Abstract
Significance: Nitric oxide (NO) plays diverse physiological roles in the central nervous system, where it modulates neuronal communication, regulates blood flow, and contributes to the innate immune responses. In a number of brain pathologies, the excessive production of NO also leads to the formation of reactive and toxic intermediates generically termed reactive nitrogen species (RNS). RNS cause irreversible or poorly reversible damage to brain cells. Recent Advances: Recent work in the field focused on the ability of NO and RNS to yield protein modifications, including the S-nitrosation of cysteine residues, which, in many instances, impact cellular functions and viability. Critical Issues: The vast majority of neuropathological studies focus on the loss of cell viability, but nitrosative stress may also strongly impair the functions of neuronal processes: axonal projections and dendritic trees. The functional integrity of axons and dendrites critically depends on local metabolism and effective delivery of metabolic enzymes and organelles. Here, we summarize the existing literature describing the effects of nitrosative stress on the major pathways of energetic metabolism: glycolysis, tricarboxylic acid cycle, and mitochondrial respiration, with the emphasis on modifications of protein thiols. Future Directions: We propose that axons and dendrites are highly vulnerable to nitrosative stress because of their low glycolytic capacity and high dependence on timely delivery of metabolic enzymes and organelles from the cell body. Thus, supplementation with the end products of glycolysis, pyruvate or lactate, may help preserve metabolism in distal neuronal processes and protect or restore synaptic function in the ailing brain. Antioxid. Redox Signal. 17, 992–1012.
Introduction
Nitric oxide (NO) is a diffusible free radical signaling molecule that regulates many physiological functions. NO is synthesized by a set of three different nitric oxide synthases (NOS) that catalyze the oxygen and NADPH-dependent oxidation of l-arginine to l-citrulline and NO (143). The brain tissue expresses all three NOS isoforms and—excluding inflammatory conditions—produces more NO for signaling purposes than the rest of the body combined (33, 203). Neuronal NOS (nNOS or NOS I) is expressed in distinct populations of neurons, where it is activated in a Ca2+/calmodulin-dependent manner, downstream of Ca2+ influx via the N-methyl-d-aspartate (NMDA) subtype of ionotropic glutamate receptors (29, 30, 32). NO signaling derived from the activity of nNOS modulates neurotransmission and regulates synaptic plasticity via a variety of mechanisms, including nitrosative modifications of cysteine moieties (31, 95, 127, 144, 238). Endothelial NOS (eNOS or NOS III) is expressed in endothelial cells of blood vessels, where its activity is controlled in a Ca2+/calmodulin-dependent manner by sheer stress and vasodilator molecules (128, 150, 207, 215). The eNOS-derived NO diffuses from endothelial to smooth muscle cells and via their relaxation, controls local vessel tone and systemic blood pressure (89, 125). Unlike nNOS and eNOS, the activity of the third member of this family, inducible NOS (iNOS or NOS II), is essentially Ca2+ independent and is regulated at the level of gene expression, typically by pro-inflammatory cytokines (169, 249, 278). This enzyme was initially cloned from macrophages (170, 274), but was subsequently found to be induced in all types of brain cells, including neurons, astrocytes, microglia, and oligodendrocytes, as well as the cells composing brain vasculature (17, 49, 90, 182, 272).
The high capacity of brain tissue that produces NO comes at a price. The unbalanced production of NO and its derivatives, collectively termed reactive nitrogen species (RNS), leads to pathological changes in brain functions, and, ultimately, to loss of neuronal cells. The contribution of RNS to neurodegeneration has received much attention (see for example ref. 142, 196, 238). In the present review, we would like to make the case that NO-mediated protein modifications, most notably S-nitrosation (or S-nitrosylation, as it relates to cellular signaling), are important contributors to metabolic deficits seen in many disorders of the central nervous system (CNS). Most importantly, we would like to propose that the distal neuronal processes and the process of synaptic transmission may be particularly vulnerable to nitrosative stress in the CNS. Such selective sensitivity is related to high dependence on timely delivery of mitochondria and metabolic enzymes that are otherwise inactivated by NO and RNS.
Nitrosative Stress Is a Common Feature of Many Brain Pathologies
There is extensive literature suggesting that unbalanced or excessive production of NO and RNS is an important component of neurodegeneration in numerous disorders of the CNS. The list of brain pathologies that have been linked to nitrosative stress includes ischemic brain injury (stroke), Alzheimer's disease, Parkinson's disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis, Huntington's disease, and traumatic brain injury. For comprehensive coverage of the role of RNS in these disorders, the reader may refer to several detailed reviews (12, 42, 61, 105, 142, 167, 186, 197, 203). The cellular and molecular mechanisms underlying the impact of nitrosative stress in the brain are diverse and likely pathology specific. Neurological deficits and loss of brain cells may be restricted to particular cell populations, be mediated by chemical modifications in a few proteins, or, alternatively, stem from global oxidative and nitrosative damage to all components of the brain, including vasculature. In this section, we give only a few specific examples, which will be relevant to our subsequent discussion of the relationship between nitrosative stress and neuronal metabolism.
One of the common approaches to track RNS production in vivo is to visualize and/or quantify protein nitrotyrosine, a stable chemical modification of tyrosine. Nitrotyrosine is frequently considered a specific fingerprint for tissue production of peroxynitrite, however, it may also be produced by other RNS (108). In the normal brain, the content of nitrotyrosine is close to detection limits; however, it is strongly elevated in a variety of brain pathologies. Increased nitrotyrosine levels have been documented in human postmortem samples obtained from patients who suffered from the Alzheimer's disease (AD), PD, MS, amyotrophic lateral sclerosis, and stroke (1, 8, 85, 100, 165, 244; reviewed in 203, 226). However, the appearance of nitrotyrosine in the human brain only indirectly confirms the elevated production of RNS, but does not provide direct evidence for the causative role of RNS in brain pathologies. Essentially all mechanistic insights on RNS involvement in neurodegeneration come from animal work.
It is worthwhile to note that the historical focus on the production of nitrotyrosine, at the expense of other modifications of biological molecules by RNS, can be explained by the availability of selective detection tools, including a number of anti-nitrotyrosine antibodies, and by a relatively high stability of this amino-acid derivative in vitro and in vivo. In contrast, monitoring the formation of other protein modifications, such as S-nitrosylation and S-glutathionylation of cysteine moieties, formation of disulfide bonds, and other amino-acid alterations proved to be much more challenging, particularly in vivo, and at the level of individual molecules. Nevertheless, these less-studied protein modifications appear to play a major role in the physiological and pathological effects of NO and RNS.
Alzheimer's disease
AD is the most common age-dependent neurodegenerative disorder that involves cognitive decline, eventually advancing to the broad loss of cerebral and bodily functions. The Alzheimer's Association indicates that there are 5.4 million individuals affected by this incurable disorder in the United States alone (www.alz.org). Development of AD pathology is accompanied by the formation of extracellular β-amyloid (or senile) plaques consisting of aggregates of partially processed amyloid precursor protein (APP) (115, 177, 234). Additionally, many degenerating neurons incorporate so-called neurofibrillary tangles, which represent aggregates of the pathologically hyperphosphorylated microtubule-associated protein tau (5, 18). Improper processing of APP to β-amyloid is critical to the AD pathology, because a number of familial cases were linked to mutations in the APP itself or in the APP-processing enzymes (235). Overexpression of human mutated proteins in the mouse brain causes the formation of β-amyloid, produces neurological deficits, and shortens the lifespan of transgenic animals (91, 229).
There is extensive experimental evidence that the damage and death of neuronal cells in AD develops due to oxidative stress promoted by either aggregated or nonaggregated forms of β-amyloid peptides (13, 168, 265), with at least a part of this damage caused by the formation of peroxynitrite (244). In support of the latter idea, a direct intracerebroventricular injection of β-amyloid peptides triggers protein tyrosine nitration (261). Monomeric and particularly oligomeric β-amyloid peptides induce inflammatory responses in glial cells—astrocytes and microglia—which start to overexpress iNOS and possibly eNOS (87, 172, 268). Increased immunoreactivity of nNOS has also been reported in degenerating neurons in postmortem sections prepared from the human brain (256). In a transgenic model of AD, the genetic disruption of iNOS reduced nitrotyrosine load and decreased amyloid plaque burden, thus directly implicating RNS production in the disease progression (198). It has been recently discovered that β-amyloid fragments stimulate S-nitrosylation of Cys644 in the dynamin-related protein 1 (Drp1), a critical factor in the mitochondrial fission and fusion (56). Both β-amyloid fragments and S-nitrosylating agents cause fragmentation of mitochondria, loss of dendritic spines, and increase in cell death in the primary cultures of cortical neurons, in a Drp1-dependent fashion. Importantly, post mortem studies detected selective loss of mitochondria in axonal projections and S-nitrosylated Drp1 in the brains of AD patients (56, 269). Another potentially relevant protein modification is S-nitrosylation of Cys83 and Cys157 in cyclin-dependent kinase 5 (Cdk5), which activates this enzyme and may lead to changes in neuronal dendritic spine density (213).
Parkinson's disease
Parkinsonism is the second most common chronic neurodegenerative disorder that affects ∼1% of individuals older than 65 years. The disease typically manifests as a severe disruption of motor coordination with bradykinesia, resting tremor, and postural instability. Motor deficits are the result of the profound loss of dopaminergic neurons within the substantia nigra pars compacta, but several other nondopaminergic neuronal populations are also affected (153). Unlike the rare familial cases of PD, the exact origins for idiopathic cases are not known. However, there is very good evidence indicating that the progression of PD is caused by oxidative and nitrosative stress and involves protein misfolding and disruption of mitochondrial energetic metabolism (154, 191). In humans and animals, PD-like symptoms and loss of striatal neurons can be induced by a number of pesticides, such as rotenone, which inhibit mitochondrial electron transport reactions, with mitochondrial complex I being the main target (71, 101). One commonly used animal model of Parkinsonism is produced by injecting animals with the synthetic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or its active metabolite 1-methyl-4-phenylpyridinium (MPP+), which cause rapid and selective loss of dopaminergic neurons via inhibiting activity of complex I, and produce the PD-like phenotype. Interestingly, the Parkinsonian effects of MPTP were initially identified in several human patients who used it as an illicit drug (155). The involvement of NO and RNS in the progression of PD is supported by a number of observations. Glutathione depletion in dopaminergic neurons and model cells, similar to that observed in PD pathology, causes dramatic accumulation of S-nitrosothiols in mitochondrial proteins (55). Pharmacological inhibitors of NOS strongly reduce neuronal loss and improve MPTP/MPP+-induced neurological deficits in rodents and primates (110, 232). Furthermore, genetic deletion of nNOS (but not eNOS) protects against the MPP+-induced striatal lesions and prevents an increase in nitrotyrosine production (179).
Several more recent publications made a fascinating connection between nitrosative stress and the intracellular E3 ubiquitin-ligase Parkin. Mutations in the Parkin gene (PARK2) have been linked to several familial cases of PD with an unusually early onset of neurological deficits (140). Several groups have found that S-nitrosylation of Parkin causes progressive inhibition of its activity and inactivation of the ubiquitin-proteosome system, and have detected S-nitrosylated Parkin in rodent models of PD and in PD patients (62, 275). It has been speculated that other ubiquitin E3 ligases with a similar protein structure may be inactivated in a comparable fashion. Subsequent work established that S-nitrosylation of protein disulfide-isomerase, the protein that facilitates maturation and proper folding of newly synthesized proteins in endoplasmic reticulum, also inhibits its activity (263). In one study, S-nitrosylated protein disulfide isomerase has been detected in all postmortem samples of PD and AD brain tissue that were examined in this context (263). These findings led to the hypothesis that nitrosative modification of Parkin and protein disulfide isomerase play a pivotal role in the dysfunction of the ubiquitin-proteosome system and in such a way contribute to PD and AD and perhaps other disorders, which are thought to involve the accumulation of misfolded proteins (197).
Multiple sclerosis
MS is an autoimmune inflammatory disease in which immune cells attack myelin sheaths insulating neuronal projections in the brain and the spinal cord. The attacks follow in a relapsing-remitting manner and are characterized by progressing demyelination, sensory deficits, and limb weakness. Advanced cases of MS lead to physical and cognitive disability and death. Although the initial triggers for MS are unknown, the recognition of its autoimmune nature led to the development of many successful therapies. Though not without side-effects, these therapies allow for the effective management of symptoms and dramatic increases in the lifespan of patients. The animal model of MS is experimental allergic encephalomyelitis (EAE), in which an attack of immune cells on myelin is triggered by the inoculation of myelin components or purified myelin proteins (248). The progression of both MS and EAE is followed not only by accumulation in the CNS of blood immune cells, lymphocytes, and macrophages, but also by the inflammatory activation of glial cells, microglia, and astrocytes (116). Inflammation upregulates the expression of iNOS and, potentially, production of peroxynitrite in many types of brain cells, as indicated by increased immunoreactivity for iNOS and nitrotyrosine (165). Furthermore, both MS and EAE cause robust S-nitrosylation of protein cysteines in the CNS (19, 20). One study demonstrated that levels of the anti-S-nitrosocysteine antibodies in plasma are elevated in EAE animals and MS patients in a manner that is predictive for the progression of the disease (28). The functional consequences of nitrosative stress in MS are still poorly understood. Several groups found that pharmacological inhibitors of iNOS, particularly relatively selective aminoguanidine, potently protect against neurological deficits in EAE models in mice and rats (66, 284). However, conflicting data with pharmacological inhibitors of iNOS and the iNOS gene deletion have also been reported (84, 132, 223). The paradoxical exacerbation of neurological deficits found in these latter studies was explained by the fact that iNOS-derived NO potently suppresses Th1-dependent immune responses, and that inhibition or removal of the enzyme prevents the natural reduction of immune responses (131). Protein S-nitrosylation in endothelial cells may also limit the infiltration of blood immune cells via changes in the expression levels of endothelial adhesion molecules (211). Overall, the role of NO and RNS in the etiology of MS is complex and involves both protective and harmful effects (for review see 243).
