plasma concentrations has a clinically significant effect.
Although small animals, such as rats and mice, are excellent models for many studies, nonhuman primates, such as cynomolgus macaques, are most closely related to humans. Because of similar physiology, skeletal formation, hormones, and metabolism, studies using the cynomolgus monkey model facilitate productive and reliable translational research in humans. The results of studies done in nonhuman primates have shown that their menstrual cycles are similar to those of humans, with comparable levels of estrogen, progesterone, estradiol, and follicle-stimulating hormone (FSH) throughout menstruation and menopause. Decreased bone mass and bone turnover after menopause in macaques occur to a similar degree as in humans, and 1 year after surgical menopause, macaques experience a level of bone loss comparable to bone loss 3–4 years after natural menopause in women.22–25 The findings of comparative research have reported that drug trials using macaque models agree with data available from drug trials in women.23,26 Macaque models, therefore, are useful in studying bone health because they allow for bone measurements difficult to obtain in women.27 Additionally, estrogen treatment (ET) has been shown to prevent bone loss and increase BMD in cynomolgus monkey models, as it does in women.28,29
The recent IOM report recognized the key role vitamin D plays in maintaining bone health and encouraged further targeted vitamin D research.10,11 It has been shown that the continuation of both calcium and vitamin D is important to influence the effectiveness of antiresorptive agents, such as bisphosphonates and hormone therapy (HT)/ET,16,30 but little is known about whether an individual's vitamin D metabolism and, hence, plasma concentration are directly related to bone health or subsequent response to antiresorptive agents. Therefore, this study sought to evaluate the plasma concentration of 25OHD3 in cynomolgus monkeys and assess its association with BMD at baseline and after being randomized to ET vs. placebo. The primary objective was to determine if monkeys with higher baseline 25OHD3 plasma concentrations have higher baseline BMD. The secondary objective was to analyze the response to ET in those with low vs. high 25OHD3 concentrations.
Materials and Methods
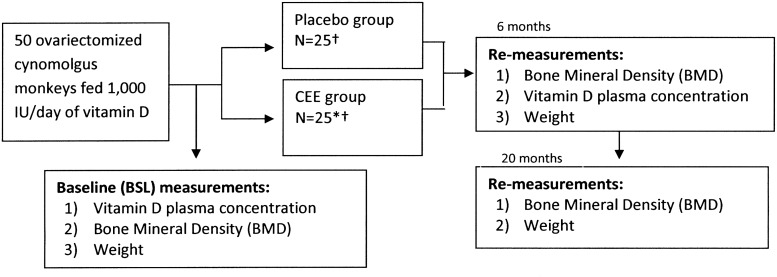
A cohort of 50 surgically menopausal cynomolgus monkeys was used for this study. Sexual maturity in this species is reached between 4 and 5 years of age, epiphyseal closure is complete between 6 and 7 years of age, and peak bone mass is attained between 9 and 10 years of age. The average age of the monkeys in this study, based on dentition, was 12 years; thus, we assured a mature baseline population was being studied. The monkeys were imported from Indonesia (the Indonesian Primate Center, the Pusat Studi Satwa Primata) at the Institute Pertanian Bogor in West Java, Indonesia. All animal procedures and protocols for this study were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services (DHHS), and guidelines established by the Wake Forest University (WFU) Institutional Animal Care and Use Committee (IACUC). All monkeys consumed an identical diet that provided them with a woman's equivalent of 1000 IU/day of 25OHD3 and 1200 mg/day of calcium for 4 months leading up to the study and then throughout the study. In addition, the monkeys randomized to ET had 0.45 mg equivalent of conjugated equine estrogen (CEE) added to their diet (Fig. 1). These specialized, weight-based, monkey chow diets were made at the WFU primate center where the monkeys were housed and where all demographic data were collected and BMD testing was performed. To confirm adequate dosing and dietary intake, all monkeys were fed 120 cal/kg of body weight per day, and body weights were monitored and obtained.
