Abstract
During embryonic cartilage development, proliferation and differentiation are tightly linked with a transient cell cycle arrest observed during determination and before main extracellular matrix production. Aim of this study was to address whether these steps are imitated during in vitro differentiation of mesenchymal stem cells (MSCs) and are crucial for a proper chondrogenesis. Human MSCs were expanded in distinct media and subjected to pellet culture in chondrogenic medium. Cells were labeled with 5-iodo-2′-deoxyuridin (IdU) or treated with mitomycin C at various time points during culture. Apoptosis was detected by cleaved caspase 3. Proliferation rate of expanded MSCs at start of pellet culture showed a positive correlation with chondrogenesis according to DNA content, proteoglycan deposition, collagen type II content, and final pellet size. Evenly distributed IdU signals at day 1 diminished and became restricted primarily to the periphery by day 3. Between days 10 and 21, IdU-positive cells were detected throughout coinciding with collagen type II positivity. Little IdU incorporation occurred after day 21 and in areas of strong matrix deposition. DNA content decreased and apoptosis was detected up to day 14. Irreversible growth arrest by mitomycin C fully blocked chondrogenic differentiation and seemed to arrest differentiation at the stage reached at treatment. In conclusion, chondrogenesis involved a transient proliferation phase appearing simultaneously with start of collagen type II deposition and growth was crucial for proper chondrogenesis. Growth and differentiation steps, thus, seemed closely coordinated and resembled, with respect to proliferation, stages known from embryonic cartilage development. Stimulation of proliferation and prevention of early apoptosis are attractive goals to further improve MSC chondrogenesis.
Introduction
Mesenchymal stem cells (MSCs) are multipotent, self-renewing cells that are promising for cartilage regeneration strategies. A still unsolved problem of chondrocyte production from MSCs is, however, the imitation of embryonic pathways of articular chondrocyte development in order to generate hyaline cartilage. MSCs are differentiated into chondrocytes by inducing them in a micromass pellet culture system in chondrogenic medium containing transforming growth factor beta (TGF-β) [1,2]. One primary advantage of MSCs is their easy availability from bone marrow aspirates [3] and other tissues like fat [4] or synovial membrane [5,6]. By using MSCs for cartilage regeneration strategies, there is no need for a joint surgery to harvest the cells and by means of extensive expansion a large number of cells can be generated for application. A remaining challenge of MSC-based cartilage repair strategies is the limited size of MSC-derived constructs, the apparent loss of cells during differentiation and the unwanted upregulation of hypertrophic markers which suggest that natural embryonic pathways of articular chondrocyte differentiation are not recapitulated [7,8].
Growth and differentiation are tightly linked during embryonic development, and when cartilage develops from mesenchymal progenitors, cells undergo extensive proliferation followed by condensation [9,10], a process that increases the cell density and leads to enhanced cell–cell contacts [11–13]. When the cell-per-volume ratio is high enough, cells that are located centrally of the condensed structure withdraw from the cell cycle, stop proliferation, and establish cartilaginous nodules [14]. These contain the chondroprogenitor cells that, after a transient exit from the cell cycle, resume cell division and produce components of the extracellular cartilage matrix, especially proteoglycans and collagen type II [15–17]. In permanent articular cartilage, chondrocytes differentiate terminally into mature chondrocytes with column formation [18,19]. In the transient cartilage formed during endochondral bone formation, these cells undergo a hypertrophic development where they increase in size and upregulate markers like collagen type X and alkaline phosphatase (ALP) [20].
It is currently unclear whether the same linkage of proliferation and differentiation found in natural chondrocyte development is recapitulated by common models of MSC in vitro chondrogenesis. While the in vitro expansion phase of MSCs may correspond to the initial proliferation of mesenchymal progenitors before condensation, the natural condensation step is imitated upon switching to the high-density pellet system. It is so far unclear, whether proliferation stops after condensation until cells are designated chondroblasts, as suggested for mesenchymal progenitors in vivo [16], and restarts during pellet growth and cartilage matrix deposition. Knowledge on the proper regulation of cell proliferation during cell determination and chondrogenesis and the relevance of growth for the success of differentiation seem mandatory for better in vitro guidance of MSCs in order to produce larger constructs of a stable chondrocytic phenotype for MSC-based tissue engineering approaches.
The aim of this study was to address whether growth and differentiation steps manifest during embryonic cartilage development are emulated during chondrogenic in vitro differentiation of MSCs, to contribute information of value toward optimizing chondrogenic induction protocols. We asked whether the proliferation rate of MSCs before high-density culture influences the outcome of chondrogenic differentiation, and whether proliferation ceases after the transition from expansion to chondrogenic differentiation conditions or restarts, as seen in vivo after prechondroblast establishment. By visualizing dividing MSCs in differentiating micromass pellets by 5-iodo-2′-deoxyuridin (IdU) labeling, we establish areas of active growth in MSC pellets. By blocking proliferation by the DNA crosslinking reagent mitomycin C, we demonstrate the relevance of proliferative capacity on the success of chondrogenesis.
