Abstract
We have previously demonstrated that hypoxia stimulates adipose-derived stem cells (ASCs) through the generation of reactive oxygen species (ROS). However, the precise mechanism involved in the ROS generation by ASCs is not well understood. We sought to investigate in this work: (1) which subtype of NADPH oxidase (Nox) is primarily expressed in ASCs; (2) where Nox4 is localized in ASCs; and (3) whether silencing of Nox4 attenuates hypoxia-enhanced function of ASC. We used 2′,7′-dichlorofluorescin diacetate (DCF-DA) as an indicator of ROS generation and found that the fluorescence intensity of DCF-DA was significantly increased after hypoxia exposure (2% oxygen). In addition, hypoxia enhanced the proliferation and migration of ASCs and upregulated the mRNA expression of Oct4 and Rex1. Quantitative analysis of mRNA expression of Nox family in ASCs demonstrated that Nox4 is primarily expressed in ASCs, while immunofluorescence assay showed that Nox4 is mainly localized in the perinuclear region and overlaps with Mitotracker, a mitochondria marker. Silencing of Nox4 by siRNA treatment downregulated the RNA and protein expression of Nox4, which significantly reduced the ROS generation under hypoxia. In addition, Nox4 silencing significantly reduced the proliferation and migration of ASCs and downregulated the mRNA expression of Oct4 and Rex1. Phosphorylation of platelet-derived growth factor receptor-β, AKT, and ERK1/2 also diminished following Nox4 silencing. In a nutshell, these results suggest that Nox4 is primarily expressed in ASCs and plays a pivotal role in the hypoxia-enhanced stimulation of ASCs.
Introduction
Adipose-derived stem cells (ASCs) offer a potential source for tissue repair and regeneration. We have recently shown that ASCs and their secretomes have diverse pharmacological effects on skin, for example, acceleration of wound healing and hair growth [1–6]. Hypoxic culturing stimulated ASCs and enhanced their regenerative potential, which is beneficial for ASC therapy [7,8]. Culturing ASCs in a hypoxic incubator (2% oxygen content) increases their proliferation, migration, and secretion of growth factor [8,9]. In a previous study, we investigated the key mediators and the signal pathways involved in the stimulation of ASCs during short-term hypoxia and found that reactive oxygen species (ROS) play a key stimulatory role (ie, ROS scavenger attenuated the stimulation). In addition, increased cellular ROS was accompanied by the activation of platelet-derived growth factor receptor (PDGFR), extracellular signal-regulated kinases (ERK), and AKT (cellular homologue of the viral v-akt oncogene product, also known as a protien kinase B, or PKB) signal pathways [10].
ROS have a significant involvement in cellular apoptosis and contribute to the pathophysiology of many degenerative diseases [11,12]. However, recent studies suggest that low level ROS could act as second messengers and activate cellular processes necessary for normal physiological functioning [13–17]. Most noteworthy of these is the stimulatory effect of ROS on stem cells physiology. Stem cells maintain a high ROS status and are responsive to ROS donors [18,19]. Intracellular ROS can be generated directly by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymes (Nox) and by the passive production of mitochondria [20–23]. These enzymes were initially described in the phagocytes, as a part of their defense against pathogens by generating a Nox-induced superoxide anion. In addition, the generation of ROS by Nox activation was reported in some nonphagocytotic cells as well [10,22–24]. In particular, Nox is one of the major sources of ROS generation in stem/progenitor cells and is activated by growth factors or hypoxia [25–27].
ROS generated from Nox play an important role in redox signaling linked to stem cell mobilization and proliferation [28–30]. Several homologues of gp91phox (also known as Nox2), Nox1, Nox3, Nox4, and Nox5, as well as the dual oxidases (Duox) 1 and 2, have been identified in a number of different tissues [31–33]. Of them, Nox4 is predominantly expressed in ASCs and functions as a switch between proliferation and differentiation of ASCs [34]. Nox4 modulates and generates ROS in insulin signaling and the expression of Nox4 is downregulated in the adipogenic differentiation of ASCs [35,36]. Although it has been known about the essential role of Nox4 in the adipogenic differentiation of ASCs, the Nox4-mediated stimulatory effect on ASCs has not been explored yet.
To clarify the important roles of ROS generation and the underlying mechanism in ASC physiology, this article investigates the following questions: (1) Which subtype of Nox is primarily expressed in ASCs? (2) Where is Nox4 localized in ASCs? (3) Does Nox4 silencing affect hypoxia-enhanced ASC functions? To answer these important questions, we investigated the involvement of Nox4 in ROS generation under hypoxia using small interfering RNA (siRNA) method in addition to the measurement of the expression and localization of Nox families in ASCs.
Materials and Methods
Cell culture and inhibition study
Sampling of human subcutaneous adipose tissue and isolation of ASCs has been previously reported [1,5]. Characterization of ASCs was performed by transdifferentiation and analysis of cell surface markers using flow cytometry. ASCs were grown in Minimum Essential Medium Alpha medium (GIBCO, Invitrogen) with 10% fetal bovine serum (GIBCO), 1% penicillin, and streptomycin (GIBCO) at 37°C in humidified normoxia (5% CO2) and hypoxia (5% CO2, 2% O2, and balanced N2). For the inhibition study of ROS generation, 100 μM N-acetyl-cysteine (NAC, ROS inhibitor; Sigma-Aldrich) and 100 nM dibenziodolium chloride (DPI, NADPH oxidase inhibitors; Sigma) were used in this study.
ROS generation assay
ROS production in ASCs was measured using 2′,7′-dichlorofluorescin diacetate (DCF-DA, Molecular Probes). ASCs (7×105 cells) were seeded in 100mm culture dishes. Cells were pretreated with NAC and DPI for 30–60 min and incubated with DCF-DA (20 μM) for 10 min at 37°C in the dark. After 10-min incubation under hypoxia or normoxia, fluorescence images of the cells were examined for detection of ROS production in cells with a fluorescence microscope (ECLIPSE E600, Nikon). In addition, fluorescence intensity of DCF-DA was measured and calculated using flow cytometry (Becon Dickinson).