Stroke
Stroke or cerebral ischemia is an acute loss of brain tissue and neurological functions that occurs due to the interruption of blood flow. Although in the United States, mortality from stroke has recently declined, it remains the fourth leading cause of death (134,000 cases in 2008) and the number one reason for the long-term disability in the nation (260). Worldwide, stroke is the second leading cause of death after heart disease (83, 260). Neurological deficits in this disorder develop due to the occlusion of one or several major brain vessels by thrombus or embolus (ischemic stroke), rupture of cerebral vessels due to either trauma or aneurism (hemorrhagic stroke), or complete cessation of cerebral blood flow such as in cardiac arrest (76). Massive depolarization of neuronal and glial cells causes release of the excitatory neurotransmitter glutamate, followed by cytosolic Ca2+ overload, and activation of numerous pathological cascades, altogether leading to the ischemic cell death and dysfunction. The mechanisms of ischemic tissue damage are extremely complex and have been described and discussed in detail elsewhere (76, 162, 167, 189). Briefly, glutamate and cell depolarization open a variety of Ca2+-permeable channels, one type of which—the NMDA subtype of glutamate receptor-channels—is of particular importance, because the channels are functionally coupled to nNOS (57, 220). NMDA receptors and nNOS are assembled in one complex with the post-synaptic density protein-95 (PSD-95) via PDZ-domain interactions (58, 60).
During ischemic episodes, nNOS activity is increased as long as there are remaining substrates, oxygen, and l-arginine, and the levels of NO and RNS synthesis may be further sustained and elevated during reperfusion (80, 174, 202). The uncontrolled production of NO and RNS by nNOS leads to irreversible tissue damage and death (76, 225). Importantly, other NOS isoforms, eNOS, and iNOS, play differing roles in ischemic brain damage. eNOS is generally protective due to vasodilatation and enhancement of collateral blood flow, while iNOS contributes to the delayed phases of brain damage, which are associated with the infiltration of blood immune cells and activation of the resident immune cells, microglia (122, 123, 225). There is extensive literature suggesting that peroxynitrite is a major damaging molecule in stroke (reviewed in 22, 203). However, the existing and emerging literature suggests that nitrosative modifications, including S-nitrosylation of cysteines, are also of key pathological importance. The negative impact of S-nitrosylation may not only involve the activation of matrix metalloproteinases that cause neuronal death and pathological disruption of the blood-brain barrier (96, 104), nitrosative modifications of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) that inhibit its glycolytic functions (for references see the following sections) but may also promote apoptotic cell death (112, 227), as well as activation of cycloxygenase-2 (257). However, the effects of S-nitrosylation are not exclusively negative. For example, S-nitrosylation of the NMDA receptors inhibits their function and downstream pathological cascades (163). An intravenous injection of S-nitrosoglutathione (GSNO) reduces ischemic brain damage via the suppression of systemic and local immune responses, and possibly the inhibition of infiltration of blood immune cells (135, 211). Vasodilating effects of S-nitrosothiols were also implicated in the neuroprotective properties of systemic nitrite in a primate model of subarachnoid hemorrhage (210). Furthermore, S-nitrosylation of proteins in the mitochondrial complex I was found to contribute to cardioprotection in animal models of heart ischemia-reperfusion injury (242).
In summary, there is extensive evidence that nitrosative stress occurs in numerous disorders of the CNS. In many cases, existing literature makes mechanistic connections between RNS production and neurological outcomes. However, our understanding of the molecular mechanisms linking nitrosative stress to the disruption of brain functions and loss of neural cell viability remains far from complete. Pinpointing the role for S-nitrosylation in vitro, but especially in vivo, has been particularly challenging, because reliable methods for S-nitrosothiol detection are still in the process of development and refining. This review focuses on chemical modifications in protein thiols, including S-nitrosylation. However, we will also cover other known chemical alterations of amino-acid residues that likely occur in vivo simultaneously with S-nitrosylation.
Biochemical Considerations for Nitrosative Stress in the Healthy and Pathological Brain
The detailed biochemical mechanisms contributing to the biological actions of nitrosative stress are described and discussed in detail elsewhere in this Forum. Here, we would like to highlight the key points related to the biological chemistry of NO and related RNS that we believe are relevant to brain pathology. This should serve to support the discussion in the next section on the role of RNS in regulating specific metabolic processes in the brain.
As already mentioned in the previous section, nitrotyrosine has been extensively utilized as a marker of nitrosative stress in many brain pathologies. The use of the term “nitrosative” in this context is, in fact, poorly descriptive and does not reflect the chemistry accounting for the formation of nitrotyrosine. This is because nitrotyrosine is not formed from the nitrosation—the addition of a nitroso group (−NO)—of tyrosine residues but from their nitration, that is, the addition of a nitro group (−NO2). NO itself is not a nitrating agent, and nitrotyrosine is instead derived mainly through a reaction with peroxynitrite or peroxidases and nitrite (for review see 203). Nitrated tyrosines, in contrast to nitrosylated cysteine residues, are relatively stable, which retrospectively allows for the development of reliable and specific nitrotyrosine antibodies and demonstration of the occurrence of peroxynitrite in vivo. However, the functional impact of protein nitration and its potential role in regulating cell signaling has been more difficult to establish. One issue is the inherent stability of nitrotyrosine and lack of a mechanism that would allow nitration-denitration reactions to occur in a regulated fashion in proteins (6). Another problem is that while peroxynitrite nitrates tyrosine residues, it also modifies other amino acids, including methionine, tryptophan, and, most importantly, cysteine. Overall, more recent studies on the physiological and pathological effects of RNS (including peroxynitrite) are increasingly focused on not only the RNS-driven S-nitrosylation but also the oxidation and S-glutathionylation of cysteine residues (Table 1), which likely occur concomitantly in vivo.
Table 1.
Some Modifications Derived from the Reaction of Cysteine Residues with Reactive Nitrogen Species and Reactive Oxygen Species
| Thiol modifications | Chemical name of modification |
|---|---|
| RSSR | Disulfidea |
| RSOH | Sulfenic |
| RS(O)OH | Sulfinic |
| RS(O)2OH | Sulfonic |
| RSNR′′R′ | Sulphenyl-amideb |
| RSNO | Nitrosothiol |
| RSNO2 | Nitrothiolc |
Intra or intermolecular disulfide may be formed between proteins. Glutathionylation is the formation of a disulfide between a cysteine residue on a protein and the tripeptide glutathione.
Sulphenic acid in proteins may form a sulphenyl-amide if a proximal nitrogen is available, as shown in the case of protein tyrosine phosphatase 1B (224, 264).
Nitrothiols may be formed from the reaction of peroxynitrite with a thiol such as glutathione (10).
The S-nitrosylation or S-nitrosation (the former term is preferred in biology, while the latter is more frequently used in chemical literature) of protein cysteine residues is increasingly recognized as a physiologically and pathologically important outcome of nitrosative stress. The brain was the first organ in which endogenous protein S-nitrosylation was detected under nonpathological conditions (127). In recent years, the major shift of interest toward the role of S-nitrosylation was driven not only by accumulating evidence of its pathological significance but also due to the emergence of new sensitive methods allowing for the quantification and characterization of protein S-nitrosothiols (86). It is now evident that protein S-nitrosylation may be mediated by NO and related RNS derived from multiple sources that include not only three isoforms of NOS but also endogenously produced nitrite, as well as RNS originating from pharmacological and nutritional inputs (86, 171). Again, it is important to keep in mind that protein modifications that are mediated by RNS are not limited to S-nitrosylation but may also involve other modifications at cysteine sites, including, but not limited to, oxidation and S-glutathionylation (152).
The chemical pathways that lead to the S-nitrosylation of cysteine residues in proteins have been relatively well characterized over the past many years (Fig. 1), but the elucidation of the physiologically relevant pathways is still a task in progress. For example, we know that the reaction of NO with molecular oxygen forms RNS such as dinitrogen trioxide (N2O3) that effectively S-nitrosylates cysteine residues in vitro (159, 271). This would suggest that molecular oxygen should represent an important modulator of NO-mediated S-nitrosylation. However, this idea is somewhat incongruent with our findings that in cultured adherent cells, the levels of protein S-nitrosothiols are strongly increased at low oxygen levels (129). One potential explanation is that the bulk of thiols in the intracellular milieu may be oxidized by NO/O2 intermediates such as nitrogen dioxide (NO2) that may be otherwise required for the formation of nitrosating species such as N2O3. In contrast to the observations just made, recent studies by the Lancaster group indicate that intracellular nitrosation by NO is, in fact, independent of molecular oxygen and may occur through the transnitrosation of thiols from dinitrosyl-iron complexes (27). Alternative oxygen-independent nitrosation pathways may also include transnitrosation reactions that require the mobilization of low-molecular-weight S-nitrosothiol pools such as S-nitrosocysteine (283). Nevertheless, S-nitrosylation in biological systems is, in essence, governed by local oxygen concentrations, because oxygen serves (i) as a substrate for NO production (250), (ii) regulator of nitrite reduction to NO (65), and (iii) source of RNS and reactive oxygen species (ROS) controlling the chemistry derived from NO (103).
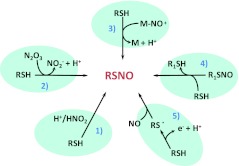
FIG. 1.
Mechanisms of nitrosothiol formation. Summary of five primary mechanisms of thiol nitrosation that may be relevant to biological systems: (1) S-nitrosation by nitrous acid (HNO2), which may occur in intracellular acidic organelles where the pH is low enough to form significant amounts of HNO2 from nitrite (NO2−); (2) S-nitrosation by dinitrogen trioxide (N2O3) derived from nitric oxide (NO) oxidation by molecular oxygen or from the anaerobic reaction of nitrite-bound methemoglobin with NO (159); (3) transfer of a nitroso group from a metal nitrosyl (M-NO+); (4) transnitrosation between thiols; and (5) radical-radical combination between NO and thiyl radicals (RS.) formed from the one-electron oxidation of thiols. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Many studies performed in vitro have established the impact of the generation of ROS on NO-mediated S-nitrosylation. For example, peroxynitrite derived from the reaction of NO with superoxide is a poor nitrosating agent, such that the generation of superoxide decreases NO-mediated cysteine S-nitrosylation (130). Thus, superoxide may serve as a sink for NO, and, in some cases, suppress the NO-mediated signaling reactions (255). Even in the absence of superoxide, the NO/O2 chemistry as it relates to thiols may be, in fact, dominated at relatively low NO concentrations by oxidation reactions rather than nitrosation (129, 231). As just mentioned, this is because excess thiols in the intracellular milieu may be oxidized by NO/O2 intermediates such as NO2 effectively scavenging S-nitrosating species. Furthermore, intracellular nitrosative chemistry may be complicated by competition for the nitrosating species between low-molecular-weight antioxidants (mainly glutathione and ascorbate) and proteins. For example, a rapid decrease in intracellular GSH may be associated with a paradoxical increase in protein S-nitrosylation (111).
An important aspect of intracellular protein S-nitrosylation is the temporal and spatial control of fluxes of RNS and ROS in the complex intracellular environment. In this context, it is increasingly recognized that compartmentalization and site specificity for nitrosation pathways can be achieved through protein-protein interactions (86). For example, NOS may co-localize and interact with specific targets, such as cyclooxygenase-2 (138). Alternatively, protein-assisted transnitrosylation, such as in the case of the interaction between caspase-3 and thioredoxin (255), may provide a way to specifically regulate S-nitrosylation in distinct signaling pathways. Furthermore, specificity of protein S-nitrosylation may be also determined, in part, by intramolecular S-nitrosylation motifs: specific amino-acid residues surrounding reactive cysteines (77, 247).
Finally, it should be noted that the relevant physiological concentrations of NO and related nitrosating species, including low-molecular-weight S-nitrosothiols, are still debated. Physiological and pathophysiological concentrations of NO, in the brain and in other tissues, may be much smaller than previously thought (106, 273). While some studies have reported tissue levels of GSNO upward of 1 μM (97, 141), others have failed to detect any significant amounts (82, 121, 282). This is obviously an important problem, because concentrations of the reactants will direct the type of chemistry imparted on the protein targets. Local levels of NO are strongly shaped by the proximity of blood vessels due to the effective scavenging of NO by hemoglobin in red blood cells (151), and may be also strongly affected by poorly characterized tissue sinks (94, 102, 107). In the same way, intracellular levels of S-nitrosothiols may be regulated through the controlled removal by specific enzymatic activities such as GSNO reductase and the thioredoxin system (14, 15).
Although as briefly outlined just now, much progress has been made to delineate the molecular mechanisms of protein S-nitrosylation, there are obviously many outstanding questions. Confirmation of the functional role for specific S-nitrosylation sites is usually attained through site-specific mutations. However, this approach does not clearly discriminate the effects of S-nitrosylation from other NO-dependent modifications at cysteine residues, such as oxidation or glutathionylation. In the case of other posttranslational protein modifications, such as phosphorylation, the evidence for their functional significance was often demonstrated via pharmacological and molecular manipulation targeting specific kinases and phosphatases. Further work on the elucidation of S-nitrosylation and denitrosylation cascades in cellular systems and in vivo should be paramount to establish S-nitrosylation as a specific regulatory mechanism in normal cell signaling and in brain pathologies.
Why Are Neuronal Cells Highly Vulnerable to Nitrosative Stress?
Neuronal cells in culture and in vivo demonstrate very high sensitivity to nitrosative stress, as compared with the non-neuronal cells in the brain and other tissues. There are multiple reasons for such a selective vulnerability, which may include but not be limited to, the following: (i) A substantial fraction of neurons synthesizes NO for signaling purposes in response to activation of the NMDA subtype of glutamate receptor channels, which are permeable to Ca2+. Several brain pathologies, particularly stroke and traumatic brain injury, lead to uncontrolled elevations in extracellular glutamate levels, causing cytosolic Ca2+ overload and sustained activation of nNOS and RNS toxicity (72, 76, 176, 267). Therefore, neurons can subject themselves to nitrosative stress. (ii) Neurons have relatively low antioxidant capacity and rely on the import of precursors of the major intracellular antioxidant glutathione by the neighboring glial cells, astrocytes (52, 78). Neuronal glutathione levels and antioxidant capacity fall rapidly in response to even mild nitrosative stress, with RNS toxicity further strongly potentiated if endogenous glutathione is depleted using pharmacological agents (23, 124). (iii) Neurons constantly undergo cyclical depolarizations and repolarizations during electrical signaling, and have a very high rate of metabolism that allows them to sustain transmembrane ionic gradients. As a result, brain tissue and neuronal cells are extremely sensitive to the inhibition of metabolic processes by NO and RNS (see next for references and discussion). (iv) Another aspect of neural physiology, which makes neurons more vulnerable, is their inability to stimulate glycolysis in response to oxidative and nitrosative stress. When treated with NO or RNS, astrocytes but not neurons respond with the activation of the rate-limiting glycolytic enzyme, 6 phosphofructo kinase-1 (PFK-1) (4). Apparently, unlike astrocytes, neurons lack an important regulatory loop that positively regulates PFK-1 upon metabolic inhibition by NO (4). (v) Neurons respond to nitrosative stress with activation of the DNA repair enzyme, poly (ADP-ribose) polymerase, and this response accelerates the depletion of cytosolic ATP and metabolic failure (279). (iv) The activation of NMDA receptors and ensuring intracellular Ca2+ overload cause RNS-dependent activation of numerous pro-apoptotic processes, including cytochrome C release from the depolarized mitochondria and activation of caspases (158, 262). It should be noted, however, that, at least in some cell types, NO and RNS can also potently block apoptosis via the direct S-nitrosylation of several members of the caspase family (139, 160).