Materials and Methods
Isolation and expansion of MSCs
MCS were isolated from fresh bone marrow aspirates of overall 10 donors undergoing total hip replacement. The studies were approved by the local ethics committee and informed consent was obtained from all individuals included in the study. Cells were fractionated by Ficoll density centrifugation and the mononuclear cell fraction was counted, divided into 4 equal parts, and seeded into culture flasks in 4 different expansion media. The control medium [21] composed of Dulbecco's modified Eagle's medium (DMEM) high glucose (Gibco, Invitrogen), 2% fetal calf serum (FCS) (Seromed/Biochrom), 40% MCDB201, 2×10−8 M dexamethasone, 10−4 M ascorbic acid-2 phosphate, 2% ITS supplement (all Sigma-Aldrich), 100 units/mL penicillin and 100 μg/mL streptomycin (Biochrom AG), 10 ng/mL recombinant human epidermal growth factor (Miltenyi), and recombinant human platelet-derived growth factor BB (Active Bioscience). The second medium, adopted from embryonic stem cell expansion (ES medium), was composed of DMEM high glucose, 12.5% FCS, 2 mM L-glutamin, 50 μM β-mercaptoethanol, 1% nonessential amino acids (all Gibco, Invitrogen), 100 units/mL penicillin and 100 μg/mL streptomycin, and 4 ng/mL basic fibroblast growth factor (bFGF) (Active Bioscience). The same medium was conditioned for 48 h by primary mouse embryo fibroblasts (PMEF-NL; Millipore) and mixed with 66% unconditioned ES medium to yield conditioned ES medium. The fourth medium was serum-free [knockout DMEM, 20% knockout serum replacement, 1 mM L-glutamin, 100 μM β-mercaptoethanol, 1% nonessential amino acids, and 4 ng/mL bFGF] mixed with 33% of PMEF-conditioned medium. The mononuclear cell fraction was seeded at a density of 0.125 Mio MNC per cm2 in 0.1% gelatine-coated flasks and maintained at 37°C in a humidified atmosphere and 6% CO2. After 24 h the cells were washed with phosphate-buffered saline (PBS) to remove nonadherent cells. MSCs were cultured up to passage 3 at a density of 5,000 cells/cm2 for the differentiation experiments. At every passage cell number and culture time were noted in order to measure the generation time.
3H-thymidine labeling
After expansion under 4 distinct conditions, 12,500 MSCs per cm2 were seeded in triplicates in adequate media. After 24 h medium was replaced by fresh medium containing 0.025 μCi 3H-thymidine. After labeling for 18 h the cells were intensively washed, lysed, and 3H-thymidine uptake was measured by a WinSpectral Analyzer.
Induction of in vitro chondrogenesis
Cells from passage 3 were harvested with trypsin/ethylenediaminetetraacetic acid (EDTA) and pellets consisting of 5×105 MSCs were formed in 1.5 mL Eppendorf reaction tubes by centrifugation (50 g for 5 min). Chondrogenic induction medium consisted of DMEM high glucose supplemented with 0.1 μM dexamethasone, 0.17 mM ascorbic acid 2-phosphate, 5 μg/mL transferrin, 5 ng/mL selenous acid, 1 mM sodium pyruvate, 0.35 mM proline, 1.25 mg/mL bovine serum albumin (BSA), 100 units/mL penicillin, 100 μg/mL streptomycin, 5 μg/mL insulin (Sanofi-Aventis), and 10 ng/mL TGF-β1 (Peprotech). Pellets were cultured for 6 weeks with medium changed 3 times a week.
Collagen type II immunohistochemistry
Pellets were fixed in 4% paraformaldehyde (PFA) for 2 h and staining procedures were performed using standard protocols. Sections (5 μm) were stained with 1% alcian blue (Chroma) for proteoglycans. Immunohistological staining was performed as described previously (Winter et al. 2003) [22]. Briefly, sections were pretreated with 2 mg/mL hyaluronidase (Merck) and 1 mg/mL pronase (Roche Diagnostics). PBS containing 5% BSA was used to block nonspecific background. Sections were incubated overnight at 4°C with a 1:1,000 diluted monoclonal mouse anti-human collagen type II antibody (II-4C11; ICN Biomedicals) in PBS containing 1% BSA. Reactivity was detected using biotinylated goat anti-mouse secondary antibody (1:500; 30 min, RT; Dianova), streptavidin-ALP (Dako; 30 min, 20°C), and fast red (Sigma-Aldrich).
Caspase 3 immunohistochemistry
PFA-fixed paraffin-embedded sections were rehydrated and microwaved with 0.01 M sodium citrate for 25 min. After blocking in 5% BSA, samples were incubated with a 1:100 diluted polyclonal-rabbit anti-active caspase 3 antibody (Abcam) for 1 h at 37°C. The secondary antibody FITC-goat anti-rabbit (Jackson Immunoresearch) was diluted 1:250 and sections were incubated for 1 h at room temperature. All sections were stained with Hoechst 33258 (Biotium) as nuclear staining and directly analyzed by fluorescence microscopy.
Quantification of proteoglycan content
Pellets (n=4–5 donors, 2 pellets per donor) were washed with PBS and fixed with 100 μL methanol at −20°C. After washing, pellets were incubated with 0.5% alcian blue solution in 1 M HCl overnight. Next day pellets were washed extensively with distilled water and alcian blue was extracted by 200 μL of 6 M guanidine hydrochloride (Sigma) for 5 h. The optical density of the extracted dye was measured at 650 nm.
Collagen type II extraction and ELISA
Pellets (n=4–5 donors, 2 pellets per donor) were digested with pepsin solution [2.5 mg pepsin/mL pepsin buffer (0.5 M acetic acid, 0.2 M NaCl)] for at least 16 h. Digest solution was neutralized with 1 M Tris Base (Roth), 4.5 M NaCl (Roth) was added, and the solution was rotated overnight at 4°C. After centrifugation, the supernatant was discarded, the pellets were resuspended in 400 μL precipitation buffer (0.1 M Tris Base and 0.4 M NaCl), and the collagens were precipitated for 4 h at −20°C with 100% ethanol. After centrifugation, the supernatant was discarded and the pellets were resuspended in lysis buffer (50 mM Tris, 150 mM NaCl, and 1% Triton X-100). The collagen type II content was measured by native type II collagen detection ELISA (Chondrex) according to manufacturer's instructions.