Proliferation assay
ASCs were plated on a 48-well plate at a density of 5,000–7,000 cells in complete media. After 24 h, the medium was replaced with serum-free medium containing 1% penicillin and streptomycin. Then, cells were incubated in normoxia (20% O2, 5% CO2) or hypoxia (2% O2, 5% CO2, and balanced N2) with or without chemical inhibitors for 72 h. After incubation, the medium was removed and cell proliferation was measured using CCK-8 assay kit (Dojindo). CCK-8 solution (150 μL) was added to each well and incubated for 2 h. Then the absorbance was measured at 450 nm using a microplate reader (TECAN, Grodig).
Migration assay
For migration assay, ASCs (5×105 cells/well) were seeded in 6-well plates with complete medium. The following day, confluent ASCs were kept in serum-free medium for 12–24 h. Then serum-starved ASCs were wounded using migration micropipette tip and were incubated in hypoxia and normoxia. Cell migration was determined by microscopic examination at 24 h after wounding. For evaluation of ASC migration, 5 randomly selected points along each wound were marked, and the horizontal distance of migrating cells from the point of initial wound was measured.
RT-PCR and quantitative real-time PCR
Total cellular RNA was extracted with TRIzol reagent (Invitrogen), followed by a reverse transcription with cDNA synthesis kit (Promega). cDNA was synthesized from 1 μg total RNA using 200 U of reverse transcriptase (M-MLV RT) and 50 ng/μL oligo(dT). The oligonucleotides used as primers are listed in Table 1. Polymerase chain reactions (PCRs) were performed in a final volume of 25 μL reaction mixture that contained 2 μL of the reverse transcription (RT) reaction mixture, 15 mM MgCl2, 1.25 mM dNTP, 20 pM of each primer, and 0.5 U of Taq polymerase (Promega). Thermal cycling over 40 cycles consisted of an initial denaturation at 94°C for 5 min, then 94°C for 30 seconds, 56°C for 30 s, and 72°C for 30 s and was terminated by a final extension at 72°C for 5 min. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA level was used for sample standardization. Quantitative real-time PCR (Q-PCR) reactions were performed in exicycler 96 cycler (Bioneer) using Quiagen QuantiTect SYBR green PCR kit. Evaluation of fold change was calculated using ΔCt value.
Table 1.
List of Primers for Polymerase Chain Reaction
|
Sequence Target gene |
Forward | Reverse |
|---|---|---|
| Nox1 | 5′-GGCCTATATGATCTGCCTAC-3′ | 5′-GAGATAGGCTGGAGAGAATG-3′ |
| Nox2 | 5′-TGATGAGGAGAAAGATGTGA-3′ | 5′-AGAGTTGGAGATGCTTTGTT-3′ |
| Nox3 | 5′-TAGCTGTTAATGCAACCATC-3′ | 5′-TTGTCCTTAGCAATTCAGTG-3′ |
| Nox4 | 5′-CTTTTGGAAGTCCATTTGAG-3′ | 5′-GTCTGTTCTCTTGCCAAAAC-3′ |
| Nox5 | 5′-TAACATCAAGTGCTACATCG-3′ | 5′-TGCCTGTACATGATACTCTG-3′ |
| Duox1 | 5′-CGACATTGAGACTGAGTTGA-3′ | 5′-CTGGAATGACGTTACCTTCT-3′ |
| Duox2 | 5′-CTCTCTGGAGTGGTGGCCTATT-3′ | 5′-GGACCTGCAGACACCTGTCT-3′ |
| Nanog | 5′-AATAACCTTGGCTGCCGTCTCT-3′ | 5′-AGCAAAGCCTCCCAATCCCAAA-3′ |
| Sox2 | 5′-GGGGAAAGTAGTTTGCTGCCTC-3′ | 5′-CCGCCGCCGATGATTGTTATT-3′ |
| Klf4 | 5′-GGGCAAGTTCGTGCTGAAGG-3′ | 5′-GAAGAGACCGCCTCCTGCTT-3′ |
| c-myc | 5′-CTTGCCGCATCCACGAAACT-3′ | 5′-TGCACCGAGTCGTAGTCGAG-3′ |
| Oct4 | 5′-ACCTTCCAATGTGGAGCATC-3′ | 5′-GAATTTGGCTGGAACTGCA-3′ |
| Rex1 | 5′-GCTTCTTCCGGGCCATTGACTG-3′ | 5′-TTGTAGGGGTCGTAACCCAGCC-3′ |
| Poldip2 | 5′-CCCTGGAAAGCAATAAAGAT-3′ | 5′-AAAGAACCCCACAATCAAAT-3′ |
| GAPDH | 5′-CGAGATCCCTCCAAAATCAA-3′ | 5′-TGTGGTCATGAGTCCTTCCA-3′ |
Antibodies
Antibodies recognizing AKT (1:3,000), phospho-AKT (1:2,000), ERK (1:3,000), phospho-ERK (1:3,000), PDGFR-β (1:2,000) and phospho-PDGFR-β (Y1009, 1:1,000) were purchased from Cell signaling Technology. Nox4 (1:1,000) was purchase from Abcam. Horseradish-peroxidase (HRP)-conjugated secondary mouse antibody (1:10,000) and HRP-conjugated secondary rabbit antibody (1:10,000) were purchased from Santa Cruz Biotechnology.