The factors just mentioned work not in isolation but rather in concert with each other. The impact of nitrosative stress on cellular metabolism feeds into all pathological cascades, at both cellular and tissue levels. Such an impact ranges from dysregulation of the ATP-dependent glutamate transport by neurons and glial cells to a global failure of ionic gradients, intracellular antioxidant defenses, and the ability of cells to maintain their integrity. In the next section, we summarize the known NO/RNS-mediated changes in activities of all the major components of energetic metabolism that may contribute to brain dysfunction in various neural pathologies. We next dedicate an additional section for discussion of the idea that nitrosative stress disproportionally affects axons and distal dendritic processes because of their unique metabolic demands, and their dependence on the long-range delivery of metabolic substrates and enzymes.
Metabolic Pathways As Targets of Nitrosative Modification and Regulation
The ability of NO to interfere with mitochondrial respiration was known long before NO was identified as a biological signaling molecule (refs in 37). NO competes with oxygen in the Fe2+/heme binding site of cytochrome c oxidase, the terminal enzymatic complex of the mitochondrial electron transport chain, and reversibly inhibits mitochondrial respiration (38, 63, 233). Such inhibition is not only thought to have physiological implications, but is also likely to cause the shutdown of mitochondrial respiration and ATP synthesis in those pathologies where NO levels remain sufficiently elevated for prolonged periods of time (37, 187). In co-cultures of neurons and glial cells (astrocytes and/or microglia), the induction of iNOS in glial cells potently suppressed mitochondrial respiration in neighboring neurons, and this phenomenon may occur in vivo (9). As discussed next, more recent findings suggest that, in contrast to NO molecule itself, other RNS have a profound impact on the metabolic processes via irreversible or poorly reversible chemical modifications of the enzymes constituting the mitochondrial electron transport chain, Krebs cycle, and glycolytic pathway. The list of relevant modifications includes mainly S-nitrosation, S-glutathionylation, oxidation, and nitration. In this section, we discuss known and putative targets for nitrosative stress in three major components of glucose metabolism and oxidation.
Glycolysis
Glycolysis is the sequence of ten reactions that catalyze the conversion of the 6-carbon glucose molecule to the 3-carbon pyruvate, with the net reaction resulting in the co-production of two molecules of ATP and two molecules of NADH. The individual reactions and relevant enzymes are depicted in Figure 2, along with a brief summary of known RNS-dependent modifications.
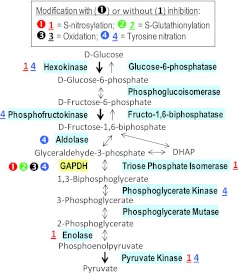
FIG. 2.
Reactive nitrogen species (RNS) modify several enzymes in the glycolytic pathway. This diagram shows the metabolism of carbohydrates in glycolysis and summarizes existing knowledge on RNS-driven modifications in glycolytic proteins. Relevant enzymes are highlighted. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is the most sensitive protein. Modifications that have been linked to the inhibition of enzymatic activities are indicated by number within a circle. When reported chemical alterations have not been linked to changes in enzymatic activities, they are represented by an underlined number. See text for details and references. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars).
There is extensive literature covering nitrosative modifications of GAPDH, the enzyme that catalyzes the conversion of glyceraldehyde-3-phosphate to 1,3-biphosphoglycerate with the production of NADH. In cultured cells, exposure to authentic NO, nitric oxide donors, or other RNS was found to inhibit GAPDH activity (75, 218, 266). The mechanisms of inhibition somewhat differ depending on the type of RNS used in experimental settings. Nitrosothiols reversibly inhibit GAPDH via S-nitrosylation (180, 185, 266) and S-glutathionylation (183). Alternatively, they irreversibly block enzymatic activity via covalent attachment of NAD+ (204). In contrast to many other cytosolic proteins, the S-nitrosylation of GAPDH was found to be resistant to the addition of reducing agents, possibly due to the limited accessibility of the relevant cysteine residues and the specific amino-acid environment (205). In addition to the impact on enzymatic activity, S-nitrosylation of GAPDH triggers translocation of this enzyme, in complex with the partner protein Siah1, to cell nucleus and activation of apoptosis (112, 114). These latter observations led to the suggestion that GAPDH may serve as an intracellular sensor of nitrosative stress (113). Peroxynitrite oxidizes GAPDH thiols situated in the active site of the enzyme, particularly Cys149 or Cys150 (species-specific), and strongly inhibits GAPDH activity (184, 245). Additionally, there are several reports of peroxynitrite-dependent tyrosine nitration of GAPDH in yeast and mammalian cells (39, 40), as well as findings of endogenous GAPDH nitration in AD, sepsis, and diabetic retinopathy (7, 134, 251). Presently, our understanding of the effects of tyrosine nitration on GAPDH activity is limited. However, one recent in vitro study demonstrated that the nitration of Tyr311 and Tyr317 inactivated purified rabbit GAPDH via a mechanism that involves the inhibition of NAD+ binding; to exclude effects on thiol groups, these experiments were done with the reversible protection of thiols (206).
The effects of RNS on other glycolytic enzymes, besides GAPDH, are only scarcely studied. In our recent work on rat brain synaptosomes, we found that preincubation with S-nitrosating agents caused long-term suppression of glycolysis, which was persistent even when GAPDH activity was restored by thiol-reducing agents (222). These findings strongly suggest that, in addition to GAPDH, the modification of other glycolytic enzymes may also be of pathological significance. Hexokinase—the first enzyme of the glycolytic pathway that traps glucose inside the cell via its phosphorylation—was identified among endogenously S-nitrosylated brain proteins and in a model experiment using S-nitrosating agents in vitro (127). This enzyme may also be nitrated at several tyrosine residues (181). Neither of these two modifications is known to affect protein activity, although purified yeast hexokinase A was shown to be partially inhibited by high concentrations of GSNO (181). Phosphofructokinase, which catalyzes another ATP-dependent and is, therefore, an irreversible step of glycolysis, is nitrated at tyrosines, with no known consequences on enzymatic activity (146). One step further, aldolase can be also nitrated, and is inhibited by up to ∼70% in cultured fibroblasts exposed to high concentrations of peroxynitrite (146). In addition, trioso phosphate isomerase and the last two enzymes of the glycolytic pathway, enolase and pyruvate kinase, are known targets for S-nitrosylation (92, 205, 239). Interestingly, the neuron-specific isoform of enolase is one of the most prominent S-nitrosylated proteins in a murine model of MS (20). It should be noted, however, that in our experiments, we were unable to find any functional effects of S-nitrosylating agents on enolase activity, even if such agents strongly inhibited GAPDH (222). Phosphoglycerate kinase and pyruvate kinase are subject to endogenous tyrosine nitration in adipocytes exposed to high glucose levels and in heart tissue after ischemia-reperfusion injury (147, 164).
In summary, with the exception of GAPDH, very little is known about the functional significance of nitrosative modifications in glycolysis. While analyzing data obtained using isolated enzymes or tissue homogenates, it is very important to keep in mind that cells usually utilize ∼1%–10% of their maximal capacity of individual glycolytic enzymes to process glucose in vivo (149). Since activity of the majority of glycolytic enzymes (including GAPDH) is not rate limiting, nitrosative stress should inhibit them by at least 70%, but perhaps as much as 90+%, in order to exert a strong net impact on glycolysis in situ and in vivo.
The tricarboxylic acid cycle
The tricarboxylic acid (TCA) cycle involves eight biochemical reactions, the main purpose of which is to supply reducing equivalents to the electron transport chain. These reactions oxidize the six-carbon molecule citrate to the four-carbon oxaloacetate and then regenerate oxaloacetate back to citrate, with the net production of three NADH molecules and one FADH2. NADH molecules are funneled to complex I, while FADH2 supplies electrons within complex II of the electron transport chain. Oxaloacetate is regenerated to citrate using the acetyl group of acetyl-CoA, which is produced from glycolytic pyruvate by the pyruvate dehydrogenase complex. These reactions and the key points for nitrosative modifications are schematically depicted in Figure 3.
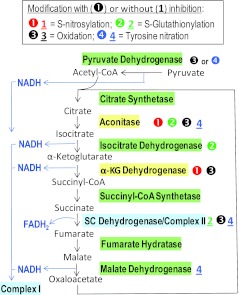
FIG. 3.
Known effects of RNS on the enzymes of the Krebs cycle. This figure summarizes mitochondrial reactions and enzymes constituting the cycle of tricarboxylic acids (TCA). Pyruvate dehydrogenase is also included as the major source of acetyl-CoA. The points of production of the reducing equivalents, NADH and FADH2, are shown as well. Relevant enzymes and the RNS-driven modifications are depicted and coded as in the preceding figure. Aconitase and α-ketoglutarate dehydrogenase are the enzymes that are most sensitive to nitrosative modifications. Succinate dehydrogenase is represented by a separate color to signify its dual role in both the TCA cycle and the electron transport chain. See text for details and references. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Pyruvate dehydrogenase is a multimeric macromolecular complex, which serves as a linking hub between glycolysis and the TCA cycle. This complex catalyzes the oxidative decarboxylation of pyruvate and adds the acetyl group to coenzyme A (CoA) with the concomitant production of NADH. Acetyl-CoA is supplied to the TCA cycle to regenerate citrate from oxaloacetate. Oxidants and peroxynitrite strongly reduce the activity of pyruvate dehydrogenase (217, 252). Brain hypoxia triggers tyrosine nitration in three of the enzymatic subunits of the pyruvate dehydrogenase complex, much similar to purified complex treated with the peroxynitrite donor SIN-1 (217). It is not firmly established whether tyrosine nitration is the cause for loss of the enzymatic activity or just an epiphenomenon. Oxidation of sulfhydryl groups may be the primary mechanism for the inactivation of pyruvate dehydrogenase. It has been found that superoxide and the hydroxyl radical potently reduce the activity of the enzyme (252). The effects of superoxide were completely reversed by addition of thiol-reducing agents, while the effects of the hydroxyl radical-generating agents were only partially reversible (252).
Among the enzymes of the TCA cycle, mitochondrial aconitase, which catalyzes the reversible isomerization of citrate to isocitrate with the formation of cis-aconitate as an intermediate, is not only heavily regulated by superoxide but is also known to be nitrosated (93, 259). Authentic NO is a very weak inhibitor of aconitase; however, peroxynitrite potently but reversibly inhibits this enzyme via disruption of the [4Fe-4S]2+ prosthetic group and removal of one of the Fe atoms from the Fe-S cluster (47). Peroxynitrite also nitrates at least two tyrosine residues, Tyr151 and Tyr472, but this modification has no apparent effect on the aconitase activity (109, 164, 259). Interestingly, GSNO inhibits aconitase activity irreversibly, via a poorly defined mechanism that may involve S-glutathionylation (259). The effects of GSNO are in contrast with those of other S-nitrosylating agents that produce a modest and reversible inhibition of the enzyme (59). Downstream of aconitase, isocitrate dehydrogenase, the NADH generating enzyme that produces α-ketoglutarate, is potently and reversibly inhibited by S-gluthathionylation (136). However, high concentrations of oxidized glutathione are necessary to achieve a substantial inhibition of this enzyme. One step further, α-ketoglutarate dehydrogenase (KGDH) complex, one of the rate limiting steps of the TCA cycle that produces succinyl-CoA and NADH, is weakly and reversibly inhibited by S-nitrosylation (59). Peroxynitrite at low micromolar concentrations potently suppresses the activity of the purified KGDH, and at higher concentrations, suppresses this enzyme in cellular assays. Inhibition occurs in a manner that is partially reversible by thiol-reducing agents, suggesting that peroxynitrite targets one of the critical thiol groups (240). In addition, malate dehydrogenase, the final enzyme of the TCA cycle that converts malate to oxaloacetate with formation of the third NADH molecule, was reported to be nitrated at tyrosine residues under conditions of hypoxia-reoxygenation (145, 164). The functional impact of tyrosine nitration in malate dehydrogenase has not been established. As discussed in the next section, succinate oxidoreductase, the enzyme that represents a part of both the TCA cycle and the electron transport chain (complex II), is also modified by RNS, with oxidation playing a major role in the modulation of the enzymatic activity.
In summary, five of the eight enzymes in the TCA cycle are modified by RNS. It appears that the major functional impact is exerted at the level of aconitase and KGDH. Aconitase activity is suppressed via disruption of the Fe-S clusters by peroxynitrite or GSNO, with the latter effect being irreversible. KGDH is potently inhibited by peroxynitrite via the oxidation of critical thiol groups. Furthermore, activity of succinate dehydrogenase/complex II is potently suppressed via thiol oxidation, thus affecting both the TCA cycle and the rate of mitochondrial respiration.
The mitochondrial electron transport chain
Mitochondrial respiration is mediated by several multi-protein complexes that utilize the energy of reducing equivalents derived from the TCA cycle for the generation of a proton gradient across the inner mitochondrial membrane. A simplified scheme of the functional organization of the electron transport complexes is depicted in Figure 4. Electrons are supplied to the respiratory chain by NADH and succinate/FADH2 via two alternative entrance points: complex I (NADH-ubiquinone oxidoreductase) and complex II (succinate-ubiquinone oxidoreductase). Electrons from both complexes are transferred to complex III (ubiquinone-cytochrome c oxidoreductase) using ubiquinone as a carrier, and then shuttled by small soluble protein cytochrome c to complex IV (cytochrome c oxidase). Complex IV transfers electrons to the final acceptor, oxygen, in the reaction that forms water. Finally, the energy stored in the proton gradient is utilized by the ATP synthetase, which is frequently called complex V, although it does not participate in electron transfer. As mentioned earlier, cytochrome c oxidase is the high-affinity target for free NO, which competes for the oxygen-binding center and reversibly inhibits enzymatic activity of the complex IV. We discuss next and summarize in Figure 4 our understanding of how various RNS may inhibit or modulate the respiratory chain at several alternative sites via S-nitrosylation, S-glutathionylation, oxidation, and, possibly, nitration of protein amino-acid residues.
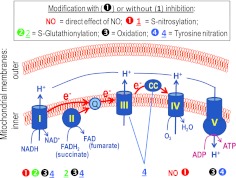
FIG. 4.