Quantification of DNA content
The DNA content of pellets prepared with MSCs derived from 3 donors was determined using the Quanti-iT PicoGreen dsDNA kit (Invitrogen) according to the manufacturer's instructions. For this purpose pellets were harvested at day 42 of chondrogenic induction and predigested in 1 mL of Tris-HCl buffer (0.05 M Tris, 1 mM CaCl2, pH 8.0) with 500 μg/mL proteinase K (Roche) overnight at 60°C. The next day samples were analyzed by mixing 20 μL of the digested sample with 80 μL TE buffer (200 mM Tris HCl and 20 mM EDTA) and PicoGreen solution. Fluorescence measurement was carried out at 485/535 nm.
IdU labeling and detection
To visualize cell proliferation in pellets at days 1, 2, 3, 7, 10, 14, 21, 28, and 35 of chondrogenic induction, cells of 5 donors were incubated with 20 μM IdU (MP Biomedicals) for 24 h at different time points, washed with PBS, and harvested for histology. The thymidine analog IdU was integrated into DNA during DNA replication and was detected by immunohistochemistry according to Teta and colleagues [23]. In brief, formalin-fixed pellets were cut into 5 μM sections and sections were rehydrated in ethanol. The nuclear membrane was permeabilized with 0.2% Triton X-100 and the sections were microwaved with 0.01 M sodium citrate. After blocking in donkey serum, samples were incubated with a mouse anti-BrdU antibody (Becton Dickison)—diluted 1:100, overnight at 4°C. The secondary antibody Cy3-donkey anti-mouse (Jackson Immunoresearch) was diluted 1:750 and sections were incubated for 1 h. The sections were stained with DAPI as nuclear staining and analyzed by fluorescence microscopy.
Mitomycin C treatment
Proliferation of MSCs, either in monolayer or in chondrogenic pellet culture of days 1, 7, 10, 14, 21, 28, and 35, was blocked by a 2-h treatment with 10 μg/mL mitomycin C (Sigma) in the corresponding culture medium [24–26]. Afterward, MSCs or pellets were washed twice with PBS while the monolayer-treated MSCs were used for pellet formation and shifted to chondrogenic culture conditions for 6 weeks. Pellet cultures were continued in chondrogenic medium until day 42 after start of induction. Influence of mitomycin C treatment on collagen type II deposition was evaluated semiquantitatively by measuring the collagen type II–stained area of mitomycin C–treated pellets compared with untreated control pellets, both harvested at day 42. Differences in stained areas between the groups were given as percentage of differentiation blocking.
Statistics
Data are presented as mean±standard deviation. Data were analyzed statistically by using Mann–Whitney U-test and P<0.05 was considered significant. Data analysis was performed with SPSS for Windows 16.0 (SPSS, Inc.).
Results
Positive correlation between proliferation and chondrogenesis
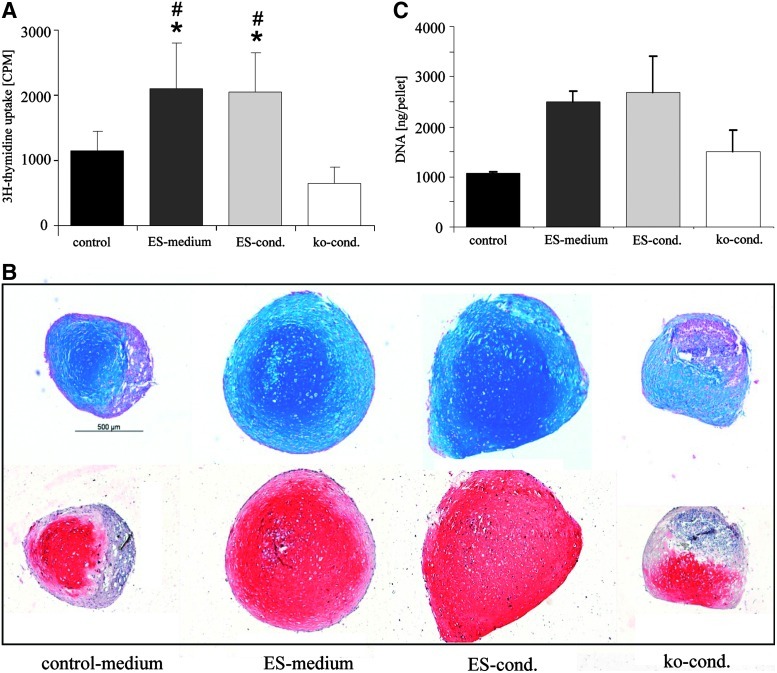
The proliferation rate of MSCs during culture expansion was modulated by growing MSCs from the same donor under 4 different culture conditions, 3 of which were adapted from embryonic stem cell expansion. According to DNA synthesis measured by 3H-thymidine uptake at passage 2 or 3 (n=3 donors per medium group), the proliferation rate of MSCs was significantly higher in ES and conditioned ES medium compared with knockout conditioned (ko-cond) medium and control medium (Fig. 1A).
FIG. 1.
