Abstract
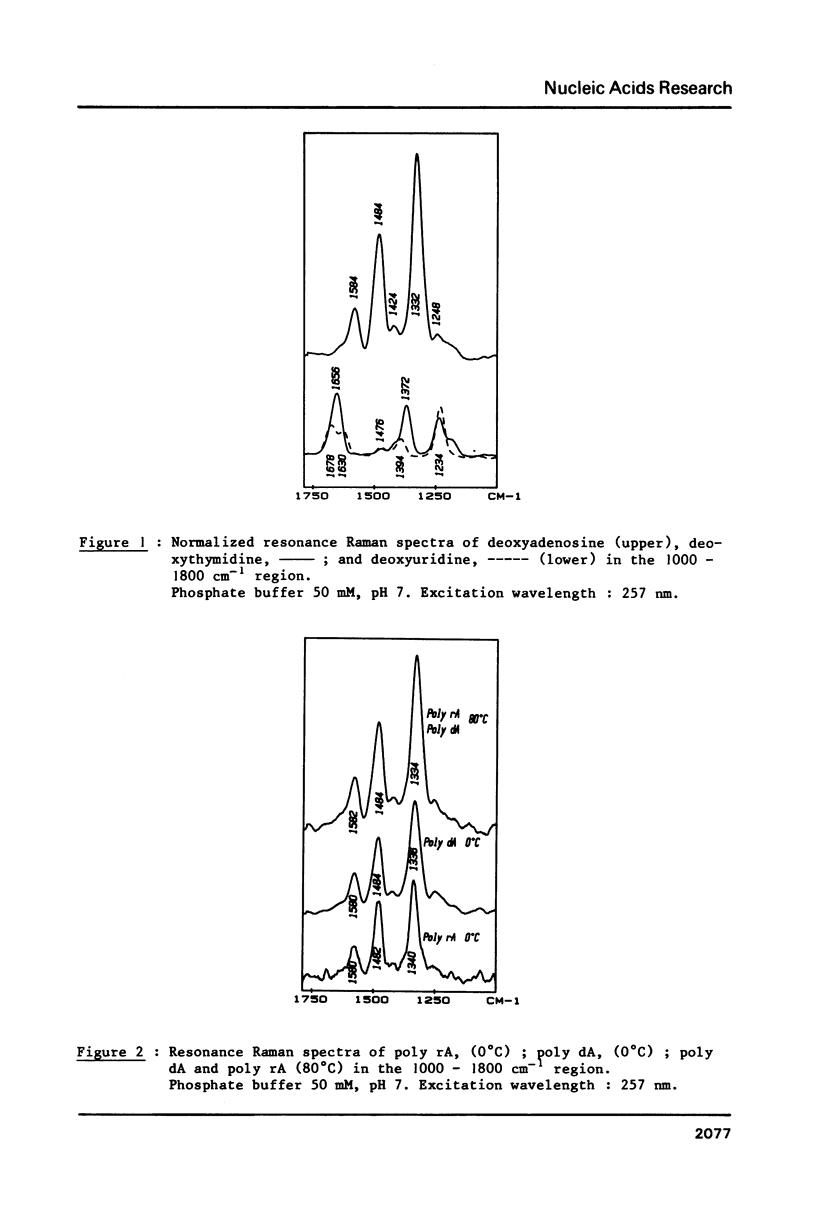
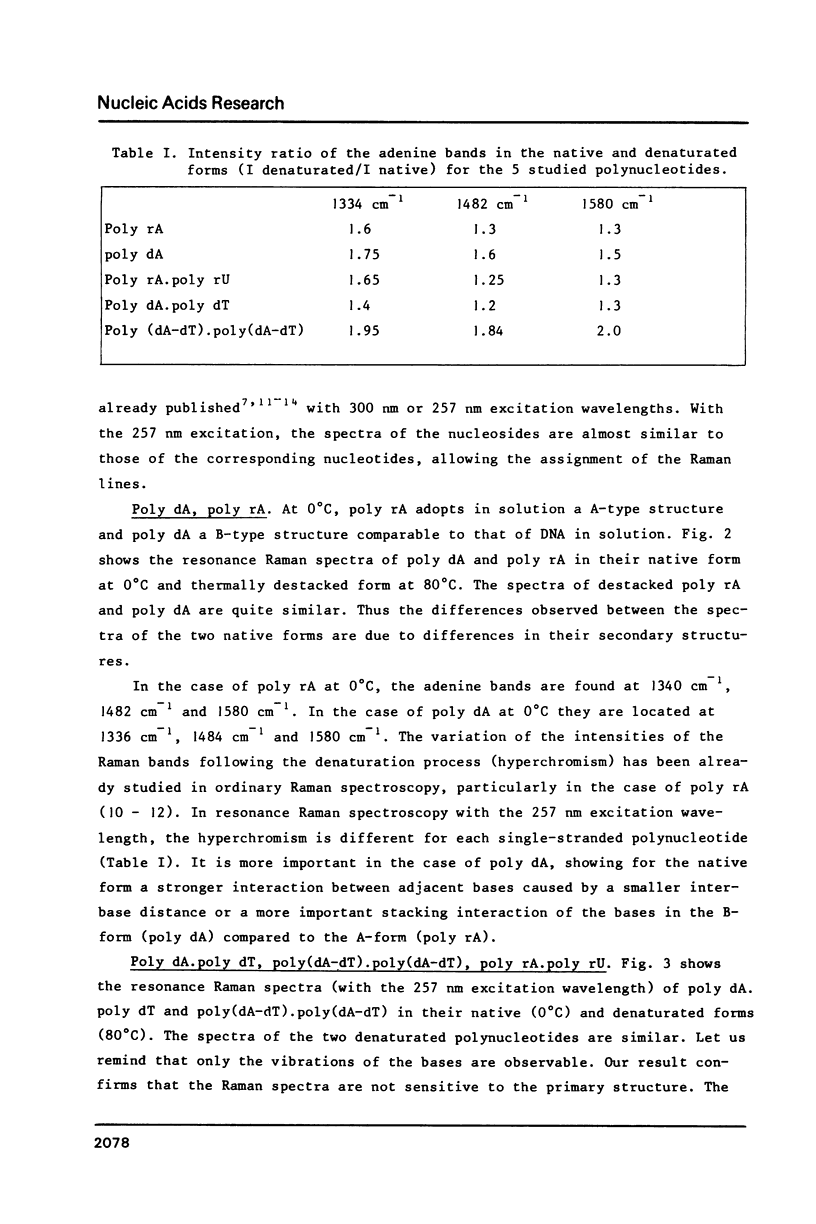
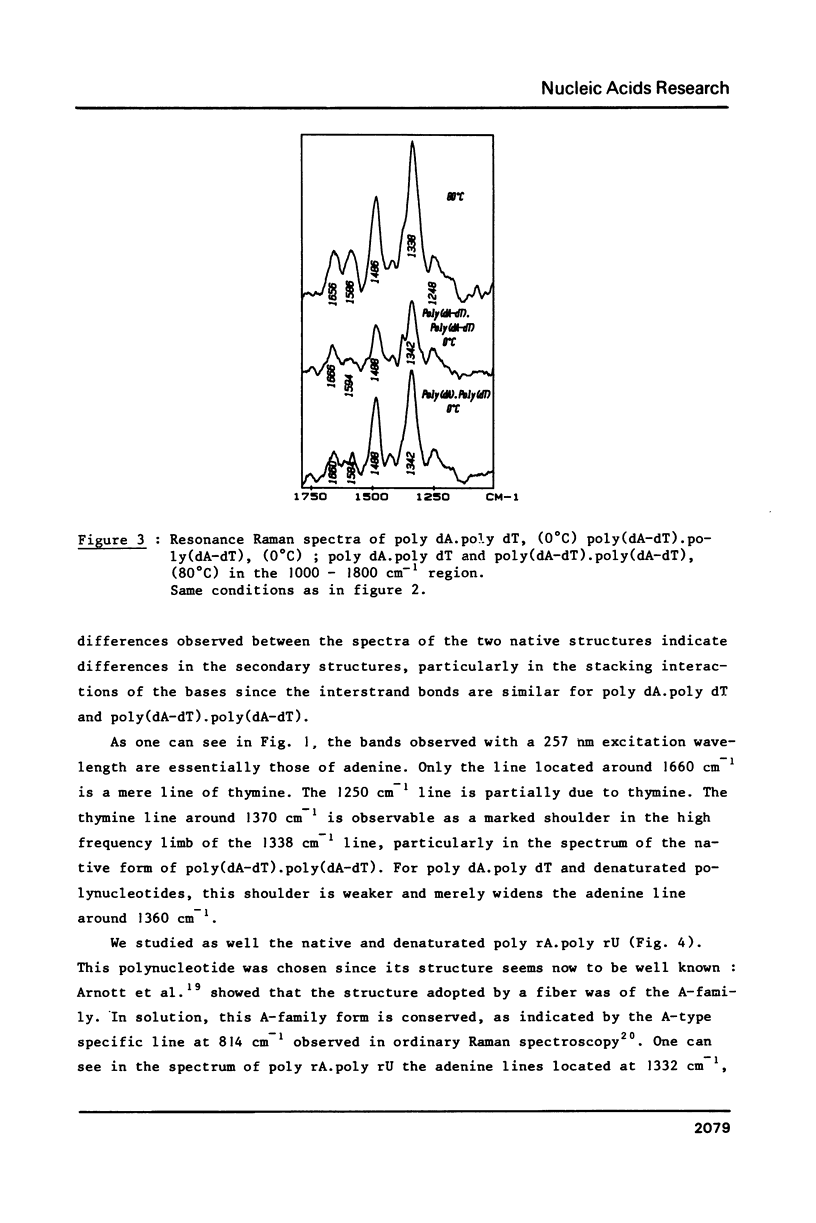
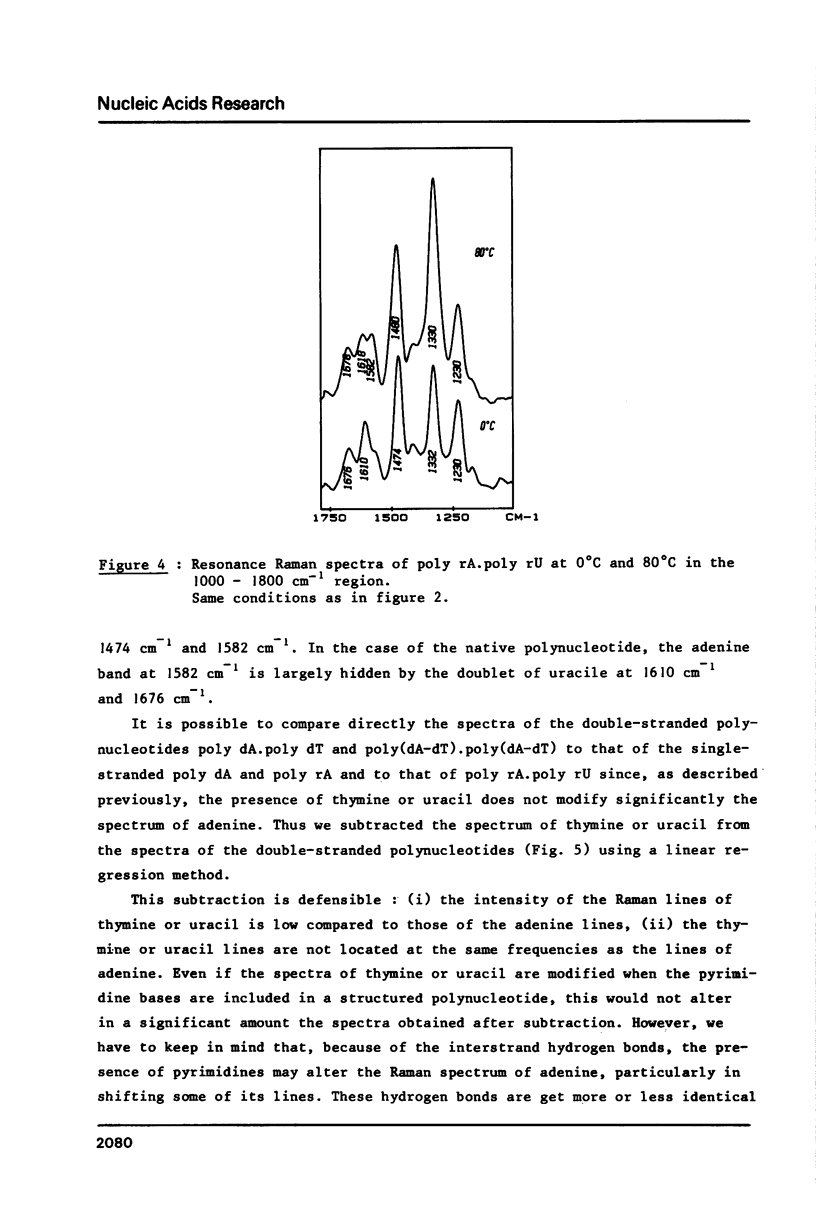
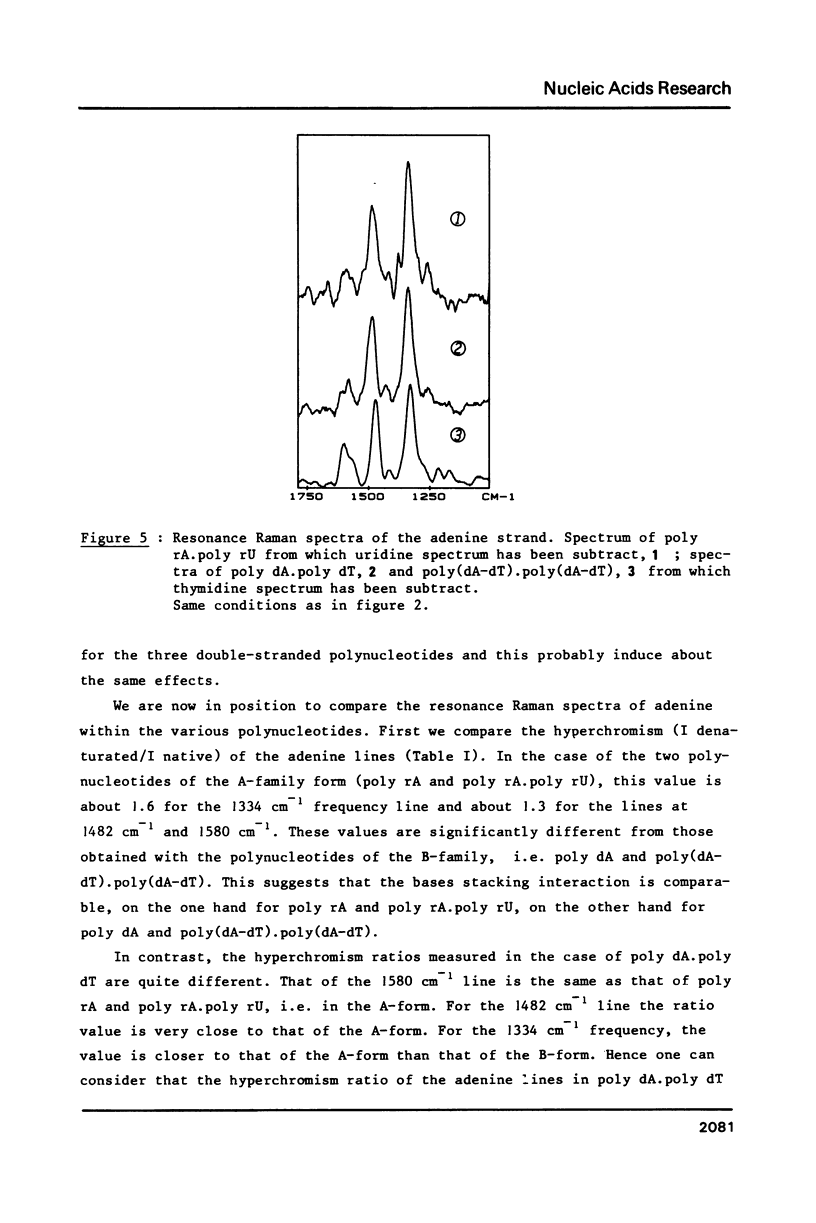
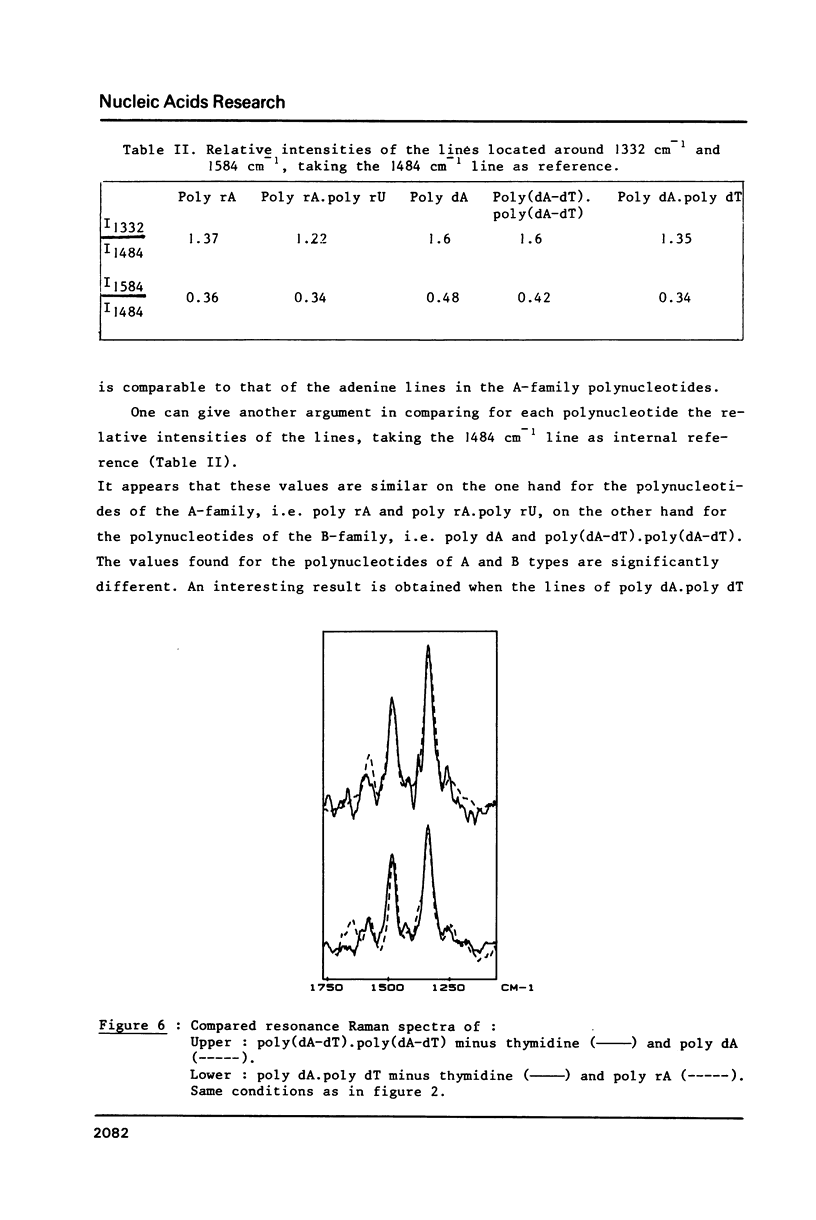
The study by resonance Raman spectroscopy with a 257 nm excitation wave-length of adenine in two single-stranded polynucleotides, poly rA and poly dA, and in three double-stranded polynucleotides, poly dA.poly dT, poly(dA-dT).poly(dA-dT) and poly rA.poly rU, allows one to characterize the A-genus conformation of polynucleotides containing adenine and thymine bases. The characteristic spectrum of the A-form of the