NO and RNS modify and inhibit several components of the mitochondrial election transport chain. This diagram depicts major enzymatic complexes (I–IV) of the mitochondrial respiratory chain. Complex I and complex II, which utilize the TCA-cycle derived NADH and FADH2 as electron donors, respectively, represent the alternative electron entrance points. Electrons are transferred to complex III by ubiquinone (Q), and from complex III to complex IV by cytochrome c (CC). The electrochemical gradient of protons, which is produced during the electron transfer, is used for ATP synthesis by the ATP synthetase (complex V). The RNS-driven modifications are depicted and coded as in the preceding figures. Complex IV is also inhibited by NO directly, via reversible NO binding to the Fe2+/heme center. See text for details and references. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars).
In mitochondria, complexes I, II, IV, and V are strongly inhibited by various RNS, particularly by peroxynitrite (reviewed in 37). Extensive experimental evidence, obtained both in vitro and in vivo, indicates that components of complex I are very sensitive to oxidative and nitrosative modifications. This concept is particularly well accepted for PD. In dopaminergic neurons and model cells, oxidative and nitrosative stress causes preferential inhibition of complex I activity and extensive accumulation of S-nitrosothiols in mitochondrial proteins, with many modifications occurring in complex I (55, 67, 71). A recent proteomics study in an animal model of PD identified 34 distinct cysteine residues in the components of complex I (26% of total thiol groups), which are modified during disease progression in vivo (68). S-nitrosating compounds, such as S-nitroso-l-cysteine, strongly inhibit mitochondrial O2 consumption and ATP production in isolated brain nerve endings (synaptosomes) and in neuronal cells (34, 81, 222). These effects are likely mediated in large part not only via direct S-nitrosylation (26, 41, 64) or S-glutathionylation (51, 253), but also perhaps via tyrosine nitration (208) of proteins in complex I. Interestingly, certain thiol modifications do not reduce complex I activity per se but instead decouple it from other components of the electron transport chain and cause the enhanced generation of ROS in mitochondria (for discussion see ref. 258).
Peroxynitrite inhibits complexes I, II, and V, and weakly suppresses the activity of complex IV via the oxidation of protein thiols and/or disruption of sulfur-iron clusters (23, 46, 166, 214, 281). The inhibitory actions of peroxynitrite and nitroxyl on complex II may be mediated by the oxidation of thiol groups, including one in the active site of succinate dehydrogenase, and the formation of intramolecular disulfide bonds (241, 281). The activity of complex III may be inhibited indirectly (for discussion see ref. 37). Interestingly, complex II can be S-glutathionylated in vivo and in vitro, particularly in ischemia and reperfusion, but such a modification produces an opposite effect as compared with that in complex I: It has been reported as protecting against the oxidative actions of peroxynitrite (50, 53). Cytochrome c oxidase is persistently inhibited by S-nitrosylation and/or oxidation of Cys196 and Cys200 in the subunit 2 of the complex located in the catalytic Cu-containing center (280). This latter effect requires prolonged exposure to high levels of NO or S-nitrosating agents, in contrast to potent inhibition caused by high-affinity NO binding to the heme/Fe2+ center. A number of studies found tyrosine nitration in various components of the electron transport chain, both in vitro and in vivo under pathological conditions, including complexes I, II, III, and V, as well as cytochrome c (2, 50, 164, 208). Several studies demonstrated a correlation between tyrosine nitration at Tyr345 and Tyr368 in the β subunit of complex V and the loss of ATP synthetase activity (88, 117). To the best of our knowledge, no reliable link was made between tyrosine nitration and the functional inhibition of complexes I, II, and III.
In summary, NO and nitrosative stress were found to strongly reduce the functional activity of the respiratory chain and ATP synthesis. Fe2+/heme-containing centers of the complex IV represent the most sensitive target for the direct actions of NO. However, the inhibition of complex IV by NO is readily reversible. Other RNS act with much lower affinity, but their effects may be of critical pathological significance, because relevant modifications of proteins are irreversible or poorly reversible. Complex I is rate limiting for the overall activity of the electron transport chain, and this complex appears to be particularly vulnerable to nitrosative modifications. To illustrate this point, complexes I, III, and IV, should be inhibited by at least 25%, 80%, and 70%, respectively, in order to see the inhibition of ATP synthesis in isolated brain synaptosomes (69).
An additional point to be considered is that nitrosative inhibition of mitochondrial electron transport and oxidative phosphorylation is not an exclusively negative event. S-nitrosylation of thiols in complex I by the systemic application of nitrite, or by using more targeted approaches, is an important mechanism that contributes to preconditioning protection against the heart ischemia-reperfusion injury (see for example refs. 212, 242). The protective effects are explained by the reduction of pathological ROS production as a result of S-nitrosylation in complex I.
Neuronal Processes and Synaptic Communication May Represent a Distinct Target for Nitrosative Stress
In order to discuss the causal link between nitrosative stress and the loss of synaptic function, we need to briefly introduce two important aspects of synaptic physiology: (i) axonal and dendritic delivery of macromolecules and organelles, and (ii) distinct features of local metabolism in the dendritic and axonal projections. Neuronal cells are unique in terms of their complexity and high specialization of their processes. A prototypical neuronal body represents <0.5% of the total cell volume, but essentially all protein synthesis and organelle assembly happen in this restricted compartment (193). The newly produced materials have to be sorted and timely and efficiently delivered to functionally distinct axonal and dendritic domains in order to sustain local signaling and metabolism. Such delivery is mediated by several transport processes, which are depicted in a simplified manner in Figure 5 and discussed next.
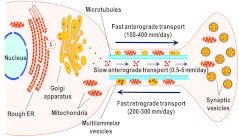
FIG. 5.
Schematic illustration of transport processes, which translocate organelles, membrane-delimited materials, and cytosolic proteins in axons and dendrites. Mitochondria and vesicular structures are moved from the cell body to the distal parts of axon and dendrites (the latter are not shown for simplicity) by the fast anterograde transport. Cytosolic proteins are moved at much slower rates by the slow anterograde transport. Mitochondria and membrane-delimited materials, but not cytosolic proteins, are returned to the cell body for recycling by the fast retrograde transport. Disruptions in local metabolic processes and the transport of newly synthesized metabolic enzymes and organelles may contribute to the selective vulnerability of neuronal projections to nitrosative stress. See text for details and references. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars).
The continuous delivery of metabolic enzymes and organelles to axons and dendrites has two important implications for the local effects of nitrosative stress. (i) If axonal and dendritic transport processes are strongly impaired, then this should result in the inability of neurons to replace mitochondria and glycolytic enzymes in the remote neuronal endings, and lead to gradual failure of dendritic but particularly axonal functions and integrity. (ii) If the transport processes are largely spared, but local metabolic enzymes and mitochondria are damaged, then delivery of the “replacement parts” should take a longer time, in proportion to the length of the neuronal projections. Critically, cytosolic proteins, including glycolytic enzymes, are delivered at rates that are ≥100-times slower than mitochondria and other organelles (for references see below). The latter difference in the delivery rates can be compared with the courier delivery on foot versus the delivery by a high-speed train (199). If this analogy is applied to the projection neurons with long axonal processes, then the “train delivery” of mitochondria to the distal segments of the axon could take hours, while the “pedestrian” delivery of cytosolic proteins could take days. This may explain why neuronal processes may selectively lose their glycolytic capacity, when subjected to nitrosative stress. In contrast, in the neuronal cell body, there are vast reserves of glycolytic enzymes, and the most sensitive part of energetic metabolism is represented by mitochondria.
Defects in axonal and dendritic transport
The translocation of cytosolic proteins and organelles to distant compartments of neuronal cells involves several specialized types of transport. Mitochondria and small vesicles, including synaptic vesicles and vesicular structures containing newly synthesized membrane lipids and proteins, are moved toward the distal part by the fast anterograde transport, at the rates of 100–400 mm/day (193). The differences in the rates are thought to be determined by size, that is, “dragging” force, of organelles. This type of transport requires specialized protein motors, kinesins, that move along microtubule scaffolds (119, 120). Some of the transported vesicles may contain secretory proteins, such as neurotransmitter peptides. The fast retrograde transport, which moves mitochondria, larger vesicles, and multivesicular (or multilammelar) bodies in the opposite direction, toward the cell body, has essentially the same rate, ∼200–300 mm/day (193). Vesicles and multilammelar bodies are typically routed to the lysosomal compartment. The fast retrograde transport is also carried out using microtubules as scaffolds, but is driven by a different class of molecular motors belonging to the dynein family (119, 120). Its functional significance is to recycle and repackage used and damaged proteins, membrane components, and mitochondria. However, it is also utilized to deliver to the cell body trophic substances and growth factors, which are locally taken from the extracellular space via endocytosis (193).
In contrast to the membrane-delimited materials and mitochondria, essentially all cytosolic proteins and partially polymerized cytoskeletal proteins are moved toward the distal part of the axon by the slow anterograde transport, which is about two orders of magnitude slower (∼0.5–5 mm/day) than the fast types of transport (193). In this case, the transport is mediated by specialized isoforms of cytosolic dyneins that are associated with the protein complex dynactin (73, 74). Early publications suggested that slow anterograde transport requires microfilament networks as scaffolds; however, more recent studies indicate that microtubules play a critical role (21, 221). Metabolic enzymes, including GAPDH, are delivered to axons and dendrites with the slow anterograde transport in macromolecular complexes with cytoskeletal proteins. Unlike components of the fast anterograde transport, all the molecules, which are associated with the slow transport, are moved in one direction and in order to be “returned” to the cell body, they have to undergo degradation or be incorporated in the multivesicular bodies (193).
Mutations in the components of molecular motors, which drive the two types of anterograde transport, such as in transgenic animals or in naturally occurring human disorders, typically cause “dying back” neuropathies, that is, neurodegeneration that starts in the distal part of the axon and progresses back to the cell body (reviewed in 54, 192). This likely occurs due to local metabolic deficits and inability to maintain structural integrity. Defects in the retrograde transport also lead to the selective loss of neuronal cells, but via different mechanisms. In the latter case, neurodegeneration is probably associated with the aggregation of misfolded proteins and/or failure to deliver endocytosed neurotrophic factors from the distal processes to the neuronal cell body (reviewed in 209). Besides molecular motors, axonal transport may be impaired by distortions in the organization of microtubules and intermediate filaments. As mentioned in one of the previous sections, Alzheimer's pathology involves the formation of neurofibrillary tangles, consisting of aggregates of the hyperphosphorylated microtubule-associated protein tau (18). It has been recently reported that tau aggregates selectively inhibit anterograde transport (133, 156).
Many neurological disorders mentioned in this review manifest both nitrosative stress and defects in axonal retrograde or anterograde transport. A list of relevant diseases includes AD, Huntington's disease, amyotrophic lateral sclerosis, PD, traumatic brain injury, and stroke (54, 192, 209). However, the connection between nitrosative stress and defects in the neuronal long-range transport remains essentially unexplored. This is particularly surprising, because NO was long thought of as serving as a physiological signal regulating axonal growth and cytoskeleton dynamics. Treatment with NO donors causes the collapse of growth cones and axonal retraction in vitro (118, 216). These effects are likely mediated by the nitrosative changes in microtubules and microtubule-associated proteins, such as nitration of α-tubulin and tau (43, 44, 79). Furthermore, α- and β-tubulins are robustly and persistently S-nitrosylated in vitro and in vivo (20, 218). However, the functional significance of S-nitrosylation of cytoskeletal proteins has not been thoroughly tested. In cultured neurons, fast anterograde axonal transport is potently inhibited by the application of NO donors and via the endogenous production of RNS by co-cultured microglial cells (246). Interestingly, the latter effects of RNS on axonal transport are mediated by the activation of c-jun NH2-terminal kinase (246), a known physiological target for NO and RNS (99, 137). Therefore, RNS may cause the dysregulation of axonal transport via the modification of molecular motors and scaffold proteins or pathological changes in intracellular signaling.
Nitrosative modifications of the synaptic communication
Experimental work over the past two or three decades strongly suggests that, in many cases, neurological deficits may be determined by the inhibition of synaptic communication and loss of synapses, rather than by death of neuronal cells. This idea is particularly well accepted in the field of AD (reviewed in 228, 236). Early quantitative morphometric studies identified a profound decrease in the numbers of cortical synapses in the post-mortem samples of AD patients. In the intermediate stages of the disease, 2–5 years after the onset of symptoms, average synapse densities were reduced by >25% in cortical layers II and III, and as much as 36% in layer V of the temporal cortex (70). Such changes could not be explained by a simple loss of neurons, because the same study found that the average numbers of synapses per neuron were decreased by 14%–38% in various parts of the cortex (70). Even in the advanced stages of the disease, loss of synaptic densities was the primary parameter that correlated with mental decline (254).
The phenomenon of pathological disruption of synaptic structure and functions is commonly described by the term “synaptopathy” (35). The failure of synaptic transmission is now thought of as serving as a basis for neurological deficits not only in the AD, but also in a variety of other brain disorders, including Hungtington's disease, schizophrenia, and, surprisingly, in learning disabilities such as autism spectrum disorders (16, 98, 161). Even in the animal models of MS, which are primarily associated with demyelination, loss of functional synapses was found both immediately after the onset of neurological symptoms and in remission (48, 175). Robust changes in the number of synapses are commonly seen in stroke, in the peri-infarct regions, where the neuronal viability is largely preserved (3, 36, 126, 148, 194). Although the loss of synapses in ischemia can be driven by death of local and distant neuronal cells, several studies found that the ischemia- and hypoxia-induced degeneration of axonal projections and dendritic trees can occur independently of the loss of cell bodies (3, 11, 126). Pathological elimination of synapses occurs nearly simultaneously with massive synaptic reorganization and synaptogenesis in neighboring brain areas. These latter processes are critical for the post-ischemic brain plasticity and repair (reviewed in 45, 195).
In addition to the direct physical loss of axonal projections and dendrites, neural pathologies may lead to synaptic deficits without gross disruption of structural integrity. This is well established in stroke. In the peri-infarct regions, where neuronal viability is largely preserved, there is a potent suppression of the excitatory neurotransmission that may last from days to weeks after recovery of blood flow (24, 25, 201). Electrophysiological experiments indicate that such inhibition of synaptic communication is due to failure of the presynaptic neurotransmitter release, while the postsynaptic responses remain largely unaffected (24, 25). The underlying mechanisms for this phenomenon remain poorly understood.