Positive correlation between proliferation and chondrogenesis. (A) MSCs of 3 donors were expanded up to passage 2 or 3 in 4 different media. About 12,500 MSCs per cm2 were seeded in triplicates and cultured for 1 day before cells were labeled with 3H-thymidine. (B) MSCs from 5 donors were cultured in 4 different media up to passage 3 and pellets consisting of 5×105 MSCs were subjected to chondrogenic induction for 6 weeks. Paraffin-embedded sections were stained for proteoglycan deposition by alcian blue shown in the upper panel and collagen type II was detected by immunohistology shown in the lower panel. (C) DNA content of representative pellets of each group was determined by PicoGreen assay. Mean±s.d., *P<0.05 compared with control medium. #P<0.05 compared with ko-cond. (Mann–Whitney U-test). MSCs, mesenchymal stem cells; ko-cond, knockout conditioned. Color images available online at www.liebertonline.com/scd
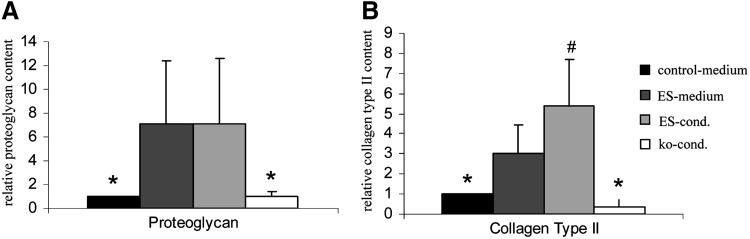
When cells of all 4 groups were subjected to chondrogenesis in micromass pellet culture for 6 weeks, the faster growing cells from both ES media exhibited a stronger differentiation according to proteoglycan and collagen type II deposition and a larger pellet size (Fig. 1B). After 6 weeks of chondrogenic differentiation, ES media–expanded groups contained more DNA than pellets consisting of cells from control or ko-cond medium (Fig. 1C). Accordingly, the proteoglycan and collagen type II content per pellet (n=4–5 donors, 2 pellets per donor and group) was significantly higher in pellets of the ES medium or conditioned ES medium groups compared with both other groups (P<0.05). Remarkably, conditioning of ES medium had a positive influence on collagen type II but not on proteoglycan deposition (Fig. 2). In summary, faster growing cells showed a superior chondrogenic differentiation suggesting a positive correlation between proliferation rate before start of high-density culture and chondrogenesis, which may be attributed to a number of factors in these highly complex media. All following experiments were performed with the nonconditioned ES medium.
FIG. 2.
Enhanced proteoglycan and collagen type II deposition by ES-expanded MSC pellets. MSCs of 5 donors were expanded up to passage 3 in 4 different media and MSCs of each medium group were subjected to chondrogenic differentiation for 6 weeks. (A) Proteoglycan deposition was estimated by alcian blue assay and (B) collagen type II deposition was determined by ELISA (each 2 pellets per donor and medium group). Data are given relatively to control medium set as 1. Mean±s.d., *P<0.05 compared with ES and conditioned ES medium. #P<0.05 compared with ES medium (P<0.05, Mann–Whitney U-test).
Rapid decline and transient restart of proliferation during chondrogenesis
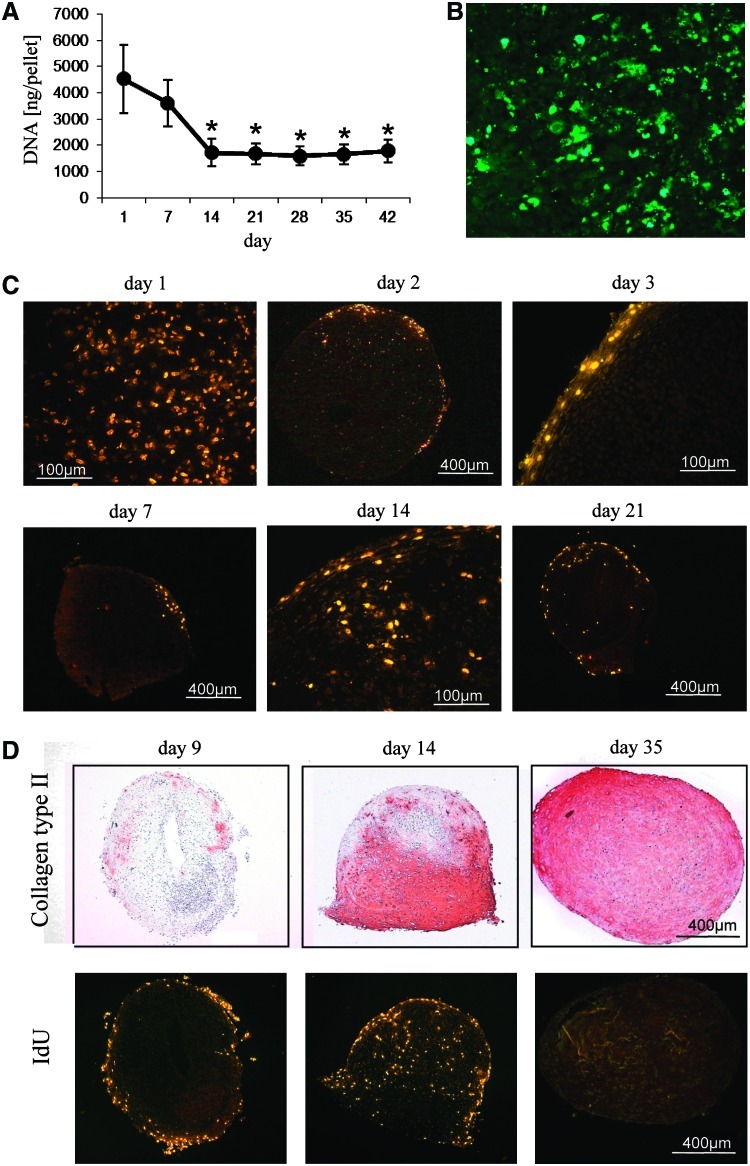
To assess the cell content of differentiating pellets, total DNA content was measured weekly during chondrogenic differentiation. Cell numbers decreased significantly from day 1 to 14 and remained on this level until 6 weeks of differentiation (Fig. 3A), providing evidence for extensive cell death within the first 2 weeks of differentiation. In line with this, cells with apoptotic bodies and cleaved caspase 3 fragments appeared throughout the pellet at days 3–4 of culture (Fig. 3B). Dead cells tended to become concentrated in one part of the pellet thereafter. While caspase 3–positive cells were still evident at day 10, no apoptosis was obvious beyond day 15 in line with the DNA content. To detect actively dividing cells at days 1, 2, 3, 7, 9, 14, 21, 28, and 35 of chondrogenesis, newly synthesized DNA was labeled for 24 h by IdU in pellets of 5 donors and culture was stopped immediately after labeling. On the first day of chondrogenic induction, IdU incorporation was evident across the whole pellet in samples of all donors, but decreased rapidly since the label was restricted primarily to the periphery of the pellet already at day 2. On days 3 and 7 no IdU-positive cells were evident except for a few cells in the outermost area of the pellet (Fig. 3C). Remarkably, cells resumed cell division in distinct regions in the inner part of the pellets as evident from IdU-positive cells in pellets labeled at day 14 or day 21 with the exact time point varying with the donor (Fig. 3C). Serial sections stained for collagen type II deposition suggested that cells divided in areas just turning positive for collagen type II protein (Figs. 3D and 4A) while no labeling was evident in regions with a strong collagen type II deposition. At day 35 of chondrogenesis proliferation had always stopped throughout the pellets (Fig. 3D).