Western blotting
Proteins were solubilized using sodium dodecyl sulfate (SDS) sampling buffer. Lysates were separated by 10% or 8% SDS-PAGE and transferred to a PVDF membrane (Millipore). The membrane was blocked with 5% skim milk for 1 h at room temperature and then incubated overnight with primary antibody at 4°C. The following day, the membrane was washed with TBS-T (0.1% Tween 20 in Tris-buffered saline) and it was incubated with HRP-conjugated secondary antibody for 1 h at room temperature. The membrane was reacted to enhanced chemiluminescence solution (Millipore) and exposed.
siRNA transfection
ASCs were seeded in 60-mm dishes with complete medium without antibiotics. The following day, confluent ASCs were kept in serum-free medium without antibiotics for 2 h. Then, 50nM Nox4 siRNA was transfected with lipofectamine 2000 (Invitrogen). Three siRNA was obtained from Invitrogen with specific sequences (Nox4 siRNA-1: sense: 5′-UUAU CCAACAAUCUCCUGGUUCUCC-3′; antisense: 5′-GGAGA ACCAGGAGAUUGUUGGAUAA-3′, Nox4 siRNA-2: sense: 5′-ACAGUGAAGACUUUGUUGAACUGAA-3′; antisense: 5′- UUCAGUUCAACAAAGUCUUCACUGU-3′, Nox4 siRNA-3: sense: 5′-CCUCAUGAUCACAGCCUCUACAUAU-3′; antisense: 5′-AUAUGUAGAGGCUGUGAUCAUGAGG-3′). Cells were incubated for 24 or 48 h after siRNA transfection. The outcomes of Nox4 silencing were evaluated by RT-PCR and western blot analysis of Nox4.
Immunostaining of Nox4 and mitochondria
ASCs were seeded on cover slips and incubated at 37°C/5% CO2 for 20 min with 100 nM of Mitotracker or ER tracker (Molecular Probes). After mitochondria staining, cells were fixed with 3.7% paraformaldehyde for 15min. Primary antibody (Nox4) was diluted at 1:200, and secondary antibody (FITC) at 1:400. Nuclear area was stained using 4′,6-diamidino-2-phenylindole (DAPI) (Sigma). Fluorescence signals of different colors were detected by florescence microcopy (ECLIPSE E600).
Fractionation of mitochondria
For fractionation of cytosol and mitochondria, ASCs were seeded in 150-mm dishes at density of 1.5×107 cells. Cells were harvested and Cytosol Extraction Buffer (BioVision) was used to isolate proteins. After lysis, cells were homogenized and centrifuged. Supernatant was collected for cytosolic fraction. Then, the pellet was resuspended and the mitochondrial fraction was isolated using Mitochondrial Extraction Buffer (BioVision). Mitochondrial fractionation was evaluated by western blot analysis using heat shock protein 60 (Hsp60, a mitochondria marker; Stressgen Biotechnology).
Statistical analysis
All data were representative of triplicate independent experiments. The statistical significance of the differences among various groups was tested using the analysis of variance or student's t-test. P<0.05 or P<0.01 were considered to be significant.
Results
ROS generation by hypoxia
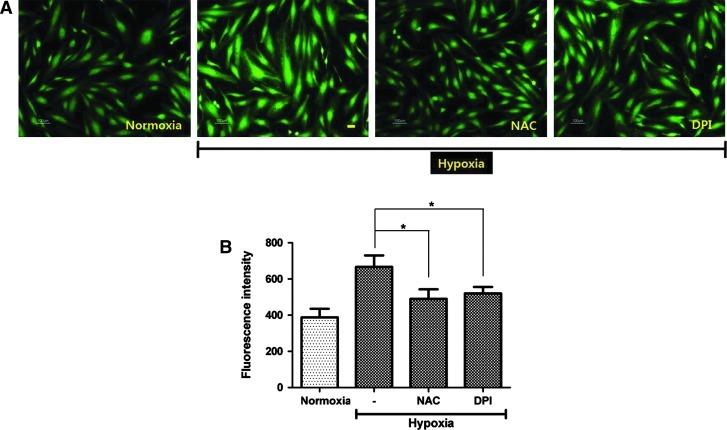
Fluorescence images in Fig. 1A show that acute hypoxia (2% O2 concentration for 10 min) significantly increased the signal intensity of DCF-DA in ASCs (green). In contrast to normoxia, fluorescence intensity of ASCs was significantly increased under hypoxia. In contrst, a NAC (a scavenger of ROS, 100 μM concentration) and a DPI (a Nox inhibitor, 100 nM concentration) significantly reduced the signal intensity of DCF-DA in ASCs. In addition, ROS generation was confirmed and calculated by flow cytometry, and the geometric mean of the fluorescence intensity of DCF-DA was significantly increased in hypoxia-incubated ASCs (Fig. 1B, P<0.05). However, staining of reactive nitrogen species (RNS) with 4,5-diaminofluorescein diacetate showed that acute hypoxia did not change the generation of RNS levels in ASCs (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd). This confirms that oxygen species are primarily generated in response to acute hypoxia in ASCs.
FIG. 1.
ROS generation by hypoxia. (A) The fluorescence microscopy images show that the signal intensity of DCF-DA in ASCs (green) significantly increased under acute hypoxia (2% O2 concentration for 10 min), which was attenuated by NAC (a ROS scavenger, 100 μM concentration) and DPI (a Nox inhibitor, 100 nM concentration) treatment. (B) Relative value for DCF-DA intensity was measured by flow cytometry. *P<0.01. ASCs, adipose-derived stem cells; DCF-DA, 2′,7′-dichlorofluorescin diacetate; DPI, dibenziodolium chloride; NAC, N-acetyl-cysteine; Nox, NADPH oxidase; ROS, reactive oxygen species.