There were several attempts to identify reasons for disruption in synaptic communication in stroke. It was suggested that neurotransmitter release may be inhibited due to the persistent defects in synapsin phosphorylation (25). However, this cannot be the sole cause, because the double-gene knockouts of synapsins I and II do not interrupt synaptic signaling but rather produce modest modulatory effects (219). Another potential mechanism for suppression of the exocytotic neurotransmitter release was identified in non-neuronal cells. S-nitrosylation of Cys21, Cys91, and Cys264 in the N-ethylmaleimide sensitive fusion protein (NSF) potently inhibits NSF function and vesicular release of the von Willebrand factor in endothelial cells (178). NSF is a hexameric ATPase that is necessary for disassembly and re-activation of protein complexes involved in membrane fusion, and, as such, is critical for sustaining membrane fusion processes, including exocytotic neurotransmitter release (173, 230, 270). We previously hypothesized that nitrosative modifications in the NSF may contribute to defects of neurotransmission in stroke (188). However, this hypothesis remains to be properly tested in the ischemic brain. Another possible mechanism for the failure of neurotransmitter release is metabolic inhibition, which may readily suppress the ATP-dependent exocytosis, as well as the uptake of neurotransmitters into the synaptic vesicles. We and others found that NO and RNS donors can strongly depolarize isolated presynaptic nerve endings (synaptosomes) and cause suppression of ATP production and vesicular neurotransmitter release (81, 190, 200, 222, 237). The acute effects of NO are determined by the reversible inhibition of cytochrome c oxidase (complex IV). However, the long-term effects of NO and RNS, which are seen long after washout of the extracellular RNS, are mediated by nitrosative modifications in numerous metabolic enzymes.
The inhibition of synaptic metabolism by NO and RNS strongly correlates with reductions in the levels of intracellular free thiols, and S-nitrosylation of presynaptic proteins (200, 222). Both mitochondrial respiration and glycolytic processes are strongly affected, based on the direct assays of metabolic reactions and measurements of plasmalemmal and mitochondrial membrane potential (81, 190, 222). As evident from the present literature analysis (see preceding sections), multiple glycolytic and mitochondrial enzymes can be modified and inhibited by RNS, but a few of them—including GAPDH, aconitase, and the respiratory complex I—likely play pivotal roles. Interestingly, the inhibition of synaptosomal ATP production by RNS can be partially rescued by supplementation with the end product of glycolysis, pyruvate (222). This is in contrast to cultured neuronal cells, in which metabolic failure is largely determined by mitochondrial defects, and pyruvate addition does not allow for additional protection (34). We speculate that such a difference can be explained by limited glycolytic capacity in nerve endings, but not in neuronal cell bodies. GAPDH appears to be the most sensitive glycolytic enzyme and can be inhibited by multiple nitrosative modifications (see refs. in the preceding sections). However, the inhibition of GAPDH in synaptosomes can be reversed by the thiol-reducing reagents, while glycolytic ATP production cannot be recovered by the same treatments (222). These findings point to additional critical modifications in glycolysis, which are yet to be identified.
In summary, although neuronal cells as a whole are highly sensitive to nitrosative stress, axonal and dendritic signaling may be at particular risk. Metabolism in axonal and dendritic compartments is highly dependent on the timely delivery and turnover of mitochondria and cytosolic enzymes. Due to the slow turnover rates and limited glycolytic capacity, even modest inhibition of glycolysis and mitochondrial respiration in these remote compartments can disproportionally affect electrical signaling and neurotransmitter release. Since glycolytic enzymes are delivered at rates that are ∼100 times slower than mitochondria, this creates an additional pressure on sustaining glycolysis under pathological conditions. The inhibition of the axonal transport machinery can further exacerbate the effects of nitrosative stress.
Conclusions and Perspectives
NO signaling is crucial for normal physiological functions in the CNS, but goes awry in many brain pathologies. NO itself possesses limited toxicity but may reversibly suppress mitochondrial respiration and ATP synthesis if produced in excess. The majority of the pathological consequences of nitrosative stress are mediated through the reaction of NO with metals or the intermediate formation of RNS, which impart many types of modifications in proteins and other biologically active molecules. As such, the S-nitrosylation, oxidation, and S-glutathionylation of critical cysteine residues can dramatically impact enzymatic activities and cellular functions. In vivo, multiple nitrosative hits in proteins may occur simultaneously, with outcomes that depend on the targeted proteins and the position of altered amino-acid residues. In spite of the substantial progress in the field, a remaining challenge is to discriminate which types of modifications or their combinations play a critical role in brain pathologies.
The objective of the present review was to analyze the pathological impact of NO-dependent modifications on proteins associated with energetic metabolism: glycolysis, the TCA cycle, and mitochondrial respiration. We propose that metabolic deficits play a major role in numerous CNS disorders. Although rarely addressed, metabolism and synaptic signaling in distal neuronal processes may be particularly vulnerable to the actions of RNS. Furthermore, metabolism in the axonal compartment may represent a separate target for therapeutic interventions. Unlike bodies of neuronal and non-neuronal cells, distal neuronal processes likely have very limited “spare” glycolytic capacity, and under conditions associated with nitrosative stress, lose their ability to sustain metabolic processes. Supplementation with the end product of glycolysis, pyruvate, or its more stable and clinically used congener, ethyl-pyruvate, may be used to at least partially rescue synaptic transmission and prevent the pathological loss of neuronal projections. Pyruvate and ethyl-pyruvate have been already tested in animal stroke models. Both compounds potently protect the animal brain against ischemic injury (157, 276, 277). However, the major focus of these latter studies was on the preservation of neuronal viability. It remains to be explored whether the structure of axonal and dendritic processes as well as synaptic function can be selectively preserved in stroke and other neurological disorders using similar pharmacological approaches.
Abbreviations Used
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- CNS
central nervous system
- Drp1
dynamin-related protein 1
- EAE
experimental allergic encephalomyelitis
- eNOS
endothelial NOS
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GSH
glutathione
- GSNO
S-nitrosoglutathione
- HNO2
nitrous acid
- iNOS
inducible NOS
- KGDH
α-ketoglutarate dehydrogenase
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS
multiple sclerosis
- N2O3
dinitrogen trioxide
- NMDA
N-methyl-d-aspartate
- NO
nitric oxide
- NO2
nitrogen dioxide
- NOS
nitric oxide synthase
- nNOS
neuronal NOS
- NSF
N-ethylmaleimide fusion protein
- PD
Parkinson's disease
- PDZ domain
postsynaptic density protein 95, Drosophila disc large tumor suppressor 1, zonula occludens-1 protein
- PFK-16
phosphofructo kinase-1
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RSNO
nitrosothiols
- TCA
tricarboxylic acids
Acknowledgments
The authors are grateful to Drs. Paul J. Feustel, Richard W. Keller Jr., and Nicole H. Bowens for a critical reading of the article. This work was supported in part by NIH grants NS052516, NS061953 to A.A.M., CA089366 to D.J., and AHA Grant-In-Aid 3860027 to D.J.
References
- 1.Abe K. Pan LH. Watanabe M. Kato T. Itoyama Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci Lett. 1995;199:152–154. doi: 10.1016/0304-3940(95)12039-7. [DOI] [PubMed] [Google Scholar]
- 2.Abriata LA. Cassina A. Tortora V. Marin M. Souza JM. Castro L. Vila AJ. Radi R. Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption. Nuclear magnetic resonance and optical spectroscopy studies. J Biol Chem. 2009;284:17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akulinin VA. Stepanov SS. Semchenko VV. Belichenko PV. Dendritic changes of the pyramidal neurons in layer V of sensory-motor cortex of the rat brain during the postresuscitation period. Resuscitation. 1997;35:157–164. doi: 10.1016/s0300-9572(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 4.Almeida A. Moncada S. Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 5.Arriagada PV. Growdon JH. Hedley-Whyte ET. Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 6.Aulak KS. Koeck T. Crabb JW. Stuehr DJ. Dynamics of protein nitration in cells and mitochondria. Am J Physiol Heart Circ Physiol. 2004;286:H30–H38. doi: 10.1152/ajpheart.00743.2003. [DOI] [PubMed] [Google Scholar]
- 7.Aulak KS. Miyagi M. Yan L. West KA. Massillon D. Crabb JW. Stuehr DJ. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Natl Acad Sci U S A. 2001;98:12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagasra O. Michaels FH. Zheng YM. Bobroski LE. Spitsin SV. Fu ZF. Tawadros R. Koprowski H. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92:12041–12045. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bal-Price A. Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balazy M. Kaminski PM. Mao K. Tan J. Wolin MS. S-Nitroglutathione, a product of the reaction between peroxynitrite and glutathione that generates nitric oxide. J Biol Chem. 1998;273:32009–32015. doi: 10.1074/jbc.273.48.32009. [DOI] [PubMed] [Google Scholar]
- 11.Baxter B. Gillingwater TH. Parson SH. Rapid loss of motor nerve terminals following hypoxia-reperfusion injury occurs via mechanisms distinct from classic Wallerian degeneration. J Anat. 2008;212:827–835. doi: 10.1111/j.1469-7580.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckman JS. Estevez AG. Crow JP. Barbeito L. Superoxide dismutase and the death of motoneurons in ALS. Trends Neurosci. 2001;24:S15–S20. doi: 10.1016/s0166-2236(00)01981-0. [DOI] [PubMed] [Google Scholar]
- 13.Behl C. Davis JB. Lesley R. Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 14.Benhar M. Forrester MT. Hess DT. Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benhar M. Forrester MT. Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 16.Betancur C. Sakurai T. Buxbaum JD. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–412. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat NR. Zhang P. Bhat AN. Cytokine induction of inducible nitric oxide synthase in an oligodendrocyte cell line: role of p38 mitogen-activated protein kinase activation. J Neurochem. 1999;72:472–478. doi: 10.1046/j.1471-4159.1999.0720472.x. [DOI] [PubMed] [Google Scholar]
- 18.Binder LI. Guillozet-Bongaarts AL. Garcia-Sierra F. Berry RW. Tau, tangles, and Alzheimer's disease. Biochim Biophys Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Bizzozero OA. DeJesus G. Bixler HA. Pastuszyn A. Evidence of nitrosative damage in the brain white matter of patients with multiple sclerosis. Neurochem Res. 2005;30:139–149. doi: 10.1007/s11064-004-9695-2. [DOI] [PubMed] [Google Scholar]
- 20.Bizzozero OA. Zheng J. Identification of major S-nitrosylated proteins in murine experimental autoimmune encephalomyelitis. J Neurosci Res. 2009;87:2881–2889. doi: 10.1002/jnr.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black MM. Lasek RJ. Slow components of axonal transport: two cytoskeletal networks. J Cell Biol. 1980;86:616–623. doi: 10.1083/jcb.86.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolanos JP. Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim Biophys Acta. 1999;1411:415–436. doi: 10.1016/s0005-2728(99)00030-4. [DOI] [PubMed] [Google Scholar]
- 23.Bolanos JP. Heales SJ. Land JM. Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- 24.Bolay H. Dalkara T. Mechanisms of motor dysfunction after transient MCA occlusion: persistent transmission failure in cortical synapses is a major determinant. Stroke. 1998;29:1988–1993. doi: 10.1161/01.str.29.9.1988. [DOI] [PubMed] [Google Scholar]
- 25.Bolay H. Gursoy-Ozdemir Y. Sara Y. Onur R. Can A. Dalkara T. Persistent defect in transmitter release and synapsin phosphorylation in cerebral cortex after transient moderate ischemic injury. Stroke. 2002;33:1369–1375. doi: 10.1161/01.str.0000013708.54623.de. [DOI] [PubMed] [Google Scholar]
- 26.Borutaite V. Budriunaite A. Brown GC. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim Biophys Acta. 2000;1459:405–412. doi: 10.1016/s0005-2728(00)00178-x. [DOI] [PubMed] [Google Scholar]
- 27.Bosworth CA. Toledo JC., Jr. Zmijewski JW. Li Q. Lancaster JR., Jr. Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc Natl Acad Sci U S A. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boullerne AI. Rodriguez JJ. Touil T. Brochet B. Schmidt S. Abrous ND. Le MM. Pua JR. Jensen MA. Mayo W. Arnason BG. Petry KG. Anti-S-nitrosocysteine antibodies are a predictive marker for demyelination in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neurosci. 2002;22:123–132. doi: 10.1523/JNEUROSCI.22-01-00123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bredt DS. Glatt CE. Hwang PM. Fotuhi M. Dawson TM. Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- 30.Bredt DS. Hwang PM. Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 31.Bredt DS. Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bredt DS. Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bredt DS. Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 34.Brorson JR. Schumacker PT. Zhang H. Nitric oxide acutely inhibits neuronal energy production. J Neurosci. 1999;19:147–158. doi: 10.1523/JNEUROSCI.19-01-00147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brose N. O'Connor V. Skehel P. Synaptopathy: dysfunction of synaptic function? Biochem Soc Trans. 2010;38:443–444. doi: 10.1042/BST0380443. [DOI] [PubMed] [Google Scholar]
- 36.Brown CE. Wong C. Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39:1286–1291. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- 37.Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 38.Brown GC. Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 39.Buchczyk DP. Briviba K. Hartl FU. Sies H. Responses to peroxynitrite in yeast: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a sensitive intracellular target for nitration and enhancement of chaperone expression and ubiquitination. Biol Chem. 2000;381:121–126. doi: 10.1515/BC.2000.017. [DOI] [PubMed] [Google Scholar]
- 40.Buchczyk DP. Grune T. Sies H. Klotz LO. Modifications of glyceraldehyde-3-phosphate dehydrogenase induced by increasing concentrations of peroxynitrite: early recognition by 20S proteasome. Biol Chem. 2003;384:237–241. doi: 10.1515/BC.2003.026. [DOI] [PubMed] [Google Scholar]