FIG. 3.
Apoptosis and proliferation of MSCs during chondrogenic differentiation. (A) At days 1, 7, 14, 21, 28, 35, and 42 of chondrogenic differentiation the DNA content of pellets (n=5 donors) was assessed by PicoGreen assay * significant different to day 1 and day 7 (p<0.05). (B) Paraffin-embedded sections of pellets were stained for the apoptosis marker cleaved caspase 3 by immunohistology at day 3 of chondrogenic differentiation. (C) At denoted time points of differentiation chondrogenic medium was supplemented with 20 μM of IdU and pellets were harvested directly after the labeling period (24 h). IdU incorporation was detected in paraffin-embedded sections by immunohistology. (D) Whole view of pellets labeled with IdU at days 9, 14, and 35 of chondrogenic differentiation. Serial sections were stained to evaluate a correlation of regions of proliferation with areas of collagen type II deposition. IdU, 5-iodo-2′-deoxyuridin. Color images available online at www.liebertonline.com/scd
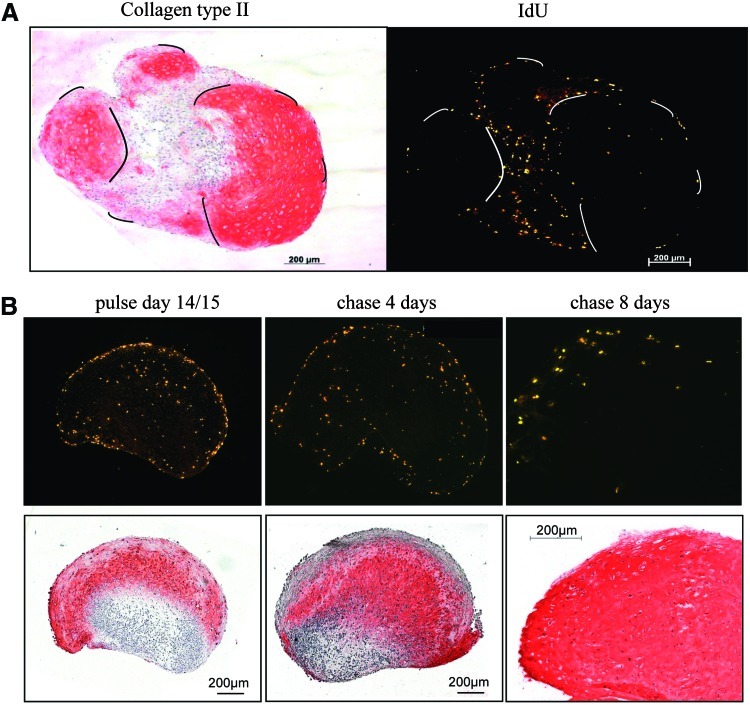
FIG. 4.
Proliferating cells in regions turning positive for collagen type II persist during ongoing differentiation. (A) Serial sections of a pellet labeled with IdU for 24 h at day 21 of chondrogenic differentiation were stained for collagen type II and IdU by immunohistology. Lines mark similar positions in the serial sections. Note the localization of labeled cells around centers of collagen type II deposition. (B) Pellets were labeled with IdU at day 14 of chondrogenic differentiation for 48 h. Pellets were harvested either directly (left) or after a chase of 4 and 8 days after labeling, respectively. Color images available online at www.liebertonline.com/scd
Label-retaining cells persist in the pellets
To determine the fate of the dividing cells in view of a constant DNA content of pellets from day 14 onward, pulse-chase experiments were performed. Cells were labeled with IdU at day 9, day 14, day 17, and day 20 of chondrogenic induction for 48 h and the fate of labeled cells was assessed after a chase of 4 and 8 days, respectively. Cells labeled at days 9/10 were still restricted to the pellet periphery (not shown). At days 14/15 much label was obvious in the inner part of the pellets in regions turning positive for collagen type II (representative data Fig. 4B). This proliferation activity was ongoing on days 17/18 but ceased around days 20/21 when collagen type II was deposited all over the pellet (data not shown). After a chase of 4 and 8 days, a high number of label-retaining cells was obvious in days 14/15 (Fig. 4B) or days 17/18 labeled pellets, suggesting that the dividing cells were cycling slowly or dropped out of the cell cycle and produced extracellular matrix. During ongoing differentiation it appeared that labeled cells were pushed apart from each other by increasing matrix deposition (Fig. 4B, chase 8 days).