Hypoxia-enhanced proliferation and migration
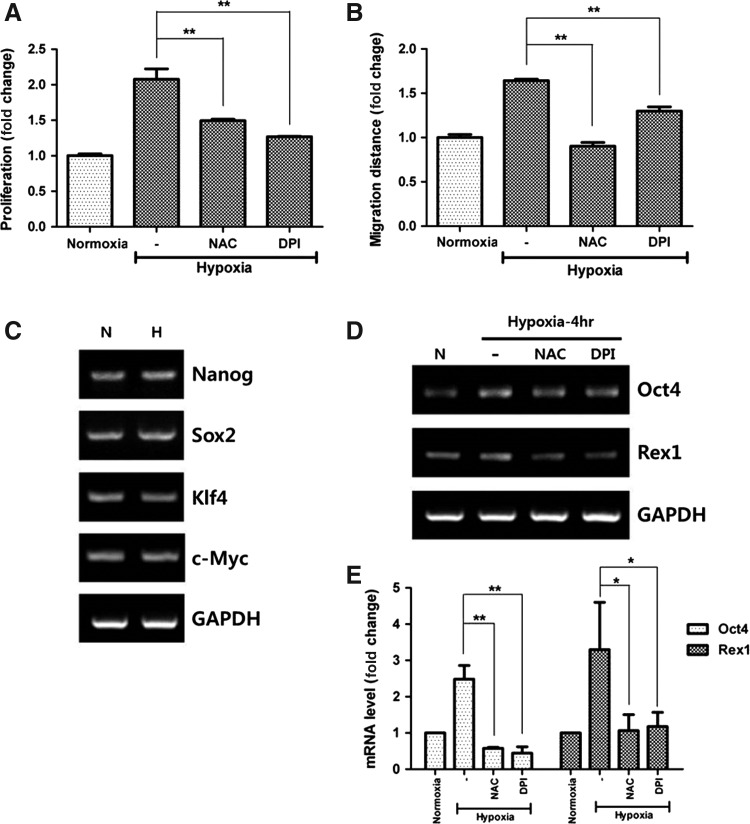
Incubation of ASCs in hypoxia (2% O2) for 72 h significantly increased the proliferation (Fig. 2A, P<0.01) of ASCs, which was in turn reduced after NAC and DPI treatment. Likewise, hypoxia (2% O2 for 24 h) significantly increased the migration of ASCs, but NAC or DPI treatment reduced their migration (Fig. 2B, P<0.01). These results suggest that hypoxia-induced ROS plays a pivotal role in the stimulation of ASCs.
FIG. 2.
ASC stimulation by hypoxia. Hypoxia increased the proliferation (A) and migration (B) of ASCs, which was attenuated by NAC and DPI treatment. In addition, mRNA expression of stemness-associated genes was measured using RT-PCR at 4 h after hypoxia, but there was no difference in Nanog, Sox2, Klf4, and c-Myc (C). However, mRNA expression of Oct4 and Rex1 was significantly upregulated and attenuated by ROS inhibitors (D). Relative mRNA levels were measured and calculated with Q-PCR (E). *P<0.05, **P<0.01. RT-PCR, reverse transcription–polymerase chain reaction; Q-PCR, quantitative real-time PCR.
Upregulation of Oct4 and Rex1 mRNA
In addition, mRNA expression of stemness-associated genes was measured by RT-PCR at 4 h after hypoxia. Even though the expression of Nanog, Sox2, Klf4, and c-Myc was unaltered (Fig. 2C), that of Oct4 and Rex1 was significantly increased by hypoxia and was diminished following NAC and DPI treatment (Fig. 2D). Therefore, mRNA expression of Oct4 and Rex1 was calculated by Q-PCR and significantly upregulated. NAC and DPI showed inhibitory effects on mRNA expression of Oct4 and Rex1 (Fig. 2E; Oct4, P<0.01; Rex1, P<0.05).
Different expression of Nox family members under hypoxia
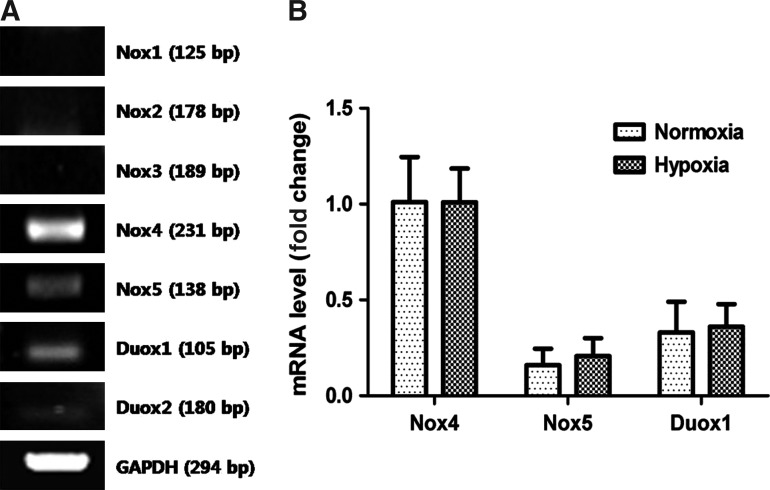
NAC or DPI treatment reduced hypoxia-induced ROS generation and disrupted ASC functioning, which suggests the potential involvement of Nox family in ROS generation by hypoxia. Therefore, the mRNA expression of Nox family in ASCs was measured to clarify which is primarily involved in ROS generation. Figure 3A shows that expression of Nox1-3 and Duox2 was negligible, while that of Nox4, Nox5, and Duox1 was detected after amplification of cDNA for 40 cycles. Relative expression of Nox4, Nox5, and Duox1 was measured by Q-PCR. The data showed that Nox4 is predominantly expressed in ASCs (Fig. 3B). Because expression of Nox family can be regulated by external stimuli such as hypoxia and insulin treatment [35–37], we investigated whether hypoxia modulates mRNA expression of Nox family. However, hypoxia (2% O2 for 4 and 24 h) did not change the mRNA expression of Nox4, Nox5, and Duox1 in ASCs (Fig. 3B).
FIG. 3.
Nox family expression in ASCs. The mRNA expression of Nox family (Nox1-5, Duox1, Doux2) was measured by RT-PCR, and signals for Nox4, Nox5, and Duox1 were detected (A). Relative expression of Nox4, Nox5, and Duox1 was measured using Q-PCR, showing that Nox4 is primarily expressed in ASCs. In addition, Nox4, Nox5, and Duox1 expression did not change under hypoxia (B). Duox, dual oxidase.