FIG. 1.
Schematic diagram of procedures and measurements performed at baseline, 6 months, and 20 months. *Two animals in this cohort had ovarian remnant syndrome, a condition well described in female monkeys31 and women.32 †BMD results were based on 24 monkeys in each group, as final BMD data were not available for 1 monkey in each group. CEE, conjugated equine estrogen.
All monkeys were housed in the same living conditions, eliminating 25OHD3 variations from ultraviolet-B radiation (UVB). All monkeys were housed in stable social groups consisting of 4–5 monkeys per pen. All pens were equipped with perches and greater than the minimal amount of floor space required by federal rules, regulations, and guidelines to allow for species-specific levels of activity and exercise. No pens were exposed to direct sunlight; therefore, UVB radiation exposure was negligible among all subjects, with no between-group differences.
Serum was transported to the Vitamin D testing center at The Reading Hospital and Medical Center (TRHMC) chemistry laboratory. We used a high performance liquid chromatography (HPLC)/tandem mass spectrometry for the 25-OH vitamin D assay, with determinations for both 25OHD3 and 25OHD2. This technology used the Shimadzu liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/2) system. The liquid LC-MS prepares the sample to be ionized through physical separation capabilities of liquid chromatography for mass analysis by injection into the AB Sciex 3200 Q Trap mass spectrometer.
To quantify BMD, we used a dual-energy x-ray absorptiometry machine (DXA) (XR-46, Norland, Fort Atkinson, WI) with DXA software (version 3.9.6b, Norland) commonly used to measure BMD in postmenopausal women and a methodology/technique previously validated to measure whole body and lumbar spinal BMD in macaques.33,34 Each monkey was sedated with ketamine (10 mg/kg) followed by isoflurane. Measurements were made of the whole body and the lumbar spine (lumbar vertebrae 2–4) using techniques described previously.33,34 Vertebral BMD changes can be detected as early as 6 months postovariectomy, and the microarchitecture of the vertebral spine is similar to that of humans. All measurements were made with the same machine, and the DXA machine was calibrated before each experimental reading. Hip BMD was not used, as it is more difficult to quantify in macaques and would, therefore, be less reliable.
Immediately before randomization (defined as baseline for this study), 25OHD3 concentrations were measured and BMD was determined for all monkeys. All monkeys underwent surgical menopause (bilateral oophorectomy). The monkeys were then randomized: 25 received CEE (a woman's equivalent of 0.45 mg/day), the ET group, and 25 received placebo. Two of the monkeys in the control group had ovarian remnant syndrome, a condition in monkeys31 and women32 that has been well documented and described. Because the ovarian remnant syndrome is common and, thus, would likely be present in some subjects if we studied a similar cohort of women, we chose to leave these 2 monkeys in the study. To assure this information did not confound our data, however, we also controlled for this information in subsequent analyses. Ovarian remnant syndrome was determined by elevated estradiol concentrations, generally >5 pg/mL, in those postoophorectomy. In this cohort, both of these monkeys had plasma estradiol concentrations of >10 pg/mL. After 6 months of treatment, 25OHD3 concentrations and BMD levels were remeasured in all monkeys. After a total of 20 months of treatment (equivalent of 6 human years), the BMD and weights were remeasured in the monkeys (Fig. 1). Associations between 25OHD3 and BMD were studied by comparing higher vs. lower concentrations of 25OHD3 (i.e.,≥vs.<the median), along with analyzing 25OHD3 as a continuous variable looking at the baseline BMD, the 20-month BMD, and the mean percent change in BMD over the 20 months. Twenty-month vitamin D concentrations were not available; based on the pharmacokinetics of vitamin D, 6-month concentrations were considered stable and steady-state.