Early irreversible growth arrest blocks chondrogenic differentiation
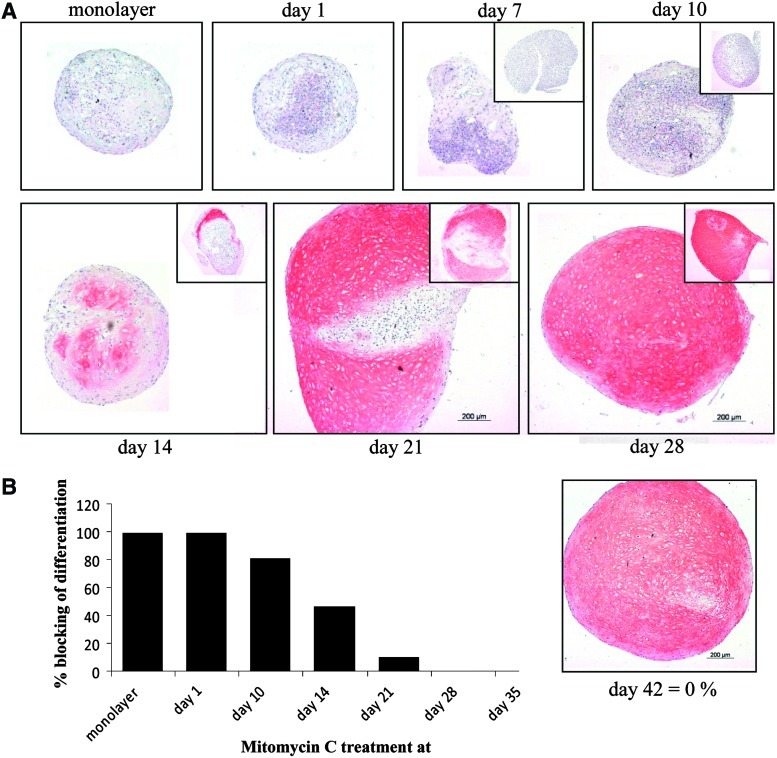
To investigate a causal relationship between proliferation and differentiation and to assess a possible value of premature growth arrest during in vitro chondrogenesis, MSCs from 5 donors were treated with mitomycin C, an alkylating agent that crosslinks DNA and therefore blocks mitosis irreversibly. The cells were exposed either during the last 2 h of monolayer expansion and then subjected to chondrogenesis or pellets were treated on days 1, 7, 10, 14, 21, 28, and 35 of chondrogenesis and differentiation culture was continued up to day 42. Mitomycin C treatment in monolayer or on day 1 of pellet culture completely inhibited differentiation and pellets consisted of an only partly viable but partly disintegrated cell mass (Fig. 5). Mitomycin C treatment at day 10 still inhibited chondrogenesis in pellets from most donors; however, part of the cells were capable to proceed to cartilaginous matrix deposition. Treatment on day 14 still resulted in inhibition of differentiation in up to half of the pellet area while treatment on day 21 affected differentiation only in about 15% of the area on average. From day 28 onward no influence of mitomycin C treatment was apparent (Fig. 5A, B).
FIG. 5.
Effect of mitomycin C treatment on chondrogenic differentiation of MSCs. (A) MSCs in monolayer or at days 1, 7, 10, 14, 21, and 28 of chondrogenic differentiation were treated with 10 μg/mL mitomycin C for 2 h to induce irreversible growth arrest and chondrogenic culture was continued up to day 42. Untreated control pellets (insets) were harvested at the time of mitomycin C treatment in order to assess the degree of differentiation before growth arrest by collagen type II immunohistochemistry. (B) Semiquantitative evaluation of the relative collagen type II–negative area of mitomycin C–treated pellets (day 42) compared with the day 42 untreated control pellets set as 0% blocking (full differentiation). Color images available online at www.liebertonline.com/scd
Discussion
In effective MSC-based tissue engineering strategies for cartilage repair, replicating natural developmental pathways of chondrocyte differentiation is desired. Up to now, this has not been fully achieved due to the unwanted loss of cells and hypertrophic development of MSCs. Therefore, a better understanding of potential differences of in vitro chondrogenesis and embryonic cartilage development [9,10] is warranted to improve chondrogenic differentiation protocols. To mimic the tightly controlled growth and differentiation steps of embryonic cartilage development, in vitro chondrogenesis should begin with fast proliferating MSCs, proceed with condensation and adaptation to high cell density with a temporary cessation in proliferation, and later restart of cell division after chondroprogenitor cells were formed. Together with cartilage matrix deposition, this cell growth would enlarge the pellet size until terminal differentiation is reached under stop of proliferation. Apoptosis and cell death do not compose natural stages of early embryonic articular cartilage development, except in areas of joint space formation, nor are they early events of the endochondral pathway in the growth plate.
This study demonstrates that, indeed, beginning differentiation with MSC populations growing faster during expansion correlated with the formation of larger pellets containing more cells, more proteoglycans, and more collagen type II. We conclude that, if cell metabolism is high, then more cells survive the shift from 2D-proliferation to condensation and determination to the chondrogenic lineage. This suggests that optimizing proliferation rate during expansion and before start of pellet culture is a means to achieve better differentiation results.