Localization of Nox4
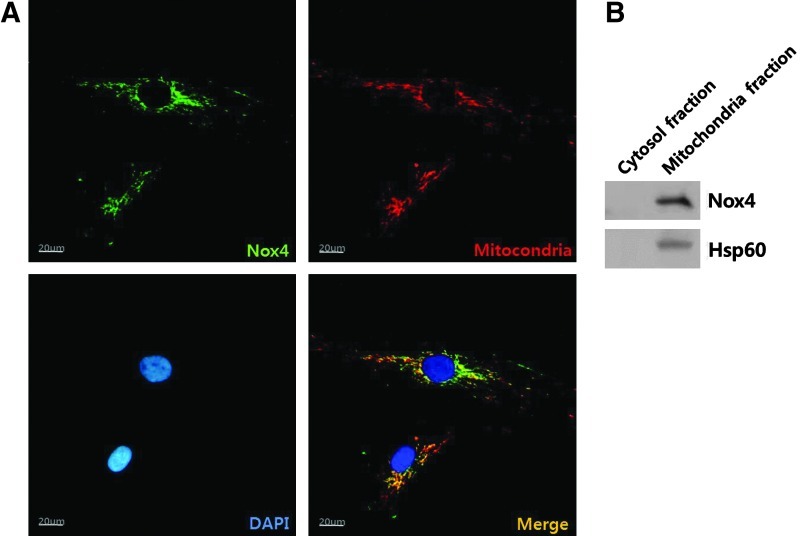
Immunofluorescence was used to detect the localization of Nox4 in ASCs. Nox4 antibody was detected with FITC antibody (green), and cellular organelles were counterstained with DAPI (blue, a nuclear marker), ER-tracker (red, an endoplasmic reticulum marker), and Mitotracker (red, a mitochondria marker). As shown in Fig. 4A, a high intensity of Nox4 signal was detected in perinuclear region and matched well with Mitotracker (orange spots in merged picture). However, Nox4 signal did not merge with ER-tracker (data not shown). In addition, cell fractionation was performed using a mitochondrial fractionation kit. Western blot analysis showed that Nox4 is predominantly localized in the mitochondrial fraction, but was present in negligible amounts in the cytosolic fraction (Fig. 4B). Our data suggest that Nox 4 is primarily expressed in mitochondria of ASCs.
FIG. 4.
Localization of Nox4 was detected by immunofluorescence method. (A) Nox4 signal (green) was high in perinuclear region. Mitochondria were counter-stained with Mitotracker (red), nucleus with DAPI (blue). Nox4 merged with mitochondria (orange spots). (B) Cell fractionation experiment has been performed and was evaluated by western blot analysis using Hsp60. Nox4 was noted to be predominantly localized in the mitochondrial fraction but negligible in the cytosolic fraction.
Decrease in hypoxia-induced ROS generation due to silencing Nox4
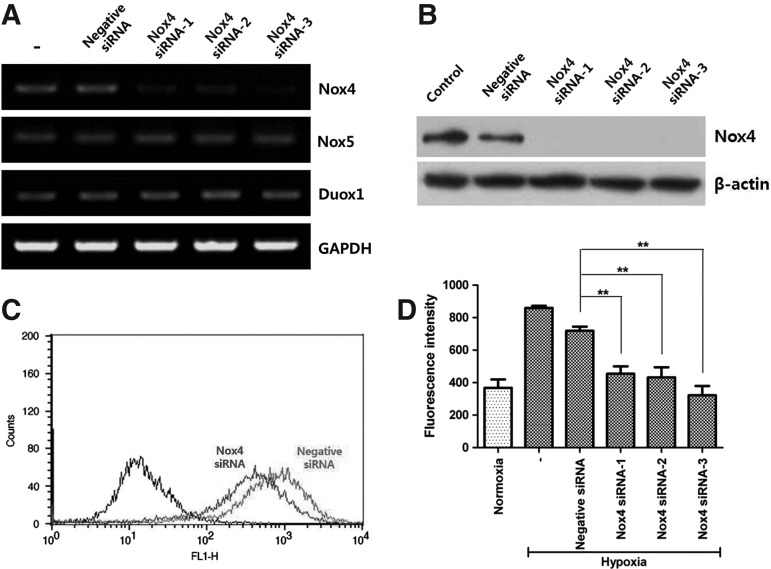
Because Nox4 is primarily expressed in ASCs, and might be a potential candidate of ROS generator, the involvement of Nox4 in ROS generation was further investigated using 3 different siRNAs for Nox4. Transfection of siRNA (50 nM each) with lipofectamine significantly decreased the Nox4 mRNA level as demonstrated by RT-PCR but did not affect Nox5 and Duox1 (Fig. 5A). In addition, Nox4 protein expression was significantly downregulated in western blot analysis (Fig. 5B). ROS generation was measured by Facs analysis and signal intensity for DCF-DA was significantly decreased by Nox4 siRNA transfection (Fig. 5C; first peak, negative control; second peak, Nox4 siRNA treatment; third peak, negative siRNA). The geometric mean of florescence intensity was measured and found that three Nox4 siRNA treatments significantly reduced the ROS generation (Fig. 5D, P<0.01).
FIG. 5.
Silencing of Nox4. Using 3 different siRNAs for Nox4, involvement of Nox4 in ROS generation was investigated. Transfection of siRNA for Nox4 with lipofectamine significantly decreased the Nox4 mRNA level in RT-PCR (A) and the protein expression in western blot analysis (B). ROS generation was measured by Facs analysis and signal intensity of DCF-DA significantly decreased following Nox4 silencing (C) (first peak, negative control; second peak, Nox4 siRNA; third peak, negative siRNA). Geometric mean of fluorescence intensity was measured and three kinds of Nox4 silencing significantly reduced the ROS generation (D). ** P<0.01.