Results
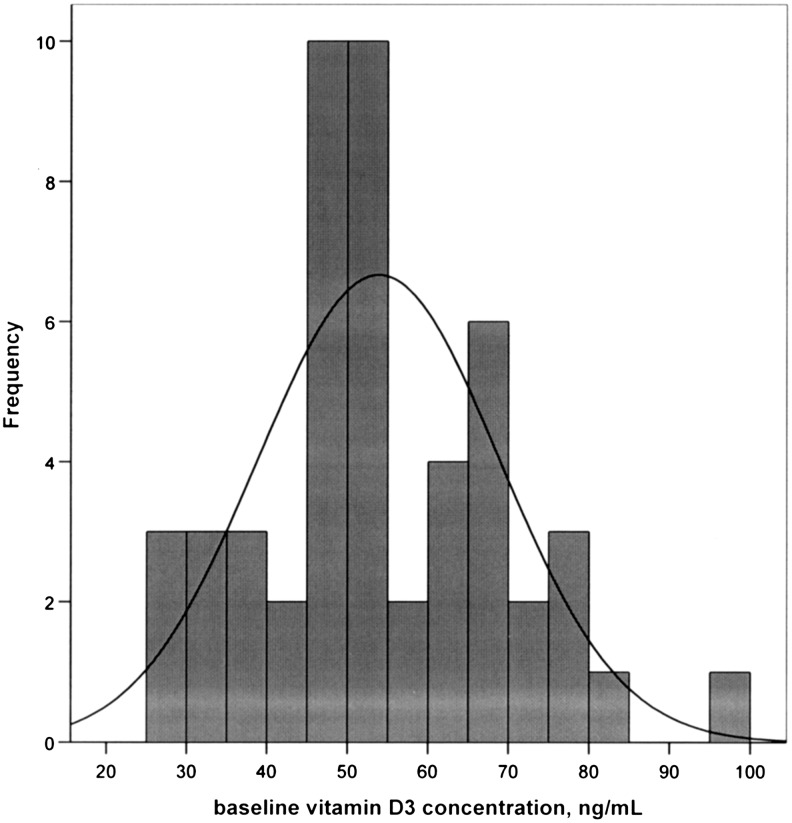
Among 50 monkeys consuming an adequate and consistent quantity of 25OHD3, the mean (standard deviation [SD], range) baseline body weight (BW) was 2.9 kg (±0.4 kg, 2.1–3.7 kg). There was no difference in body weights throughout the study between groups, providing a high degree of confidence that each monkey was receiving adequate dietary intake of 25OHD3. The mean baseline 25OHD3 concentration was 53.9 ng/mL (±15.0 ng/mL, 26.4–95.2 ng/mL) (Fig. 2). The mean percent change of 25OHD3 concentrations (from baseline to 6-month values) was 3.9% (±23.3%, −44.9% to 71.7%). Six-month BMD data were available for all monkeys, but the 20-month BMD data were not available for 1 monkey in each group, yielding cohorts of n=24 for both groups.
FIG. 2.
The frequency of vitamin D3 concentrations in cynomolgus monkeys (Macaca fascicularis) after controlled diet consumption of a woman's equivalent of 1000 IU/day of oral vitamin D3 for 4 months (baseline).