- 41.Burwell LS. Nadtochiy SM. Tompkins AJ. Young S. Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calabrese V. Cornelius C. Rizzarelli E. Owen JB. Dinkova-Kostova AT. Butterfield DA. Nitric oxide in cell survival: a Janus molecule. Antioxid Redox Signal. 2009;11:2717–2739. doi: 10.1089/ars.2009.2721. [DOI] [PubMed] [Google Scholar]
- 43.Cappelletti G. Maggioni MG. Ronchi C. Maci R. Tedeschi G. Protein tyrosine nitration is associated with cold- and drug-resistant microtubules in neuronal-like PC12 cells. Neurosci Lett. 2006;401:159–164. doi: 10.1016/j.neulet.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Cappelletti G. Tedeschi G. Maggioni MG. Negri A. Nonnis S. Maci R. The nitration of tau protein in neurone-like PC12 cells. FEBS Lett. 2004;562:35–39. doi: 10.1016/S0014-5793(04)00173-5. [DOI] [PubMed] [Google Scholar]
- 45.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 46.Cassina A. Radi R. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- 47.Castro L. Rodriguez M. Radi R. Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem. 1994;269:29409–29415. [PubMed] [Google Scholar]
- 48.Centonze D. Muzio L. Rossi S. Cavasinni F. De C V. Bergami A. Musella A. D'Amelio M. Cavallucci V. Martorana A. Bergamaschi A. Cencioni MT. Diamantini A. Butti E. Comi G. Bernardi G. Cecconi F. Battistini L. Furlan R. Martino G. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29:3442–3452. doi: 10.1523/JNEUROSCI.5804-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chao CC. Hu S. Molitor TW. Shaskan EG. Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 50.Chen CL. Chen J. Rawale S. Varadharaj S. Kaumaya PP. Zweier JL. Chen YR. Protein tyrosine nitration of the flavin subunit is associated with oxidative modification of mitochondrial complex II in the post-ischemic myocardium. J Biol Chem. 2008;283:27991–28003. doi: 10.1074/jbc.M802691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CL. Zhang L. Yeh A. Chen CA. Green-Church KB. Zweier JL. Chen YR. Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry. 2007;46:5754–5765. doi: 10.1021/bi602580c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y. Vartiainen NE. Ying W. Chan PH. Koistinaho J. Swanson RA. Astrocytes protect neurons from nitric oxide toxicity by a glutathione- dependent mechanism. J Neurochem. 2001;77:1601–1610. doi: 10.1046/j.1471-4159.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- 53.Chen YR. Chen CL. Pfeiffer DR. Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- 54.Chevalier-Larsen E. Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim Biophys Acta. 2006;1762:1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Chinta SJ. Andersen JK. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: implications for Parkinson's disease. Free Radic Biol Med. 2006;41:1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Cho DH. Nakamura T. Fang J. Cieplak P. Godzik A. Gu Z. Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 58.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 59.Chouchani ET. Hurd TR. Nadtochiy SM. Brookes PS. Fearnley IM. Lilley KS. Smith RA. Murphy MP. Identification of S-nitrosated mitochondrial proteins by S-nitrosothiol difference in gel electrophoresis (SNO-DIGE): implications for the regulation of mitochondrial function by reversible S-nitrosation. Biochem J. 2010;430:49–59. doi: 10.1042/BJ20100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christopherson KS. Hillier BJ. Lim WA. Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 61.Chung KK. Dawson TM. Dawson VL. Nitric oxide, S-nitrosylation and neurodegeneration. Cell Mol Biol (Noisy -le-grand) 2005;51:247–254. [PubMed] [Google Scholar]
- 62.Chung KK. Thomas B. Li X. Pletnikova O. Troncoso JC. Marsh L. Dawson VL. Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 63.Cleeter MW. Cooper JM. rley-Usmar VM. Moncada S. Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 64.Clementi E. Brown GC. Feelisch M. Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cosby K. Partovi KS. Crawford JH. Patel RP. Reiter CD. Martyr S. Yang BK. Waclawiw MA. Zalos G. Xu X. Huang KT. Shields H. Kim-Shapiro DB. Schechter AN. Cannon RO., III Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 66.Cross AH. Misko TP. Lin RF. Hickey WF. Trotter JL. Tilton RG. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994;93:2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danielson SR. Andersen JK. Oxidative and nitrative protein modifications in Parkinson's disease. Free Radic Biol Med. 2008;44:1787–1794. doi: 10.1016/j.freeradbiomed.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danielson SR. Held JM. Oo M. Riley R. Gibson BW. Andersen JK. Quantitative mapping of reversible mitochondrial Complex I cysteine oxidation in a Parkinson disease mouse model. J Biol Chem. 2011;286:7601–7608. doi: 10.1074/jbc.M110.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davey GP. Peuchen S. Clark JB. Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem. 1998;273:12753–12757. doi: 10.1074/jbc.273.21.12753. [DOI] [PubMed] [Google Scholar]
- 70.Davies CA. Mann DM. Sumpter PQ. Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 71.Dawson TM. Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 72.Dawson VL. Dawson TM. Bartley DA. Uhl GR. Snyder SH. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dillman JF., III Dabney LP. Karki S. Paschal BM. Holzbaur EL. Pfister KK. Functional analysis of dynactin and cytoplasmic dynein in slow axonal transport. J Neurosci. 1996;16:6742–6752. doi: 10.1523/JNEUROSCI.16-21-06742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dillman JF., III Dabney LP. Pfister KK. Cytoplasmic dynein is associated with slow axonal transport. Proc Natl Acad Sci U S A. 1996;93:141–144. doi: 10.1073/pnas.93.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dimmeler S. Lottspeich F. Brune B. Nitric oxide causes ADP-ribosylation and inhibition of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1992;267:16771–16774. [PubMed] [Google Scholar]
- 76.Dirnagl U. Iadecola C. Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 77.Doulias PT. Greene JL. Greco TM. Tenopoulou M. Seeholzer SH. Dunbrack RL. Ischiropoulos H. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc Natl Acad Sci U S A. 2010;107:16958–16963. doi: 10.1073/pnas.1008036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dringen R. Pfeiffer B. Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eiserich JP. Estevez AG. Bamberg TV. Ye YZ. Chumley PH. Beckman JS. Freeman BA. Microtubule dysfunction by posttranslational nitrotyrosination of alpha-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc Natl Acad Sci U S A. 1999;96:6365–6370. doi: 10.1073/pnas.96.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eliasson MJ. Huang Z. Ferrante RJ. Sasamata M. Molliver ME. Snyder SH. Moskowitz MA. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19:5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Erecinska M. Nelson D. Vanderkooi JM. Effects of NO-generating compounds on synaptosomal energy metabolism. J Neurochem. 1995;65:2699–2705. doi: 10.1046/j.1471-4159.1995.65062699.x. [DOI] [PubMed] [Google Scholar]
- 82.Eu JP. Liu L. Zeng M. Stamler JS. An apoptotic model for nitrosative stress. Biochemistry. 2000;39:1040–1047. doi: 10.1021/bi992046e. [DOI] [PubMed] [Google Scholar]
- 83.Feigin VL. Stroke epidemiology in the developing world. Lancet. 2005;365:2160–2161. doi: 10.1016/S0140-6736(05)66755-4. [DOI] [PubMed] [Google Scholar]
- 84.Fenyk-Melody JE. Garrison AE. Brunnert SR. Weidner JR. Shen F. Shelton BA. Mudgett JS. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160:2940–2946. [PubMed] [Google Scholar]
- 85.Forster C. Clark HB. Ross ME. Iadecola C. Inducible nitric oxide synthase expression in human cerebral infarcts. Acta Neuropathol. 1999;97:215–220. doi: 10.1007/s004010050977. [DOI] [PubMed] [Google Scholar]
- 86.Foster MW. Hess DT. Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frautschy SA. Yang F. Irrizarry M. Hyman B. Saido TC. Hsiao K. Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- 88.Fujisawa Y. Kato K. Giulivi C. Nitration of tyrosine residues 368 and 345 in the beta-subunit elicits FoF1-ATPase activity loss. Biochem J. 2009;423:219–231. doi: 10.1042/BJ20090594. [DOI] [PubMed] [Google Scholar]
- 89.Furchgott RF. Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 90.Galea E. Feinstein DL. Reis DJ. Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures. Proc Natl Acad Sci U S A. 1992;89:10945–10949. doi: 10.1073/pnas.89.22.10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Games D. Adams D. Alessandrini R. Barbour R. Berthelette P. Blackwell C. Carr T. Clemens J. Donaldson T. Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 92.Gao C. Guo H. Wei J. Mi Z. Wai PY. Kuo PC. Identification of S-nitrosylated proteins in endotoxin-stimulated RAW264.7 murine macrophages. Nitric Oxide. 2005;12:121–126. doi: 10.1016/j.niox.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 93.Gardner PR. Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 94.Gardner PR. Martin LA. Hall D. Gardner AM. Dioxygen-dependent metabolism of nitric oxide in mammalian cells. Free Radic Biol Med. 2001;31:191–204. doi: 10.1016/s0891-5849(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 95.Garthwaite J. Charles SL. Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 96.Gasche Y. Copin JC. Sugawara T. Fujimura M. Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 97.Gaston B. Reilly J. Drazen JM. Fackler J. Ramdev P. Arnelle D. Mullins ME. Sugarbaker DJ. Chee C. Singel DJ, et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993;90:10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Glantz LA. Gilmore JH. Lieberman JA. Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006;81:47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 99.Go YM. Patel RP. Maland MC. Park H. Beckman JS. Darley-Usmar VM. Jo H. Evidence for peroxynitrite as a signaling molecule in flow-dependent activation of c-Jun NH(2)-terminal kinase. Am J Physiol. 1999;277:H1647–H1653. doi: 10.1152/ajpheart.1999.277.4.H1647. [DOI] [PubMed] [Google Scholar]
- 100.Good PF. Hsu A. Werner P. Perl DP. Olanow CW. Protein nitration in Parkinson's disease. J Neuropathol Exp Neurol. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 101.Greenamyre JT. Sherer TB. Betarbet R. Panov AV. Complex I and Parkinson's disease. IUBMB Life. 2001;52:135–141. doi: 10.1080/15216540152845939. [DOI] [PubMed] [Google Scholar]
- 102.Griffiths C. Garthwaite J. The shaping of nitric oxide signals by a cellular sink. J Physiol. 2001;536:855–862. doi: 10.1111/j.1469-7793.2001.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grisham MB. Jourd'heuil D. Wink DA. Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites:implications in inflammation. Am J Physiol. 1999;276:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 104.Gu Z. Kaul M. Yan B. Kridel SJ. Cui J. Strongin A. Smith JW. Liddington RC. Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 105.Guix FX. Uribesalgo I. Coma M. Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 106.Hall CN. Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21:92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Halligan KE. Jourd'heuil FL. Jourd'heuil D. Cytoglobin is expressed in the vasculature and regulates cell respiration and proliferation via nitric oxide dioxygenation. J Biol Chem. 2009;284:8539–8547. doi: 10.1074/jbc.M808231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–160. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- 109.Han D. Canali R. Garcia J. Aguilera R. Gallaher TK. Cadenas E. Sites and mechanisms of aconitase inactivation by peroxynitrite: modulation by citrate and glutathione. Biochemistry. 2005;44:11986–11996. doi: 10.1021/bi0509393. [DOI] [PubMed] [Google Scholar]
- 110.Hantraye P. Brouillet E. Ferrante R. Palfi S. Dolan R. Matthews RT. Beal MF. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- 111.Haqqani AS. Do SK. Birnboim HC. The role of a formaldehyde dehydrogenase-glutathione pathway in protein S-nitrosation in mammalian cells. Nitric Oxide. 2003;9:172–181. doi: 10.1016/j.niox.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 112.Hara MR. Agrawal N. Kim SF. Cascio MB. Fujimuro M. Ozeki Y. Takahashi M. Cheah JH. Tankou SK. Hester LD. Ferris CD. Hayward SD. Snyder SH. Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 113.Hara MR. Cascio MB. Sawa A. GAPDH as a sensor of NO stress. Biochim Biophys Acta. 2006;1762:502–509. doi: 10.1016/j.bbadis.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 114.Hara MR. Thomas B. Cascio MB. Bae BI. Hester LD. Dawson VL. Dawson TM. Sawa A. Snyder SH. Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc Natl Acad Sci U S A. 2006;103:3887–3889. doi: 10.1073/pnas.0511321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hardy JA. Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 116.Hauser SL. Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 117.Haynes V. Traaseth NJ. Elfering S. Fujisawa Y. Giulivi C. Nitration of specific tyrosines in FoF1 ATP synthase and activity loss in aging. Am J Physiol Endocrinol Metab. 2010;298:E978–E987. doi: 10.1152/ajpendo.00739.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.He Y. Yu W. Baas PW. Microtubule reconfiguration during axonal retraction induced by nitric oxide. J Neurosci. 2002;22:5982–5991. doi: 10.1523/JNEUROSCI.22-14-05982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 120.Hirokawa N. Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- 121.Hoffmann J. Haendeler J. Zeiher AM. Dimmeler S. TNFalpha and oxLDL reduce protein S-nitrosylation in endothelial cells. J Biol Chem. 2001;276:41383–41387. doi: 10.1074/jbc.M107566200. [DOI] [PubMed] [Google Scholar]
- 122.Huang Z. Huang PL. Ma J. Meng W. Ayata C. Fishman MC. Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 123.Iadecola C. Zhang F. Casey R. Nagayama M. Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ibi M. Sawada H. Kume T. Katsuki H. Kaneko S. Shimohama S. Akaike A. Depletion of intracellular glutathione increases susceptibility to nitric oxide in mesencephalic dopaminergic neurons. J Neurochem. 1999;73:1696–1703. doi: 10.1046/j.1471-4159.1999.731696.x. [DOI] [PubMed] [Google Scholar]
- 125.Ignarro LJ. Buga GM. Wood KS. Byrns RE. Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iyirhiaro GO. Brust TB. Rashidian J. Galehdar Z. Osman A. Phillips M. Slack RS. MacVicar BA. Park DS. Delayed combinatorial treatment with flavopiridol and minocycline provides longer term protection for neuronal soma but not dendrites following global ischemia. J Neurochem. 2008;105:703–713. doi: 10.1111/j.1471-4159.2007.05166.x. [DOI] [PubMed] [Google Scholar]
- 127.Jaffrey SR. Erdjument-Bromage H. Ferris CD. Tempst P. Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 128.Janssens SP. Shimouchi A. Quertermous T. Bloch DB. Bloch KD. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem. 1992;267:14519–14522. [PubMed] [Google Scholar]