Attenuation of hypoxia-enhanced function by Nox4 silencing
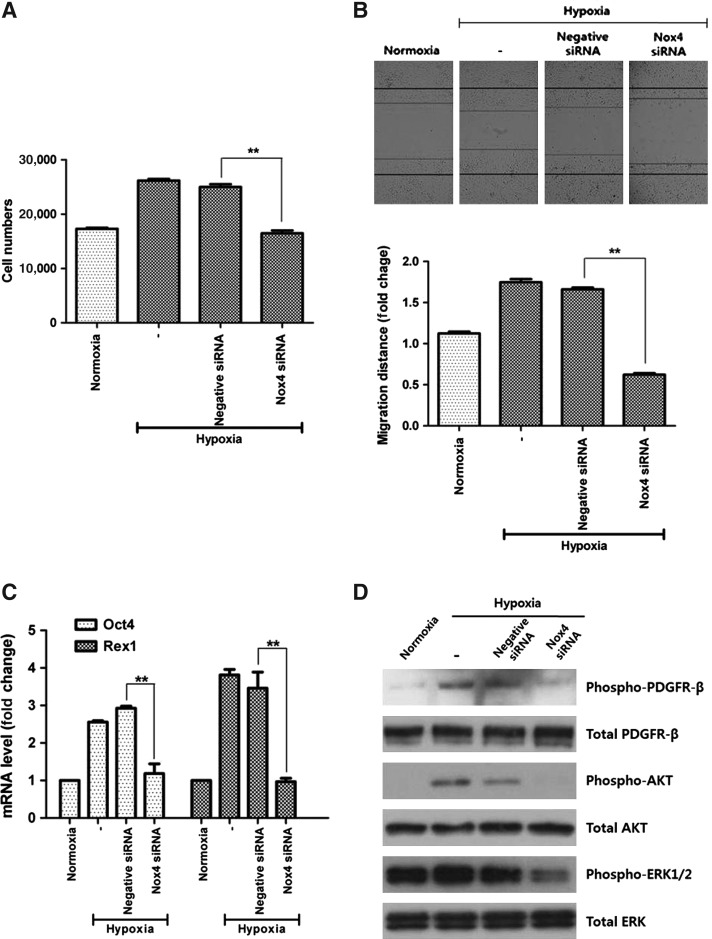
We further examined the involvement of Nox4 in hypoxia-enhanced function of ASCs. As expected, Nox4 silencing by siRNA significantly decreased the hypoxia-enhanced proliferation (Fig. 6A, P<0.01) and migration (Fig. 6B, P<0.01) of ASCs. In addition, mRNA expression of Oct4 and Rex1 was measured by Q-PCR and was seen to be significantly decreased by siRNA transfection (Fig. 6C, P<0.01). These results indicate that Nox4-induced ROS generation plays a significant role in the hypoxia-induced stimulation of ASCs.
FIG. 6.
Functional inhibition by Nox4 silencing. Nox4 silencing by siRNA significantly decreased the hypoxia-enhanced proliferation (A) and migration (B) of ASCs. In addition, mRNA expression of Oct4 and Rex1 also diminished significantly after Nox4 silencing (C). Nox4 silencing significantly attenuated the hypoxia-enhanced phosphorylation of PDGFR-β, AKT, and ERK1/2 molecules (D). **P<0.01. PDGFR, platelet-derived growth factor receptor
Signal pathways mediating Nox4 function
Previously it was shown that hypoxia-induced ROS activated/stimulated the PDGFR, AKT, and ERK-related signal pathways [10]. Therefore, we investigated whether these signal pathways are affected by the silencing of Nox4 enzyme. As shown in Fig. 6D, acute hypoxia (10 min) increased the phosphorylation of PDGFR-β, AKT, and ERK1/2 molecules. However, Nox4 silencing by siRNA transfection significantly reduced the hypoxia-enhanced phosphorylation of PDGFR-β, AKT, and ERK1/2.
Discussion
Low oxygen tension (2–8% of O2) is an important characteristic of the stem cell niche, and hypoxia provides an adequate environment for the maintenance of stem cell properties [38,39]. In addition, hypoxia enhances the proliferative and self-renewal capacities of mesenchymal stem cells in vitro [40]. Suga et al. established surgically induced ischemia models by disrupting blood supply, which induced injury/death of cells including adipocytes, vascular endothelial cells, and blood-derived cells. However, hypoxia induced the proliferation of ASCs, indicating that hypoxia also acts as a stimulator in vivo [39]. Although hypoxia is considered to be beneficial for ASC maintenance, ASCs are routinely cultured in normoxia (20% O2). As both hypoxia and ROS generation stimulates the proliferation, migration, and regenerative potential of ASCs under well-controlled conditions, it could be beneficial to expose ASCs to hypoxia during cell manipulation. In addition to the economic benefit in cost reduction for ASC culture expansion, hypoxic preconditioning is potentially beneficial for clinical application owing to the enhancement of ASC regenerative capacity.
ROS are known to serve as a second messenger in the intracellular signal transduction pathway for a variety of cellular processes, including inflammation, cell cycle progression, apoptosis, aging, and cancer [29,41,42]. Although excess accumulation of ROS exhibits adverse effects on the cellular homeostasis, there is conflicting evidence that ROS at low levels promote cell proliferation and survival [10,43,44]. Therefore, better understanding the complex role of ROS in ASCs physiology is essential for clarify the key factors involved in the dual aspects of ROS. It is likely that the determining factor as to whether ROS is harmful or beneficial to ASCs is primarily dependent on the concentration. The low/moderate ROS generation may act as a stimulus for ASC function. To test this hypothesis, we investigated the effects of various ROS donors whether they stimulate or damage ASCs. As expected, a low dose of antimycin (0.01–10 μM concentration) significantly increased, while a higher dose (>100 μM concentration) decreased, the proliferation and migration of ASCs (our unpublished data). Likewise, low dose rotenone (<1 nM concentration) significantly increased the proliferation and migration of ASCs. Therefore, intracellular level of ROS is the key factor for ASC proliferation and migration of ASCs, which should be considered for ASC maintenance.