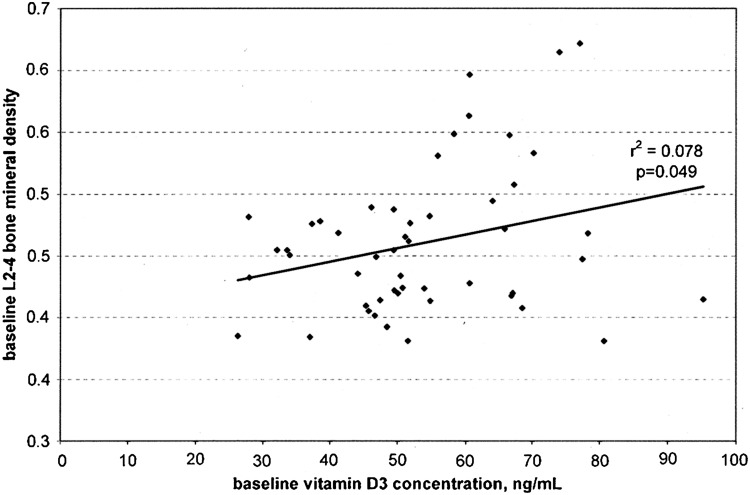
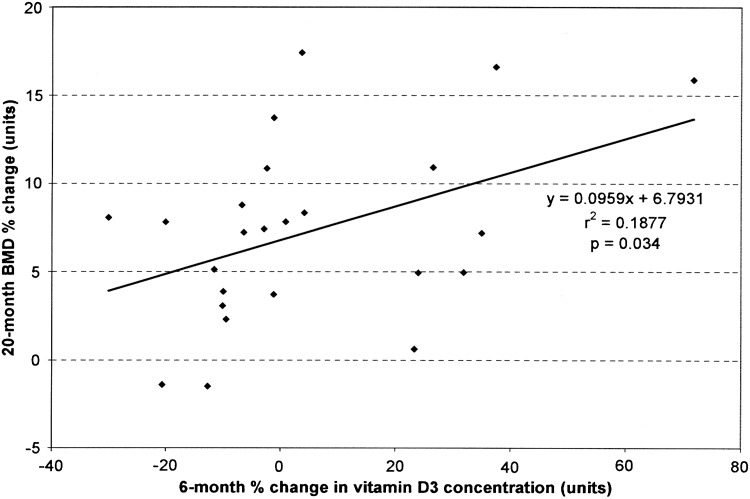
At baseline, as the concentration of 25OHD3 increased, the BMD significantly increased in a linear fashion (p=0.049) (Fig. 3). As the percent change in 25OHD3 increased (from baseline to 6 months), the percent change in BMD over 20 months increased in a linear fashion (p=0.034) (Fig. 4). Body weights and 25OHD3 concentrations were compared for each cohort at baseline, and there were no differences in the mean value of each variable between the ET and control monkeys.
FIG. 3.
Baseline vitamin D3 concentration as a function of baseline lumbar spine (L2–4) BMD in cynomolgus monkeys (Macaca fascicularis). Baseline, for this study, was after a 4-month prestudy period consuming a controlled diet of a women's equivalent of 1000 IU/day of 25OHD3.
FIG. 4.
Comparison of the percent change in lumbar spine (L2–4) BMD from baseline to 20 months in the cynomolgus monkeys (Macaca fascicularis) on CEE as the percent change in vitamin D3 plasma concentration increased (from baseline to 6 months).
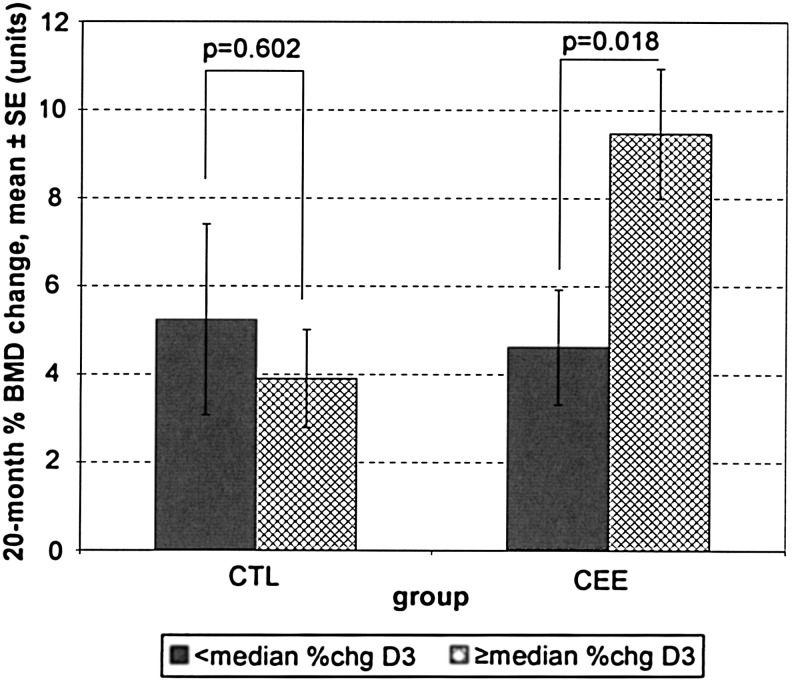
When comparing the monkeys receiving ET to the control monkeys after 20 months, those on ET had a greater mean change in BMD measurements (0.034 g/cm2 vs. 0.020 g/cm2), although the difference failed to achieve statistical significance (p=0.064). For monkeys on ET, those with a mean percentage change in 25OHD3 concentrations at or above the median (using the average of the baseline to 6 month 25OHD3 concentration) had greater absolute 20-month BMD values compared to monkeys with a lower percentage change in 25OHD3 concentrations (0.045±0.029 g/cm2 vs. 0.021±0.017 g/cm2, p=0.027). For monkeys on ET, those with a mean percentage change in 25OHD3 concentrations at or above the median experienced a greater BMD percent change over 20 months compared to monkeys with a lower percentage change in 25OHD3 concentrations (9.47±5.31 g/cm2 vs. 4.62±3.69 g/cm2, p=0.018) (Fig. 5).
FIG. 5.
Comparison of the mean percent change in lumbar spine (L2–4) BMD from baseline to 20 months in the cynomolgus monkeys (Macaca fascicularis) on CEE vs. control (CTL), broken into two groups, those in the lower (<median) and upper (≥median) median of vitamin D3 concentrations (based on the percent change from baseline to 6 months).
Because of the possibility that the results could have been affected by the 2 monkeys with ovarian remnant syndrome, the data subsequently were analyzed with these subjects removed. The results of having excluded data from these 2 monkeys showed no differences between groups in any of the comparisons.