adenine strand is observed, except small differences, for poly rA, poly rA.poly rU and poly dA.poly dT. Our results prove that it is the adenine strand which adopts the A-family conformation in poly dA.poly dT.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Hall I. H., Puigjaner L. C. Heteronomous DNA. Nucleic Acids Res. 1983 Jun 25;11(12):4141–4155. doi: 10.1093/nar/11.12.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W., Dover S. D., Fuller W., Hodgson A. R. Structures of synthetic polynucleotides in the A-RNA and A'-RNA conformations: x-ray diffraction analyses of the molecular conformations of polyadenylic acid--polyuridylic acid and polyinosinic acid--polycytidylic acid. J Mol Biol. 1973 Dec 5;81(2):107–122. doi: 10.1016/0022-2836(73)90183-6. [DOI] [PubMed] [Google Scholar]
- Arnott S., Selsing E. Structures for the polynucleotide complexes poly(dA) with poly (dT) and poly(dT) with poly(dA) with poly (dT). J Mol Biol. 1974 Sep 15;88(2):509–521. doi: 10.1016/0022-2836(74)90498-7. [DOI] [PubMed] [Google Scholar]
- Chinsky L., Jolles B., Laigle A., Turpin P. Y., Taboury J., Taillandier E. Identification of a new electronic transition in the Z form of poly(dG-dC).poly(dG-dC) by infrared absorption and resonance Raman spectroscopy. Biopolymers. 1984 Oct;23(10):1931–1942. doi: 10.1002/bip.360231009. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Peticolas W. L. Melting and premelting phenomenon in DNA by laser Raman scattering. Biopolymers. 1975 Feb;14(2):247–264. doi: 10.1002/bip.1975.360140202. [DOI] [PubMed] [Google Scholar]
- Gupta G., Sarma M. H., Dhingra M. M., Sarma R. H., Rajagopalan M., Sasisekharan V. Poly(dA-dT).poly(dA-dT) in low salt appears to be a left-handed B-helix combined use of chemical theory, fiber diffraction and NMR spectroscopy. J Biomol Struct Dyn. 1983 Oct;1(2):395–416. doi: 10.1080/07391102.1983.10507450. [DOI] [PubMed] [Google Scholar]
- Jollès B., Chinsky L., Laigle A. Contribution of the resonance Raman spectroscopy to the identification of Z DNA. J Biomol Struct Dyn. 1984 Jun;1(6):1335–1346. doi: 10.1080/07391102.1984.10507524. [DOI] [PubMed] [Google Scholar]
- Klug A., Jack A., Viswamitra M. A., Kennard O., Shakked Z., Steitz T. A. A hypothesis on a specific sequence-dependent conformation of DNA and its relation to the binding of the lac-repressor protein. J Mol Biol. 1979 Jul 15;131(4):669–680. doi: 10.1016/0022-2836(79)90196-7. [DOI] [PubMed] [Google Scholar]
- Laigle A., Chinsky L., Turpin P. Y. Recognition of base pairs by polar peptides in double stranded DNA. Nucleic Acids Res. 1982 Mar 11;10(5):1707–1720. doi: 10.1093/nar/10.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E. W., Peticolas W. L. Conformational dependence of the Raman scattering intensities from polynucleotides. 3. Order-disorder changes in helical structures. Biopolymers. 1971;10(8):1377–1418. doi: 10.1002/bip.360100811. [DOI] [PubMed] [Google Scholar]
- Small E. W., Peticolas W. L. Conformational dependence of the Raman scattering intensities from polynucleotides. Biopolymers. 1971;10(1):69–88. doi: 10.1002/bip.360100107. [DOI] [PubMed] [Google Scholar]
- Tomlinson B. L., Peticolas W. L. Conformational dependence of Raman scattering intensities in polyadenylic acid. J Chem Phys. 1970 Feb 15;52(4):2154–2156. doi: 10.1063/1.1673270. [DOI] [PubMed] [Google Scholar]