Vascular endothelial growth factor (VEGF) stimulates the growth of newly formed blood vessels and plays a role in the tissue repair system by restoring the oxygen supply to the tissues. In addition, VEGF acts as a key paracrine mediator of ASC function and an increased VEGF secretion is closely correlated with enhanced paracrine effect of ASCs. Interestingly, VEGF expression is regulated by ROS generation [10,45]. For example, hypoxia markedly increases the VEGF mRNA level, which was attenuated by the ROS scavenging and the inhibition of NADPH oxidase (Supplementary Fig. S2A, B). In addition, lovastatin inhibited ROS generation by Nox4 enzyme and downregulated VEGF expression [45]. Therefore, we investigated whether Nox4 affected VEGF expression in ASCs and found that Nox4 silencing reduced the mRNA expression of VEGF (Supplementary Fig. S2C). These results suggest that Nox4 is involved in the upregulation of VEGF and may modulate the paracrine effects of ASCs.
There exists a controversial view on the subcellular localization of Nox4 [46–48]. Chen et al. detected the Nox4 expression in endoplasmic reticulum (ER) of endothelial cells and insisted that ER localization of Nox4 is critical for the regulation of protein tyrosine phosphatase 1B [46]. However, Nox4 signal did not match with ER marker in ASCs in our study (data not shown), indicating that Nox4 is not highly expressed in ER. Instead, Block et al. generated specific Nox4 antibody and reported that Nox 4 is highly expressed in mitochondria of kidney cells using immunoblot anlysis and immunofluorescence assay [48]. They merged Nox4 signal with an ER marker and a Golgi marker, but the Nox4 signal did not match with these organelles. Graham et al. also reported that Nox4 is overexpressed in breast cancer cell lines and is mainly localized in the mitochondria [47]. Our immunofluorescence data (Fig. 4) provides evidence that a functional Nox4 is largely localized to and regulated by mitochondria in ASCs. Therefore, this organelle may be an important source of ROS.
We have previously demonstrated that hypoxia stimulates ASCs through ROS generation [10]. However, the precise mechanism involved in the ROS synthesis of ASCs has not yet been established. Therefore, we looked for the specific subtype of Nox that is primarily expressed and localized in ASCs, and whether Nox4 silencing affected hypoxia-enhanced ASC functions. Quantitative analysis of Nox family in ASCs demonstrated the predominant Nox4 expression. Immunofluorescence showed that Nox4 is highly localized in the perinuclear region, especially in mitochondria. Nox4 silencing reduced the generation of ROS, notably reduced the proliferation/migration of ASCs, and downregulated the mRNA expression of Oct4 and Rex1. Phosphorylation of PDGFR-β, AKT, and ERK1/2 was reduced by Nox4 silencing. Collectively, these results espouse that Nox4 is primarily involved in the hypoxia-enhanced stimulation of ASCs.
Supplementary Material
Acknowledgments
This study was primarily supported by a grant of basic Science Research Program through the National Research Foundation of Korea (2011-0019636). Y.X. is partially supported by NIH (HD-034852, AT-004422).
Author Disclosure
The authors declare no competing financial interests.
References
- 1.Kim WS. Park BS. Sung JH. Yang JM. Park SB. Kwak SJ. Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Kim WS. Park BS. Sung JH. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin Biol Ther. 2009;9:879–887. doi: 10.1517/14712590903039684. [DOI] [PubMed] [Google Scholar]
- 3.Won CH. Yoo HG. Kwon OS. Sung MY. Kang YJ. Chung JH. Park BS. Sung JH. Kim WS. Kim KH. Hair growth promoting effects of adipose tissue-derived stem cells. J Dermatol Sci. 2010;57:134–137. doi: 10.1016/j.jdermsci.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Yang JA. Chung HM. Won CH. Sung JH. Potential application of adipose-derived stem cells and their secretory factors to skin: discussion from both clinical and industrial viewpoints. Expert Opin Biol Ther. 2010;10:495–503. doi: 10.1517/14712591003610598. [DOI] [PubMed] [Google Scholar]
- 5.Kim WS. Park BS. Park SH. Kim HK. Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009;53:96–102. doi: 10.1016/j.jdermsci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Kim WS. Park SH. Ahn SJ. Kim HK. Park JS. Lee GY. Kim KJ. Whang KK. Kang SH. Park BS. Sung JH. Whitening effect of adipose-derived stem cells: a critical role of TGF-beta 1. Biol Pharm Bull. 2008;31:606–610. doi: 10.1248/bpb.31.606. [DOI] [PubMed] [Google Scholar]
- 7.Chung HM. Won CH. Sung JH. Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential. Expert Opin Biol Ther. 2009;9:1499–1508. doi: 10.1517/14712590903307362. [DOI] [PubMed] [Google Scholar]
- 8.Lee EY. Xia Y. Kim WS. Kim MH. Kim TH. Kim KJ. Park BS. Sung JH. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 9.Song SY. Chung HM. Sung JH. The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther. 2010;10:1529–1537. doi: 10.1517/14712598.2010.522987. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH. Park SH. Park SG. Choi JS. Xia Y. Sung JH. The Pivotal Role of Reactive Oxygen Species Generation in the Hypoxia-Induced Stimulation of Adipose-Derived Stem Cells. Stem Cells Dev. 2011;2011;20:1753–1761. doi: 10.1089/scd.2010.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Follezou JY. Emerit J. Bricaire F. Neuro-degenerative diseases: role of reactive oxygen species and of apoptosis. Presse Med. 1999;28:1661–1666. [PubMed] [Google Scholar]
- 12.Knight JA. Reactive oxygen species and the neurodegenerative disorders. Ann Clin Lab Sci. 1997;27:11–25. [PubMed] [Google Scholar]
- 13.Vieira HL. Alves PM. Vercelli A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog Neurobiol. 2011;93:444–455. doi: 10.1016/j.pneurobio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Hamanaka RB. Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z. Li Y. Sarkar FH. Signaling mechanism(s) of reactive oxygen species in epithelial-mesenchymal transition reminiscent of cancer stem cells in tumor progression. Curr Stem Cell Res Ther. 2010;5:74–80. doi: 10.2174/157488810790442813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccoli C. D'Aprile A. Scrima R. Ripoli M. Boffoli D. Tabilio A. Capitanio N. Role of reactive oxygen species as signal molecules in the pre-commitment phase of adult stem cells. Ital J Biochem. 2007;56:295–301. [PubMed] [Google Scholar]