Discussion
The results of this study indicate that large interindividual variations in 25OHD3 concentrations exist even when the amount of 25OHD3 consumed is tightly controlled. A major advantage of our methodology and use of the macaque model is the ability to eliminate the multiple confounding variables seen in human cohorts. With this in mind, our data in this translational model suggest that an individual's serum 25OHD3 concentration is dependent on more than diet, oral 25OHD3 supplementation doses, sun exposure, or menopausal status. The individual variations are presumably largely dependent on genetics. These findings also raise the question of what role 25OHD3 supplementation plays. How much is one able to respond to vitamin D deficiency through pharmacologic supplementation (vs. sun exposure), or are there certain genetically determined individuals who are able to respond more favorably?
These results showed a clear association between those macaques with higher 25OHD3 concentrations and higher BMD measurement. The natural question is: Can 25OHD3 supplementation translate, over time, into improved BMD? Alternatively, it could be that increased 25OHD3 is simply a marker for genetic predisposition to other metabolic phenomena that cause increased BMD. This possibility is especially intriguing, given the fact that all monkeys were receiving an identical diet, with identical 25OHD3 supplementation and similar sun exposure.
It has been well established that HT/ET leads to increased BMD because of its antiresorptive properties.35,36 Although all monkeys receiving ET had improved BMD, there was clearly an advantage in those receiving CEE in the higher 25OHD3 group compared with the lower 25OHD3 group. Looking at the absolute difference, those receiving CEE with increased 25OHD3 concentrations had approximately double the BMD of those with lower 25OHD3 concentrations (Fig. 5). Interestingly, in addition to those receiving CEE who had increased plasma concentrations of 25OHD3, all other groups had approximately the same BMD findings after 20 months (those receiving placebo, regardless of the 25OHD3 concentration, and those receiving CEE with lower 25OHD3 concentrations). If these results persist in larger-scale studies and translational findings, they could imply that increased 25OHD3 concentrations may target those who will have positive or enhanced bone effects with CEE and help delineate what role 25OHD3 replacement may have. It could be that 25OHD3 in the plasma works synergistically with CEE to yield positive skeletal results.