- 129.Jourd'heuil D. Jourd'heuil FL. Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free redical mechanism. J Biol Chem. 2003;278:15720–15726. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 130.Jourd'heuil D. Miranda KM. Kim SM. Espey MG. Vodovotz Y. Laroux S. Mai CT. Miles AM. Grisham MB. Wink DA. The oxidative and nitrosative chemistry of the nitric oxide/superoxide reaction in the presence of bicarbonate. Arch Biochem Biophys. 1999;365:92–100. doi: 10.1006/abbi.1999.1143. [DOI] [PubMed] [Google Scholar]
- 131.Kahl KG. Schmidt HH. Jung S. Sherman P. Toyka KV. Zielasek J. Experimental autoimmune encephalomyelitis in mice with a targeted deletion of the inducible nitric oxide synthase gene: increased T-helper 1 response. Neurosci Lett. 2004;358:58–62. doi: 10.1016/j.neulet.2003.12.095. [DOI] [PubMed] [Google Scholar]
- 132.Kahl KG. Zielasek J. Uttenthal LO. Rodrigo J. Toyka KV. Schmidt HH. Protective role of the cytokine-inducible isoform of nitric oxide synthase induction and nitrosative stress in experimental autoimmune encephalomyelitis of the DA rat. J Neurosci Res. 2003;73:198–205. doi: 10.1002/jnr.10649. [DOI] [PubMed] [Google Scholar]
- 133.Kanaan NM. Morfini GA. LaPointe NE. Pigino GF. Patterson KR. Song Y. Andreadis A. Fu Y. Brady ST. Binder LI. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci. 2011;31:9858–9868. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kanwar M. Kowluru RA. Role of glyceraldehyde 3-phosphate dehydrogenase in the development and progression of diabetic retinopathy. Diabetes. 2009;58:227–234. doi: 10.2337/db08-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khan M. Sekhon B. Giri S. Jatana M. Gilg AG. Ayasolla K. Elango C. Singh AK. Singh I. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab. 2005;25:177–192. doi: 10.1038/sj.jcbfm.9600012. [DOI] [PubMed] [Google Scholar]
- 136.Kil IS. Park JW. Regulation of mitochondrial NADP+-dependent isocitrate dehydrogenase activity by glutathionylation. J Biol Chem. 2005;280:10846–10854. doi: 10.1074/jbc.M411306200. [DOI] [PubMed] [Google Scholar]
- 137.Kim H. Shim J. Han PL. Choi EJ. Nitric oxide modulates the c-Jun N-terminal kinase/stress-activated protein kinase activity through activating c-Jun N-terminal kinase kinase. Biochemistry. 1997;36:13677–13681. doi: 10.1021/bi970837f. [DOI] [PubMed] [Google Scholar]
- 138.Kim SF. Huri DA. Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 139.Kim YM. Talanian RV. Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem. 1997;272:31138–31148. doi: 10.1074/jbc.272.49.31138. [DOI] [PubMed] [Google Scholar]
- 140.Kitada T. Asakawa S. Hattori N. Matsumine H. Yamamura Y. Minoshima S. Yokochi M. Mizuno Y. Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 141.Kluge I. Gutteck-Amsler U. Zollinger M. Do KQ. S-nitrosoglutathione in rat cerebellum: identification and quantification by liquid chromatography-mass spectrometry. J Neurochem. 1997;69:2599–2607. doi: 10.1046/j.1471-4159.1997.69062599.x. [DOI] [PubMed] [Google Scholar]
- 142.Knott AB. Bossy-Wetzel E. Nitric oxide in health and disease of the nervous system. Antioxid Redox Signal. 2009;11:541–554. doi: 10.1089/ars.2008.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Knowles RG. Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Knowles RG. Palacios M. Palmer RM. Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Koeck T. Fu X. Hazen SL. Crabb JW. Stuehr DJ. Aulak KS. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J Biol Chem. 2004;279:27257–27262. doi: 10.1074/jbc.M401586200. [DOI] [PubMed] [Google Scholar]
- 146.Koeck T. Levison B. Hazen SL. Crabb JW. Stuehr DJ. Aulak KS. Tyrosine nitration impairs mammalian aldolase A activity. Mol Cell Proteomics. 2004;3:548–557. doi: 10.1074/mcp.M300141-MCP200. [DOI] [PubMed] [Google Scholar]
- 147.Koeck T. Willard B. Crabb JW. Kinter M. Stuehr DJ. Aulak KS. Glucose-mediated tyrosine nitration in adipocytes: targets and consequences. Free Radic Biol Med. 2009;46:884–892. doi: 10.1016/j.freeradbiomed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kovalenko T. Osadchenko I. Nikonenko A. Lushnikova I. Voronin K. Nikonenko I. Muller D. Skibo G. Ischemia-induced modifications in hippocampal CA1 stratum radiatum excitatory synapses. Hippocampus. 2006;16:814–825. doi: 10.1002/hipo.20211. [DOI] [PubMed] [Google Scholar]
- 149.Kubler W. Spieckermann PG. Regulation of glycolysis in the ischemic and the anoxic myocardium. J Mol Cell Cardiol. 1970;1:351–377. doi: 10.1016/0022-2828(70)90034-9. [DOI] [PubMed] [Google Scholar]
- 150.Lamas S. Marsden PA. Li GK. Tempst P. Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992;89:6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lancaster JR., Jr. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci U S A. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lancaster JR., Jr. Nitroxidative, nitrosative, and nitrative stress: kinetic predictions of reactive nitrogen species chemistry under biological conditions. Chem Res Toxicol. 2006;19:1160–1174. doi: 10.1021/tx060061w. [DOI] [PubMed] [Google Scholar]
- 153.Lang AE. Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 154.Lang AE. Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med. 1998;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 155.Langston JW. Ballard P. Tetrud JW. Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 156.LaPointe NE. Morfini G. Pigino G. Gaisina IN. Kozikowski AP. Binder LI. Brady ST. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J Neurosci Res. 2009;87:440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee JY. Kim YH. Koh JY. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 2001;21:RC171. doi: 10.1523/JNEUROSCI.21-20-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Leist M. Volbracht C. Kuhnle S. Fava E. Ferrando-May E. Nicotera P. Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Mol Med. 1997;3:750–764. [PMC free article] [PubMed] [Google Scholar]
- 159.Lewis R. Tannenbaum SR. Deen WM. Kinetics of N-nitrosation in oxygenated nitric oxide solutions at physiological pH: role of nitrous anhydride and efefcts of phosphate and chloride. J Am Chem Soc. 1995;117:3933–3999. [Google Scholar]
- 160.Li J. Billiar TR. Talanian RV. Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;240:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 161.Li JY. Plomann M. Brundin P. Huntington's disease: a synaptopathy? Trends Mol Med. 2003;9:414–420. doi: 10.1016/j.molmed.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 162.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 163.Lipton SA. Choi YB. Pan ZH. Lei SZ. Chen HS. Sucher NJ. Loscalzo J. Singel DJ. Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 164.Liu B. Tewari AK. Zhang L. Green-Church KB. Zweier JL. Chen YR. He G. Proteomic analysis of protein tyrosine nitration after ischemia reperfusion injury: mitochondria as the major target. Biochim Biophys Acta. 2009;1794:476–485. doi: 10.1016/j.bbapap.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu JS. Zhao ML. Brosnan CF. Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158:2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lizasoain I. Moro MA. Knowles RG. Darley-Usmar V. Moncada S. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem J. 1996;314:877–880. doi: 10.1042/bj3140877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lo EH. Dalkara T. Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 168.Lorenzo A. Yankner BA. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci U S A. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lowenstein CJ. Alley EW. Raval P. Snowman AM. Snyder SH. Russell SW. Murphy WJ. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci U S A. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lowenstein CJ. Glatt CS. Bredt DS. Snyder SH. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992;89:6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lundberg JO. Weitzberg E. Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 172.Luth HJ. Holzer M. Gartner U. Staufenbiel M. Arendt T. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer's disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res. 2001;913:57–67. doi: 10.1016/s0006-8993(01)02758-5. [DOI] [PubMed] [Google Scholar]
- 173.Malhotra V. Orci L. Glick BS. Block MR. Rothman JE. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988;54:221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- 174.Malinski T. Bailey F. Zhang ZG. Chopp M. Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1993;13:355–358. doi: 10.1038/jcbfm.1993.48. [DOI] [PubMed] [Google Scholar]
- 175.Mandolesi G. Grasselli G. Musumeci G. Centonze D. Cognitive deficits in experimental autoimmune encephalomyelitis: neuroinflammation and synaptic degeneration. Neurol Sci. 2010;31:S255–S259. doi: 10.1007/s10072-010-0369-3. [DOI] [PubMed] [Google Scholar]
- 176.Manev H. Favaron M. Guidotti A. Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989;36:106–112. [PubMed] [Google Scholar]
- 177.Masters CL. Simms G. Weinman NA. Multhaup G. McDonald BL. Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Matsushita K. Morrell CN. Cambien B. Yang SX. Yamakuchi M. Bao C. Hara MR. Quick RA. Cao W. O'Rourke B. Lowenstein JM. Pevsner J. Wagner DD. Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Matthews RT. Beal MF. Fallon J. Fedorchak K. Huang PL. Fishman MC. Hyman BT. MPP+ induced substantia nigra degeneration is attenuated in nNOS knockout mice. Neurobiol Dis. 1997;4:114–121. doi: 10.1006/nbdi.1997.0141. [DOI] [PubMed] [Google Scholar]
- 180.McDonald LJ. Moss J. Stimulation by nitric oxide of an NAD linkage to glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1993;90:6238–6241. doi: 10.1073/pnas.90.13.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miller S. Ross-Inta C. Giulivi C. Kinetic and proteomic analyses of S-nitrosoglutathione-treated hexokinase A: consequences for cancer energy metabolism. Amino Acids. 2007;32:593–602. doi: 10.1007/s00726-006-0424-9. [DOI] [PubMed] [Google Scholar]
- 182.Minc-Golomb D. Tsarfaty I. Schwartz JP. Expression of inducible nitric oxide synthase by neurones following exposure to endotoxin and cytokine. Br J Pharmacol. 1994;112:720–722. doi: 10.1111/j.1476-5381.1994.tb13136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mohr S. Hallak H. de Boitte A. Lapetina EG. Brune B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1999;274:9427–9430. doi: 10.1074/jbc.274.14.9427. [DOI] [PubMed] [Google Scholar]
- 184.Mohr S. Stamler JS. Brune B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett. 1994;348:223–227. doi: 10.1016/0014-5793(94)00596-6. [DOI] [PubMed] [Google Scholar]
- 185.Mohr S. Stamler JS. Brune B. Posttranslational modification of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosylation and subsequent NADH attachment. J Biol Chem. 1996;271:4209–4214. doi: 10.1074/jbc.271.8.4209. [DOI] [PubMed] [Google Scholar]
- 186.Moncada S. Bolanos JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- 187.Moncada S. Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 188.Mongin AA. Nitric oxide may contribute to the long-term impairment of synaptic transmission after transient ischemia. Stroke. 2002;33:2348–2350. doi: 10.1161/01.str.0000033074.40202.8e. [DOI] [PubMed] [Google Scholar]
- 189.Mongin AA. Disruption of ionic and cell volume homeostasis in cerebral ischemia: The perfect storm. Pathophysiology. 2007;14:183–193. doi: 10.1016/j.pathophys.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mongin AA. Nedvetsky PI. Fedorovich SV. Depolarization of isolated brain nerve endings by nitric oxide donors: Membrane mechanisms. Biochemistry (Mosc) 1998;63:662–670. [PubMed] [Google Scholar]
- 191.Moore DJ. West AB. Dawson VL. Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 192.Morfini GA. Burns M. Binder LI. Kanaan NM. Lapointe N. Bosco DA. Brown RH., Jr. Brown H. Tiwari A. Hayward L. Edgar J. Nave KA. Garberrn J. Atagi Y. Song Y. Pigino G. Brady ST. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Morfini GA. Stenoien DL. Brady ST. Axonal transport. In: Siegel GJ, editor; Albers RW, editor; Brady ST, editor; Price DL, editor. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. London: Elsevier Academic Press; 2006. pp. 485–501. [Google Scholar]
- 194.Mudrick LA. Baimbridge KG. Long-term structural changes in the rat hippocampal formation following cerebral ischemia. Brain Res. 1989;493:179–184. doi: 10.1016/0006-8993(89)91014-7. [DOI] [PubMed] [Google Scholar]
- 195.Murphy TH. Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 196.Nakamura T. Lipton SA. Emerging roles of S-nitrosylation in protein misfolding and neurodegenerative diseases. Antioxid Redox Signal. 2008;10:87–101. doi: 10.1089/ars.2007.1858. [DOI] [PubMed] [Google Scholar]
- 197.Nakamura T. Lipton SA. Cell death: protein misfolding and neurodegenerative diseases. Apoptosis. 2009;14:455–468. doi: 10.1007/s10495-008-0301-y. [DOI] [PubMed] [Google Scholar]
- 198.Nathan C. Calingasan N. Nezezon J. Ding A. Lucia MS. La PK. Fuortes M. Lin M. Ehrt S. Kwon NS. Chen J. Vodovotz Y. Kipiani K. Beal MF. Protection from Alzheimer's-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J Exp Med. 2005;202:1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 200.Nedvetsky PI. Konev SV. Rakovich AA. Petrenko SV. Mongin AA. Effects of nitric oxide donors on Ca2+-dependent [14C]GABA release from brain synaptosomes: The role of SH-groups. Biochemistry (Mosc) 2000;65:1027–1035. [PubMed] [Google Scholar]
- 201.Neumann-Haefelin T. Witte OW. Periinfarct and remote excitability changes after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20:45–52. doi: 10.1097/00004647-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 202.Osuka K. Feustel PJ. Mongin AA. Tranmer BI. Kimelberg HK. Tamoxifen inhibits nitrotyrosine formation after reversible middle cerebral artery occlusion in the rat. J Neurochem. 2001;76:1842–1850. doi: 10.1046/j.1471-4159.2001.00198.x. [DOI] [PubMed] [Google Scholar]
- 203.Pacher P. Beckman JS. Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Padgett CM. Whorton AR. S-nitrosoglutathione reversibly inhibits GAPDH by S-nitrosylation. Am J Physiol. 1995;269:C739–C749. doi: 10.1152/ajpcell.1995.269.3.C739. [DOI] [PubMed] [Google Scholar]
- 205.Paige JS. Xu G. Stancevic B. Jaffrey SR. Nitrosothiol reactivity profiling identifies S-nitrosylated proteins with unexpected stability. Chem Biol. 2008;15:1307–1316. doi: 10.1016/j.chembiol.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Palamalai V. Miyagi M. Mechanism of glyceraldehyde-3-phosphate dehydrogenase inactivation by tyrosine nitration. Protein Sci. 2010;19:255–262. doi: 10.1002/pro.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Palmer RMJ. Ashton DS. Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 208.Pearce LL. Kanai AJ. Epperly MW. Peterson J. Nitrosative stress results in irreversible inhibition of purified mitochondrial complexes I and III without modification of cofactors. Nitric Oxide. 2005;13:254–263. doi: 10.1016/j.niox.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 209.Perlson E. Maday S. Fu MM. Moughamian AJ. Holzbaur EL. Retrograde axonal transport: pathways to cell death? Trends Neurosci. 2010;33:335–344. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pluta RM. Dejam A. Grimes G. Gladwin MT. Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. JAMA. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 211.Prasad R. Giri S. Nath N. Singh I. Singh AK. GSNO attenuates EAE disease by S-nitrosylation-mediated modulation of endothelial-monocyte interactions. Glia. 2007;55:65–77. doi: 10.1002/glia.20436. [DOI] [PubMed] [Google Scholar]