- 17.Sauer H. Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal. 2005;7:1423–1434. doi: 10.1089/ars.2005.7.1423. [DOI] [PubMed] [Google Scholar]
- 18.Valle-Prieto A. Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19:1885–1893. doi: 10.1089/scd.2010.0093. [DOI] [PubMed] [Google Scholar]
- 19.Dernbach E. Urbich C. Brandes RP. Hofmann WK. Zeiher AM. Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y. Lou MF. The regulation of NADPH oxidase and its association with cell proliferation in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:2291–2300. doi: 10.1167/iovs.08-2568. [DOI] [PubMed] [Google Scholar]
- 21.Hu R. Wang YL. Edderkaoui M. Lugea A. Apte MV. Pandol SJ. Ethanol augments PDGF-induced NADPH oxidase activity and proliferation in rat pancreatic stellate cells. Pancreatology. 2007;7:332–340. doi: 10.1159/000105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambeth JD. Krause KH. Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 23.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 24.Sundaresan M. Yu ZX. Ferrans VJ. Irani K. Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 25.Ushio-Fukai M. Urao N. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal. 2009;11:2517–2533. doi: 10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange S. Heger J. Euler G. Wartenberg M. Piper HM. Sauer H. Platelet-derived growth factor BB stimulates vasculogenesis of embryonic stem cell-derived endothelial cells by calcium-mediated generation of reactive oxygen species. Cardiovasc Res. 2009;81:159–168. doi: 10.1093/cvr/cvn258. [DOI] [PubMed] [Google Scholar]
- 27.Urao N. Inomata H. Razvi M. Kim HW. Wary K. McKinney R. Fukai T. Ushio-Fukai M. Role of Nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res. 2008;103:212–220. doi: 10.1161/CIRCRESAHA.108.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novo E. Busletta C. Bonzo LV. Povero D. Paternostro C. Mareschi K. Ferrero I. David E. Bertolani C, et al. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells. J Hepatol. 2011;54:964–974. doi: 10.1016/j.jhep.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Coant N. Ben Mkaddem S. Pedruzzi E. Guichard C. Treton X. Ducroc R. Freund JN. Cazals-Hatem D. Bouhnik Y, et al. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroder K. Kohnen A. Aicher A. Liehn EA. Buchse T. Stein S. Weber C. Dimmeler S. Brandes RP. NADPH oxidase Nox2 is required for hypoxia-induced mobilization of endothelial progenitor cells. Circ Res. 2009;105:537–544. doi: 10.1161/CIRCRESAHA.109.205138. [DOI] [PubMed] [Google Scholar]
- 31.Glian'ko AK. Ishchenko AA. Structural and functional characteristics of plant NADPH oxidase: a review. Prikl Biokhim Mikrobiol. 2010;46:509–518. [PubMed] [Google Scholar]
- 32.Leusen JH. Verhoeven AJ. Roos D. Interactions between the components of the human NADPH oxidase: a review about the intrigues in the phox family. Front Biosci. 1996;1:d72–90. doi: 10.2741/a117. [DOI] [PubMed] [Google Scholar]
- 33.Rada BK. Geiszt M. Hably C. Ligeti E. Consequences of the electrogenic function of the phagocytic NADPH oxidase. Philos Trans R Soc Lond B Biol Sci. 2005;360:2293–2300. doi: 10.1098/rstb.2005.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroder K. Wandzioch K. Helmcke I. Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29:239–245. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- 35.Mouche S. Mkaddem SB. Wang W. Katic M. Tseng YH. Carnesecchi S. Steger K. Foti M. Meier CA, et al. Reduced expression of the NADPH oxidase NOX4 is a hallmark of adipocyte differentiation. Biochim Biophys Acta. 2007;1773:1015–1027. doi: 10.1016/j.bbamcr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Mahadev K. Motoshima H. Wu X. Ruddy JM. Arnold RS. Cheng G. Lambeth JD. Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diebold I. Petry A. Hess J. Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohyeldin A. Garzon-Muvdi T. Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Suga H. Eto H. Aoi N. Kato H. Araki J. Doi K. Higashino T. Yoshimura K. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010;126:1911–1923. doi: 10.1097/PRS.0b013e3181f4468b. [DOI] [PubMed] [Google Scholar]
- 40.Wang DW. Fermor B. Gimble JM. Awad HA. Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–191. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 41.Wang J. Essner E. Shichi H. Ultrastructural and immunocytochemical studies of smooth muscle cells in iris arterioles of rats with experimental autoimmune uveoretinitis. Exp Mol Pathol. 1994;61:153–163. doi: 10.1006/exmp.1994.1033. [DOI] [PubMed] [Google Scholar]
- 42.Jee MK. Kim JH. Han YM. Jung SJ. Kang KS. Kim DW. Kang SK. DHP-derivative and low oxygen tension effectively induces human adipose stromal cell reprogramming. PloS One. 2010;5:e9026. doi: 10.1371/journal.pone.0009026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiarugi P. Fiaschi T. Redox signalling in anchorage-dependent cell growth. Cell Signal. 2007;19:672–682. doi: 10.1016/j.cellsig.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal. 2006;8:1765–1774. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- 45.Li J. Wang JJ. Yu Q. Chen K. Mahadev K. Zhang SX. Inhibition of reactive oxygen species by Lovastatin downregulates vascular endothelial growth factor expression and ameliorates blood-retinal barrier breakdown in db/db mice: role of NADPH oxidase 4. Diabetes. 2010;59:1528–1538. doi: 10.2337/db09-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen K. Kirber MT. Xiao H. Yang Y. Keaney JF., Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham KA. Kulawiec M. Owens KM. Li X. Desouki MM. Chandra D. Singh KK. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther. 2010;10:223–231. doi: 10.4161/cbt.10.3.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Block K. Gorin Y. Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.