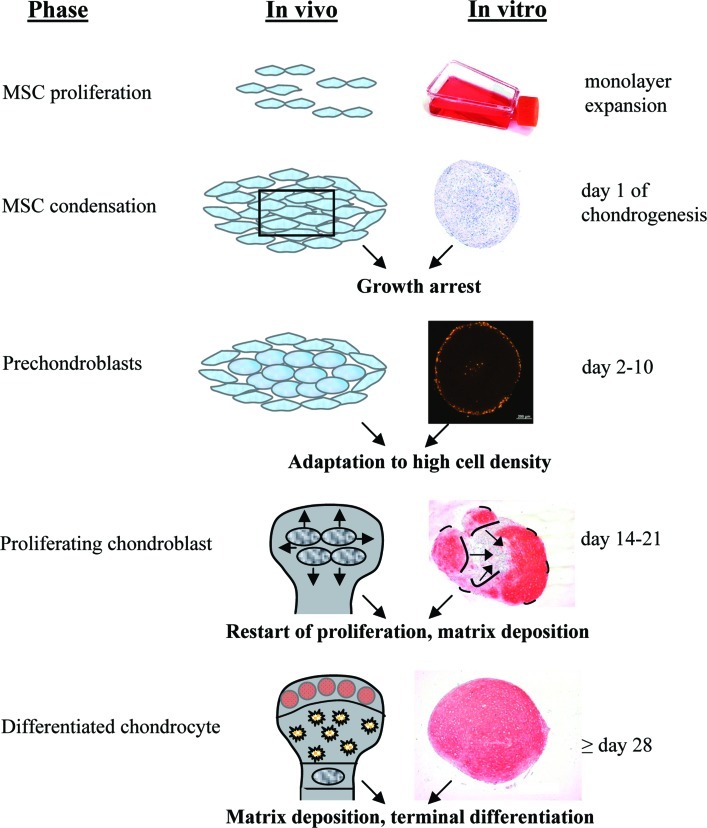
Regarding early events after start of pellet culture, a previous study on cell proliferation during in vitro chondrogenesis of MSCs reported about a 30% increase in cell number between day 0 and 7 and a loss of cells, apparently through apoptosis, thereafter [27]. These results implied that MSC pellets undergoing in vitro chondrogenesis via common protocols lack the natural sequence of transient growth arrest and appropriate control of growth and differentiation steps during chondrocyte development, which could be a main reason for small construct size and suboptimal differentiation results. The current study challenges this view, demonstrating that the shift of MSCs from fast proliferation in monolayer to high-density pellet culture indeed imitated the embryonic in vivo condensation step of mesenchymal progenitors associated with transient withdrawal from the cell cycle [15] until chondroprogenitor cells are established [16]. MSCs rapidly stopped proliferating within 1 day of pellet culture and, except for few residual cells in the outermost layer, remained growth arrested until days 10–14, with the exact time point depending on the donor (Fig. 6).
FIG. 6.
Steps of in vivo embryonic cartilage development and in vitro chondrogenesis of MSCs. Color images available online at www.liebertonline.com/scd
Like in natural development, chondroprogenitors prepared to become chondroblasts then clearly resumed cell division at the same time as synthesizing large amounts of cartilaginous matrix, evident by the fact that they were localized in pellet areas turning positive for collagen type II. DNA labeling of parallel pellets on days 9, 14, 17, and 20 demonstrated that proliferation of chondroblasts lasted for about 1 week. Remarkably, the high fraction of label-retaining cells in pulse-chase experiments suggested that dividing chondroblasts were cycling slowly or would drop out of the cell cycle altogether. Thus, some of the cells may resume cell division for only one cycle while a portion of the cells may undergo few cycles or do not divide at all. From serial sections it appeared that cells in one area of a pellet may precede cells in another area in starting to proliferate and turning collagen type II positive, suggesting that some cells may enter the chondroblast stage earlier than others. Most interestingly, reappearance of dividing cells at 10–14 days coincides with a drop of parathyroid hormone-related protein (PTHrP) levels and induction of Indian hedgehog (IHH) expression in pellets, according to our previous studies [28]. This suggests a possible regulation of proliferation by changing PTHrP/IHH levels, as known from chondrocytes in the growth plate [29,30]. In some pellets, layers of proliferating cells surrounded centers of strong collagen type II deposition (Fig. 4A) in line with molecular gradient organizing differentiation, such as in (curved) primitive growth plates moving through the pellet. Consistently, proliferation ceased when the pellet was differentiated throughout, again in line with the natural terminal differentiation to chondrocytes in articular cartilage and growth plate.
According to irreversible growth arrest of cells with mitomycin C before start of pellet culture, proliferation ability of MSCs is a requirement for successful in vitro chondrogenesis. At later time points loss of proliferation ability apparently arrested chondrogenic differentiation at the stage reached at treatment (Fig. 5A) suggesting a tight linkage between growth and differentiation also in this in vitro model. Since a similar incubation of monolayer MSCs with the irreversible mitosis inhibitor mitomycin C did in no way impair mineral deposition and ALP activation as markers of in vitro osteogenesis in our previous study [31], we exclude that toxic effects of mitomycin C alone may explain our results.
In conclusion, growth and differentiation were closely coordinated during chondrogenesis of MSCs and resembled, with respect to proliferation, stages known from embryonic cartilage development (Fig. 6). This is remarkable in light of unaltered medium conditions throughout chondrogenesis and suggests that a self-sustaining intrinsic differentiation program was triggered, which should be further characterized in future studies.
The inappropriate and unnatural part of in vitro chondrogenesis according to our study was, however, the high cell death observed from day 3 up to day 15 in line with previous reports [32]. About 50% of the cells are finally lost by apoptosis or other forms of cell death and the rapid peak of cell death around days 3–4 suggests that the shift to high cell density and low nutrient and oxygen conditions is a delicate step and one main reason for cell loss. Most likely, it is a result of a selection process whereby the fittest and most adaptable cells survive at the cost of the remaining cell mass that degenerated especially in the pellet center, while even some cell proliferation continued in the periphery throughout the first 3 weeks. Overall it is, thus, appealing to modify and facilitate growth of MSCs at discrete steps to allow more cells to enter and successfully pass the chondrogenic pathway. Possibly this could also avoid the waste of about half of the cells after a time-consuming and expensive in vitro production.