- 212.Prime TA. Blaikie FH. Evans C. Nadtochiy SM. James AM. Dahm CC. Vitturi DA. Patel RP. Hiley CR. Abakumova I. Requejo R. Chouchani ET. Hurd TR. Garvey JF. Taylor CT. Brookes PS. Smith RA. Murphy MP. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2009;106:10764–10769. doi: 10.1073/pnas.0903250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Qu J. Nakamura T. Cao G. Holland EA. McKercher SR. Lipton SA. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by {beta}-amyloid peptide. Proc Natl Acad Sci U S A. 2011;108:14330–14335. doi: 10.1073/pnas.1105172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Radi R. Rodriguez M. Castro L. Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 215.Rees DD. Palmer RM. Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Renteria RC. Constantine-Paton M. Exogenous nitric oxide causes collapse of retinal ganglion cell axonal growth cones in vitro. J Neurobiol. 1996;29:415–428. doi: 10.1002/(SICI)1097-4695(199604)29:4<415::AID-NEU1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 217.Richards EM. Rosenthal RE. Kristian T. Fiskum G. Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity. Free Radic Biol Med. 2006;40:1960–1970. doi: 10.1016/j.freeradbiomed.2006.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Romero JM. Bizzozero OA. Intracellular glutathione mediates the denitrosylation of protein nitrosothiols in the rat spinal cord. J Neurosci Res. 2009;87:701–709. doi: 10.1002/jnr.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rosahl TW. Spillane D. Missler M. Herz J. Selig DK. Wolff JR. Hammer RE. Malenka RC. Sudhof TC. Essential functions of synapsin-I and synapsin-II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- 220.Rothman SM. Olney JW. Glutamate and the pathophysiology of hypoxic—ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 221.Roy S. Winton MJ. Black MM. Trojanowski JQ. Lee VM. Cytoskeletal requirements in axonal transport of slow component-b. J Neurosci. 2008;28:5248–5256. doi: 10.1523/JNEUROSCI.0309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rudkouskaya A. Sim V. Shah AA. Feustel PJ. Jourd'heuil D. Mongin AA. Long-lasting inhibition of presynaptic metabolism and neurotransmitter release by protein S-nitrosylation. Free Radic Biol Med. 2010;49:757–769. doi: 10.1016/j.freeradbiomed.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ruuls SR. Van Der Linden S. Sontrop K. Huitinga I. Dijkstra CD. Aggravation of experimental allergic encephalomyelitis (EAE) by administration of nitric oxide (NO) synthase inhibitors. Clin Exp Immunol. 1996;103:467–474. doi: 10.1111/j.1365-2249.1996.tb08304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Salmeen A. Andersen JN. Myers MP. Meng TC. Hinks JA. Tonks NK. Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 225.Samdani AF. Dawson TM. Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 226.Sarchielli P. Galli F. Floridi A. Floridi A. Gallai V. Relevance of protein nitration in brain injury: a key pathophysiological mechanism in neurodegenerative, autoimmune, or inflammatory CNS diseases and stroke. Amino Acids. 2003;25:427–436. doi: 10.1007/s00726-003-0028-6. [DOI] [PubMed] [Google Scholar]
- 227.Sawa A. Khan AA. Hester LD. Snyder SH. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci U S A. 1997;94:11669–11674. doi: 10.1073/pnas.94.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Scheff SW. Price DA. Synaptic pathology in Alzheimer's disease: a review of ultrastructural studies. Neurobiol Aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 229.Scheuner D. Eckman C. Jensen M. Song X. Citron M. Suzuki N. Bird TD. Hardy J. Hutton M. Kukull W. Larson E. Levy-Lahad E. Viitanen M. Peskind E. Poorkaj P. Schellenberg G. Tanzi R. Wasco W. Lannfelt L. Selkoe D. Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 230.Schiavo G. Gmachl MJ. Stenbeck G. Sollner TH. Rothman JE. A possible docking and fusion particle for synaptic transmission. Nature. 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- 231.Schrammel A. Gorren AC. Schmidt K. Pfeiffer S. Mayer B. S-nitrosation of glutathione by nitric oxide, peroxynitrite, and (*)NO/O(2)(*-) Free Radic Biol Med. 2003;34:1078–1088. doi: 10.1016/s0891-5849(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 232.Schulz JB. Matthews RT. Muqit MM. Browne SE. Beal MF. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995;64:936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- 233.Schweizer M. Richter C. Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem Biophys Res Commun. 1994;204:169–175. doi: 10.1006/bbrc.1994.2441. [DOI] [PubMed] [Google Scholar]
- 234.Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 235.Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer's disease. J Biol Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 236.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 237.Sequeira SM. Ambrosio AF. Malva JO. Carvalho AP. Carvalho CM. Modulation of glutamate release from rat hippocampal synaptosomes by nitric oxide. Nitric Oxide. 1997;1:315–329. doi: 10.1006/niox.1997.0144. [DOI] [PubMed] [Google Scholar]
- 238.Shahani N. Sawa A. Nitric oxide signaling and nitrosative stress in neurons: role for s-nitrosylation. Antioxid Redox Signal. 2011;14:1493–1504. doi: 10.1089/ars.2010.3580. [DOI] [PubMed] [Google Scholar]
- 239.Shi Q. Feng J. Qu H. Cheng YY. A proteomic study of S-nitrosylation in the rat cardiac proteins in vitro. Biol Pharm Bull. 2008;31:1536–1540. doi: 10.1248/bpb.31.1536. [DOI] [PubMed] [Google Scholar]
- 240.Shi Q. Xu H. Yu H. Zhang N. Ye Y. Estevez AG. Deng H. Gibson GE. Inactivation and reactivation of the mitochondrial alpha-ketoglutarate dehydrogenase complex. J Biol Chem. 2011;286:17640–17648. doi: 10.1074/jbc.M110.203018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shiva S. Crawford JH. Ramachandran A. Ceaser EK. Hillson T. Brookes PS. Patel RP. Darley-Usmar VM. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem J. 2004;379:359–366. doi: 10.1042/BJ20031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shiva S. Sack MN. Greer JJ. Duranski M. Ringwood LA. Burwell L. Wang X. MacArthur PH. Shoja A. Raghavachari N. Calvert JW. Brookes PS. Lefer DJ. Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Smith KJ. Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 244.Smith MA. Richey Harris PL. Sayre LM. Beckman JS. Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Souza JM. Radi R. Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch Biochem Biophys. 1998;360:187–194. doi: 10.1006/abbi.1998.0932. [DOI] [PubMed] [Google Scholar]
- 246.Stagi M. Dittrich PS. Frank N. Iliev AI. Schwille P. Neumann H. Breakdown of axonal synaptic vesicle precursor transport by microglial nitric oxide. J Neurosci. 2005;25:352–362. doi: 10.1523/JNEUROSCI.3887-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Stamler JS. Toone EJ. Lipton SA. Sucher NJ. (S)NO signals: translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 248.Steinman L. Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 249.Stuehr DJ. Cho HJ. Kwon NS. Weise MF. Nathan CF. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991;88:7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Stuehr DJ. Santolini J. Wang ZQ. Wei CC. Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 251.Sultana R. Poon HF. Cai J. Pierce WM. Merchant M. Klein JB. Markesbery WR. Butterfield DA. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis. 2006;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 252.Tabatabaie T. Potts JD. Floyd RA. Reactive oxygen species-mediated inactivation of pyruvate dehydrogenase. Arch Biochem Biophys. 1996;336:290–296. doi: 10.1006/abbi.1996.0560. [DOI] [PubMed] [Google Scholar]
- 253.Taylor ER. Hurrell F. Shannon RJ. Lin TK. Hirst J. Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 254.Terry RD. Masliah E. Salmon DP. Butters N. DeTeresa R. Hill R. Hansen LA. Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 255.Thomas DD. Ridnour LA. Espey MG. Donzelli S. Ambs S. Hussain SP. Harris CC. DeGraff W. Roberts DD. Mitchell JB. Wink DA. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem. 2006;281:25984–25993. doi: 10.1074/jbc.M602242200. [DOI] [PubMed] [Google Scholar]
- 256.Thorns V. Hansen L. Masliah E. nNOS expressing neurons in the entorhinal cortex and hippocampus are affected in patients with Alzheimer's disease. Exp Neurol. 1998;150:14–20. doi: 10.1006/exnr.1997.6751. [DOI] [PubMed] [Google Scholar]
- 257.Tian J. Kim SF. Hester L. Snyder SH. S-nitrosylation/activation of COX-2 mediates NMDA neurotoxicity. Proc Natl Acad Sci U S A. 2008;105:10537–10540. doi: 10.1073/pnas.0804852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tompkins AJ. Burwell LS. Digerness SB. Zaragoza C. Holman WL. Brookes PS. Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim Biophys Acta. 2006;1762:223–231. doi: 10.1016/j.bbadis.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 259.Tortora V. Quijano C. Freeman B. Radi R. Castro L. Mitochondrial aconitase reaction with nitric oxide, S-nitrosoglutathione, and peroxynitrite: mechanisms and relative contributions to aconitase inactivation. Free Radic Biol Med. 2007;42:1075–1088. doi: 10.1016/j.freeradbiomed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 260.Towfighi A. Saver JL. Stroke declines from third to fourth leading cause of death in the United States: historical perspective and challenges ahead. Stroke. 2011;42:2351–2355. doi: 10.1161/STROKEAHA.111.621904. [DOI] [PubMed] [Google Scholar]
- 261.Tran MH. Yamada K. Nakajima A. Mizuno M. He J. Kamei H. Nabeshima T. Tyrosine nitration of a synaptic protein synaptophysin contributes to amyloid beta-peptide-induced cholinergic dysfunction. Mol Psychiatry. 2003;8:407–412. doi: 10.1038/sj.mp.4001240. [DOI] [PubMed] [Google Scholar]
- 262.Uehara T. Kikuchi Y. Nomura Y. Caspase activation accompanying cytochrome c release from mitochondria is possibly involved in nitric oxide-induced neuronal apoptosis in SH-SY5Y cells. J Neurochem. 1999;72:196–205. doi: 10.1046/j.1471-4159.1999.0720196.x. [DOI] [PubMed] [Google Scholar]
- 263.Uehara T. Nakamura T. Yao D. Shi ZQ. Gu Z. Ma Y. Masliah E. Nomura Y. Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 264.Van Montfort RL. Congreve M. Tisi D. Carr R. Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 265.Varadarajan S. Yatin S. Aksenova M. Butterfield DA. Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000;130:184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 266.Vedia L. McDonald B. Reep B. Brune B. Di Silvio M. Billiar TR. Lapetina EG. Nitric oxide-induced S-nitrosylation of glyceraldehyde-3-phosphate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem. 1992;267:24929–24932. [PubMed] [Google Scholar]
- 267.Wahl F. Obrenovitch TP. Hardy AM. Plotkine M. Boulu R. Symon L. Extracellular glutamate during focal cerebral ischaemia in rats: time course and calcium dependency. J Neurochem. 1994;63:1003–1011. doi: 10.1046/j.1471-4159.1994.63031003.x. [DOI] [PubMed] [Google Scholar]
- 268.Wallace MN. Geddes JG. Farquhar DA. Masson MR. Nitric oxide synthase in reactive astrocytes adjacent to beta-amyloid plaques. Exp Neurol. 1997;144:266–272. doi: 10.1006/exnr.1996.6373. [DOI] [PubMed] [Google Scholar]
- 269.Wang X. Su B. Lee HG. Li X. Perry G. Smith MA. Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wilson DW. Wilcox CA. Flynn GC. Chen E. Kuang WJ. Henzel WJ. Block MR. Ullrich A. Rothman JE. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989;339:355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- 271.Wink DA. Darbyshire JF. Nims RW. Saavedra JE. Ford PC. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993;6:23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 272.Wong ML. Rettori V. al-Shekhlee A. Bongiorno PB. Canteros G. McCann SM. Gold PW. Licinio J. Inducible nitric oxide synthase gene expression in the brain during systemic inflammation. Nat Med. 1996;2:581–584. doi: 10.1038/nm0596-581. [DOI] [PubMed] [Google Scholar]
- 273.Wood KC. Batchelor AM. Bartus K. Harris KL. Garthwaite G. Vernon J. Garthwaite J. Picomolar nitric oxide signals from central neurons recorded using ultrasensitive detector cells. J Biol Chem. 2011;286:43172–43181. doi: 10.1074/jbc.M111.289777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Xie QW. Cho HJ. Calaycay J. Mumford RA. Swiderek KM. Lee TD. Ding A. Troso T. Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 275.Yao D. Gu Z. Nakamura T. Shi ZQ. Ma Y. Gaston B. Palmer LA. Rockenstein EM. Zhang Z. Masliah E. Uehara T. Lipton SA. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yi JS. Kim TY. Kyu KD. Koh JY. Systemic pyruvate administration markedly reduces infarcts and motor deficits in rat models of transient and permanent focal cerebral ischemia. Neurobiol Dis. 2007;26:94–104. doi: 10.1016/j.nbd.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 277.Yu YM. Kim JB. Lee KW. Kim SY. Han PL. Lee JK. Inhibition of the cerebral ischemic injury by ethyl pyruvate with a wide therapeutic window. Stroke. 2005;36:2238–2243. doi: 10.1161/01.STR.0000181779.83472.35. [DOI] [PubMed] [Google Scholar]
- 278.Yui Y. Hattori R. Kosuga K. Eizawa H. Hiki K. Kawai C. Purification of nitric oxide synthase from rat macrophages. J Biol Chem. 1991;266:12544–12547. [PubMed] [Google Scholar]
- 279.Zhang J. Dawson VL. Dawson TM. Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 280.Zhang J. Jin B. Li L. Block ER. Patel JM. Nitric oxide-induced persistent inhibition and nitrosylation of active site cysteine residues of mitochondrial cytochrome-c oxidase in lung endothelial cells. Am J Physiol Cell Physiol. 2005;288:C840–C849. doi: 10.1152/ajpcell.00325.2004. [DOI] [PubMed] [Google Scholar]
- 281.Zhang L. Chen CL. Kang PT. Garg V. Hu K. Green-Church KB. Chen YR. Peroxynitrite-mediated oxidative modifications of complex II: relevance in myocardial infarction. Biochemistry. 2010;49:2529–2539. doi: 10.1021/bi9018237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhang Y. Hogg N. Formation and stability of S-nitrosothiols in RAW 264.7 cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L467–L474. doi: 10.1152/ajplung.00350.2003. [DOI] [PubMed] [Google Scholar]
- 283.Zhang Y. Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci U S A. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zhao W. Tilton RG. Corbett JA. McDaniel ML. Misko TP. Williamson JR. Cross AH. Hickey WF. Experimental allergic encephalomyelitis in the rat is inhibited by aminoguanidine, an inhibitor of nitric oxide synthase. J Neuroimmunol. 1996;64:123–133. doi: 10.1016/0165-5728(95)00158-1. [DOI] [PubMed] [Google Scholar]