When the baseline 25OHD3 concentrations were measured, the monkeys had been on a consistent diet with the equivalent of 1000 IU/day of 25OHD3 for 4 months. By calculating a percent change in 25OHD3 from baseline to 6 months, we are likely identifying those individuals that are responsive or amenable to 25OHD3 supplementation. A major finding of this study is that the responsiveness of 25OHD3 supplementation may have prognostic value in terms of the potential beneficial effects of ET. The clinical implication of these findings, therefore, may be the following: For women given CEE for bone protection, knowledge of the 25OHD3 concentration and an attempt to raise it into an adequate range may be beneficial or prognostic. Of note, similar to trends observed in humans, baseline 25OHD3 was significantly correlated with both 6-month 25OHD3 and 6-month percent change in 25OHD3 (p<0.001), although there was no difference in this relationship in the estrogen group (control [CTL] vs. CEE). Monkeys in the CTL group showed a significant correlation between baseline 25OHD3 and 6-month 25OHD3 (r=0.472, p=0.017) and between baseline 25OHD3 and percent change in 25OHD3 (r=−0.595, p=0.002). Monkeys in the CEE group showed a significant correlation between baseline 25OHD3 and 6-month 25OHD3 (r=0.759, p<0.001) and between baseline 25OHD3 and percent change in 25OHD3 (r=− 0.405, p=0.044).
Limitations of this study include the small sample size. Whether these findings will translate from the monkey model to a human cohort will need to be demonstrated. However, cynomolgus monkeys are a well-documented model in which to study these findings.22–26 Using human models, it is nearly impossible to recreate the tightly controlled environment achieved in this cohort. As we do not know the ideal 25OHD3 concentrations for optimum health in humans or monkeys, we analyzed the data based on the relationships between higher vs. lower 25OHD3 concentrations as surrogates for adequate vs. inadequate. As we are aware of few studies evaluating 25OHD3 concentrations in nonhuman primates, we are unable to compare our data to prior studies. The amount of sunlight among these animals, by design, was negligible. The amount of oral 25OHD3 in the diets, however, was adequate to meet the physiologic needs. The 25OHD3 concentrations in these monkeys may be higher than those found in young adult women, and interpretation of human 25OHD3 concentrations in epidemiologic studies is difficult, considering the multiple confounding environmental factors, such as diet, sunlight, concomitant medical conditions, medications and supplements, and compliance. In order to provide a more controlled environment than human studies, therefore, we chose to eliminate UVB (direct sunlight exposure) as a major potential confounding factor. One of the most clinically relevant finding in this study is that individual differences and wide variations between 25OHD3 concentrations exist despite equal and adequate dietary supplementation, similar exercise, and minimal sun exposure.
A major strength of this study is the strict, natural compliance of the study model, with regard to diet, compliance with medication vs. placebo, timing of testing and medications, and other study protocol. The fact that we were able to see such clear results and trends despite a small sample size is quite impressive. Certainly, repeating this study, along with a larger sample, in translational findings would be ideal.
In conclusion, cynomolgus monkeys fed a diet containing a woman's equivalent of 1000 IU/day of 25OHD3 have a wide range of plasma 25OHD3 concentrations, likely due to genetic variations. Despite consuming the same amount of dietary 25OHD3, those with higher 25OHD3 plasma concentrations have higher BMDs. Those consuming CEE, in the upper half of the median of plasma 25OHD3 concentrations, had the greatest increase in BMD over time. These results suggest that 25OHD3 and CEE may have synergistic effects on BMD.
Acknowledgments
The funding sources for this work were the research budgets of the Wake Forest University Primate Center, The Reading Hospital and Medical Center, and the NIH (AG027847, S.E.A.; T32 training grant RR 07009-32, K.E.).
Preliminary 6-month results were presented in abstract form October 8, 2010, at the NAMS 21st annual meeting in Chicago, Illinois.