Altogether, our data demonstrate that in vitro chondrogenesis of MSCs partially imitates growth and differentiation steps known from embryonic cartilage development and suggest that proliferation before and during chondrogenesis is important for success. Thus, it seems not too oversimplified but rather a highly attractive model based on an almost unlimited human cell source that can help to dissect the role of important growth regulators for chondrogenesis. With this new knowledge, sequential alteration of culture conditions to support and optimize the here-described self-sustaining growth and differentiation phases seems an appropriate development program for in vitro chondrogenesis.
Acknowledgments
This study was partially funded by the German Research Foundation (grant DFG R/707/7-1) and the Forschungsfonds of the Orthopaedic University Hospital Heidelberg. The authors thank Simone Gantz for statistical analysis, Birgit Frey for technical support, Jake Kushner for providing the IdU immunohistochemistry protocol, and Julianne McCall for proofreading.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Johnstone B. Hering TM. Caplan AI. Goldberg VM. Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 2.Weiss S. Hennig T. Bock R. Steck E. Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ. Petrakova KV. Kurolesova AI. Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 4.Zuk PA. Zhu M. Ashjian P. De Ugarte DA. Huang JI. Mizuno H. Alfonso ZC. Fraser JK. Benhaim P. Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bari C. Dell'Accio F. Tylzanowski P. Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.Dickhut A. Pelttari K. Janicki P. Wagner W. Eckstein V. Egermann M. Richter W. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219–226. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 7.Pelttari K. Winter A. Steck E. Goetzke K. Hennig T. Ochs BG. Aigner T. Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 8.Mueller MB. Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldring MB. Tsuchimochi K. Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 10.Hall BK. Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Coelho CN. Kosher RA. Gap junctional communication during limb cartilage differentiation. Dev Biol. 1991;144:47–53. doi: 10.1016/0012-1606(91)90477-k. [DOI] [PubMed] [Google Scholar]
- 12.Coelho CN. Kosher RA. A gradient of gap junctional communication along the anterior-posterior axis of the developing chick limb bud. Dev Biol. 1991;148:529–535. doi: 10.1016/0012-1606(91)90271-4. [DOI] [PubMed] [Google Scholar]
- 13.Kelley RO. Fallon JF. Identification and distribution of gap junctions in the mesoderm of the developing chick limb bud. J Embryol Exp Morphol. 1978;46:99–110. [PubMed] [Google Scholar]
- 14.ten Berge D. Brugmann SA. Helms JA. Nusse R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development. 2008;135:3247–3257. doi: 10.1242/dev.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solursh M. Reiter RS. Determination of limb bud chondrocytes during a transient block of the cell cycle. Cell Differ. 1975;4:131–137. doi: 10.1016/0045-6039(75)90034-2. [DOI] [PubMed] [Google Scholar]
- 16.Minkoff R. Martin RE. Cell cycle analysis of facial mesenchyme in the chick embryo. II. Label dilution studies and developmental fate of slow cycling cells. J Embryol Exp Morphol. 1984;81:61–73. [PubMed] [Google Scholar]
- 17.Flickinger RA. Muscle and cartilage differentiation in small and large explants from the chick embryo limb bud. Dev Biol. 1974;41:202–208. doi: 10.1016/0012-1606(74)90294-2. [DOI] [PubMed] [Google Scholar]
- 18.DeLise AM. Fischer L. Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu H. Yokoyama S. Asahara H. Growth and differentiation of the developing limb bud from the perspective of chondrogenesis. Dev Growth Differ. 2007;49:449–454. doi: 10.1111/j.1440-169X.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 20.Poole AR. The growth plate: cellular physiology, cartilage assembly and mineralization. In: Hall B, editor; Newman S, editor. Cartilage Molecular Aspects. CRC Press; Boco Raton, FL: 1991. pp. 179–211. [Google Scholar]
- 21.Reyes M. Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci. 2001;938:231–233. doi: 10.1111/j.1749-6632.2001.tb03593.x. [DOI] [PubMed] [Google Scholar]
- 22.Winter A. Breit S. Parsch D. Benz K. Steck E. Hauner H. Weber RM. Ewerbeck V. Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 23.Teta M. Rankin MM. Long SY. Stein GM. Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Tomasz M. Mitomycin C: small, fast and deadly (but very selective) Chem Biol. 1995;2:575–579. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 25.Waring MJ. Drugs which affect the structure and function of DNA. Nature. 1968;219:1320–1325. doi: 10.1038/2191320a0. [DOI] [PubMed] [Google Scholar]
- 26.Iyer VN. Szybalski W. Mitomycins and porfiromycin: chemical mechanism of activation and cross-linking of DNA. Science. 1964;145:55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- 27.Sekiya I. Vuoristo JT. Larson BL. Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer J. Dickhut A. Rickert M. Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 29.Minina E. Wenzel HM. Kreschel C. Karp S. Gaffield W. McMahon AP. Vortkamp A. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development. 2001;128:4523–4534. doi: 10.1242/dev.128.22.4523. [DOI] [PubMed] [Google Scholar]
- 30.Chen X. Macica CM. Nasiri A. Broadus AE. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58:3788–3797. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janicki P. Boeuf S. Steck E. Egermann M. Kasten P. Richter W. Prediction of in vivo bone forming potency of bone marrow-derived human mesenchymal stem cells. Eur Cell Mater. 2011;21:488–507. doi: 10.22203/ecm.v021a37. [DOI] [PubMed] [Google Scholar]
- 32.Ichinose S. Tagami M. Muneta T. Sekiya I. Morphological examination during in vitro cartilage formation by human mesenchymal stem cells. Cell Tissue Res. 2005;322:217–226. doi: 10.1007/s00441-005-1140-6. [DOI] [PubMed] [Google Scholar]