Disclosure Statement
T.B.C. is a member of an advisory committee to Pfizer pharmaceuticals and has been supported with a research grant from Pfizer. The other authors have no conflicts of interest to report.
References
- 1.Pittas AG. Dawson-Hughes B. Vitamin D and diabetes. J Steroid Biochem Mol Biol. 2010;121:425–429. doi: 10.1016/j.jsbmb.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Hurst PR. Stonehouse W. Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—A randomized, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 3.Wong KE. Szeto FL. Zhang W, et al. Involvement of the vitamin D receptor in energy metabolism: Regulation of uncoupling proteins. Am J Physiol Endocrinol Metab. 2009;296:E820–828. doi: 10.1152/ajpendo.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S. Glenn DJ. Ni W, et al. Expression of the vitamin D receptor is increased in the hypertrophic heart. Hypertension. 2008;52:1106–1112. doi: 10.1161/HYPERTENSIONAHA.108.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh J. Weng S. Felton SK, et al. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–698. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TJ. Pencina MJ. Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otani T. Iwasaki M. Sasazuki S. Inoue M. Tsugame S. Plasma vitamin D and risk of colorectal cancer: The Japan public health center-based prospective study. Br J Cancer. 2007;97:446–451. doi: 10.1038/sj.bjc.6603892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H. Stampfer MJ. Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007:4e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garland CF. Gorham ED. Mohr SB, et al. Vitamin D and prevention of breast cancer: Pooled analysis. J Steroid Biochem Mol Biol. 2007;103:708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Ross AC, editor; Taylor CL, editor; Yaktine AL, editor; Del Valle HB, editor. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary reference intakes for calcium and vitamin D. books.nap.edu/openbook.php?record_id=13050&page=R1. [Mar 28;2012 ]. books.nap.edu/openbook.php?record_id=13050&page=R1
- 11.Institute of Medicine, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Brief report. Dietary reference intakes for calcium and vitamin D. Nov, 2010. www.iom.edu/∼/media/Files/Report%20Files/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Vitamin%20D%20and%20Calcium%202010%20Report%20Brief.pdf. [Mar 28;2012 ]. www.iom.edu/∼/media/Files/Report%20Files/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Vitamin%20D%20and%20Calcium%202010%20Report%20Brief.pdf
- 12.Yusupov E. Li-Ng M. Pollack S. Yeh JK. Mikhail M. Aloia JF. Vitamin D and serum cytokines in a randomized clinical trial. Int J Endocrinol. 2010:7. doi: 10.1155/2010/305054. Article ID 305054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Osteoporosis Foundation (IOF) IOF position statement: Vitamin D recommendations for older adults—Position paper. www.natap.org/2010/HIV/072310_01.htm. [Mar 28;2012 ]. www.natap.org/2010/HIV/072310_01.htm [DOI] [PubMed]
- 14.National Osteoporosis Foundation. Prevention: Vitamin D and bone health. www.nof.org/aboutosteoporosis/prevention/vitamind. [Mar 28;2012 ]. www.nof.org/aboutosteoporosis/prevention/vitamind
- 15.Holick MF. Binkley NC. Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 16.North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17:25–54. doi: 10.1097/gme.0b013e3181c617e6. [DOI] [PubMed] [Google Scholar]
- 17.Ginde AA. Liu MC. Camargo CA. Demographic differences and trends of vitamin D insufficiency in the U.S. population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter D. De Lange M. Snieder H, et al. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res. 2001;16:371–378. doi: 10.1359/jbmr.2001.16.2.371. [DOI] [PubMed] [Google Scholar]
- 19.Karohl C. Su S. Kumari M, et al. Heritability and seasonal variability of vitamin D concentrations in male twins. Am J Clin Nutr. 2010;92:1393–1398. doi: 10.3945/ajcn.2010.30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang TJ. Zhang F. Richards JB, et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutiérrez OM. Farwell WR. Kermah D. Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. www.natap.org/2010/HIV/aframericansbone.pdf. [Mar 28;2012 ]. www.natap.org/2010/HIV/aframericansbone.pdf [DOI] [PMC free article] [PubMed]
- 22.Jerome C. Hormonal therapies and osteoporosis. ILAR J. 2004;45:170–178. doi: 10.1093/ilar.45.2.170. [DOI] [PubMed] [Google Scholar]
- 23.Smith SY. Jollette J. Turner CH. Skeletal health: Model of postmenopausal osteoporosis. Am J Primatol. 2009;71:752–765. doi: 10.1002/ajp.20715. [DOI] [PubMed] [Google Scholar]
- 24.Jerome CP. Turner CH. Lees CJ. Decreased bone mass and strength in ovariectomized cynomolgus monkeys (Macaca fascicularis) Calcif Tissue Int. 1997;60:265–271. doi: 10.1007/s002239900227. [DOI] [PubMed] [Google Scholar]
- 25.Jerome CP. Peterson PE. Nonhuman primate models in skeletal research. Bone. 2001;29:1–6. doi: 10.1016/s8756-3282(01)00477-x. [DOI] [PubMed] [Google Scholar]
- 26.Burr DB. Hirano T. Turner CH. Hotchkiss C. Brommage R. Hock JM. Intermittently administered human parathyroid hormone (1–34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res. 2001;16:157–165. doi: 10.1359/jbmr.2001.16.1.157. [DOI] [PubMed] [Google Scholar]
- 27.Fox J. Newman MK. Turner CH. Guldberg RE. Varela A. Smith SY. Effects of treatment with parathyroid hormone 1–84 on quantity and biomechanical properties of thoracic vertebral trabecular bone in ovariectomized rhesus monkeys. Calcif Tissue Int. 2008;82:212–220. doi: 10.1007/s00223-008-9108-7. [DOI] [PubMed] [Google Scholar]
- 28.Jayo MJ. Register TC. Carlson CS. Effects on bone of oral hormone replacement therapy initiated 2 years after ovariectomy in young adult monkeys. Bone. 1998;23:361–366. doi: 10.1016/s8756-3282(98)00106-9. [DOI] [PubMed] [Google Scholar]
- 29.Jerome CP. Carlson CS. Register TC, et al. Bone functional changes in intact, ovariectomized, and ovariectomized, hormone-supplemented adult cynomolgus monkeys (Macaca fascicularis) evaluated by serum markers and dynamic histomorphometry. J Bone Miner Res. 1994;9:527–540. doi: 10.1002/jbmr.5650090413. [DOI] [PubMed] [Google Scholar]
- 30.The North American Menopause Society. Menopause practice: A clinician's guide. 4th. Mayfield Heights, OH: NAMS; 2010. Disease risk: Osteoporosis; pp. 5.9–5.20. [Google Scholar]
- 31.Kuwamura Y. Kakehi K. Hirakawa K. Miyajima H. Ectopic uterine ovarian tissue in cynomolgus monkeys. Toxicol Pathol. 2006;34:220–222. doi: 10.1080/01926230600695482. [DOI] [PubMed] [Google Scholar]
- 32.Steege JF. Ovarian remnant syndrome. Obstet Gynecol. 1987;70:64–67. [PubMed] [Google Scholar]
- 33.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194(Suppl):S3–11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 34.Lees CJ. Kaplan JR. Chen H. Jerome CP. Register TC. Franke AA. Bone mass and soy isoflavones in socially housed, premenopausal macaques. Am J Clin Nutr. 2007;86:245–250. doi: 10.1093/ajcn/86.1.245. [DOI] [PubMed] [Google Scholar]
- 35.Wells G. Tugwell P. Shea B, et al. for the Osteoporosis Methodology Group and the Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr Rev. 2002;23:529–539. doi: 10.1210/er.2001-5002. [DOI] [PubMed] [Google Scholar]
- 36.Cauley JA. Robbins J. Chen Z, et al. for the Women's Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women's Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]