Abstract
Background
Delivering affordable cancer care is becoming increasingly important. Bevacizumab (BEV) is a costly molecular targeted agent effective for a variety of cancer including lung cancer. The objective of this review is to assess published economic evaluation of BEV in the treatment of non-small cell lung cancer (NSCLC).
Methods
A literature search in PubMed, Cochrane, and the Health Technology Assessment reports for English-language publications before February 2012 was performed. Studies were independently screened by two reviewers, and eight publications were included in the review. The results of these eight articles were tabulated and all cost estimates were reported in 2011 US dollars.
Results
Among the eight articles, three were cost studies and five were cost-effectiveness/utility analysis. For first-line treatment, BEV-containing regimen was reported to be the most costly regimen in one study but cost saving when compared with pemetrexed/cisplatin in another study. When compared with other regimens, BEV-containing regimen was reported to be cost effective in two cost-effectiveness studies (incremental cost-effectiveness ratio [ICER] in the range of US$30,318–US$54,317 per life year) but not cost effective in the other three studies (ICER over US$300,000 per life year).
Conclusion
In this review of economic evaluation of BEV in the treatment of NSCLC, it was found that the literature was not conclusive on the economic benefit of BEV. The role of BEV in other treatment settings for NSCLC was unknown. Further studies, such as clinical trials with adequate power to compare the efficacy between low dose and high dose BEV, potential impact of predictive biomarkers for BEV, and comprehensive economic evaluation will strengthen the current state of knowledge on the economic value of BEV in NSCLC.
Keywords: health economic evaluation, cancer care, cost effectiveness
Introduction
The high costs associated with emerging oncology products have led to a faster pace of growth in the costs of cancer compared with other diseases. Delivering affordable cancer care is a growing concern globally, as is highlighted in a recent paper.1 Non-small cell lung cancer (NSCLC) is the leading cause of cancer death worldwide. Although recent advances in medical technologies and pharmaceutical innovations have improved the outcome of patients with NSCLC, the cost-effectiveness of some emerging treatments for NSCLC, such as bevacizumab (BEV), remains inconclusive.2–4
BEV (Hoffmann-La Roche Ltd, Avastin, Basel, Switzerland), a molecular targeted agent in a class of drugs known as monoclonal antibodies, is one of the major advances in recent treatment of NSCLC.5 BEV was first approved by the Food and Drug Administration (FDA) for the treatment of metastatic colorectal cancer in 2004 in the United States. The FDA approval was extended to first-line treatment in combination with chemotherapy for common types of metastatic NSCLC in 2006, and then further extended to second-line treatment of glioblastoma and metastatic renal cell cancer.
In a randomized Phase 3 study, Sandler et al reported that BEV in combination with chemotherapy was associated with a hazard ratio (of death) of 0.79 (P = 0.003) when compared with chemotherapy alone.6 The dosage of BEV approved by the FDA for the treatment of non-squamous, unresectable, locally advanced, recurrent or metastatic NSCLC is 15 mg/kg, administered intravenously every 3 weeks in combination with carboplatin and paclitaxel.7 Although the efficacy of BEV has been demonstrated in clinical trials, costs associated with BEV raise a major concern regarding the affordability of this novel agent among clinicians and payers, as well as patients. Drug cost alone for BEV-containing regimens is about twice as high as that for regimens without BEV among patients with metastatic colorectal cancer.8 Another study estimated that the initial treatment cost per person for BEV-containing regimens in metastatic colorectal cancer is around US$80,000.9 While the costs and cost-effectiveness of BEV in the treatment of metastatic colorectal cancer have been explored in numerous studies, less is known about the economics of BEV in NSCLC. The objective of this study was to provide a critical review of economic evaluation of BEV in the treatment of NSCLC.
Methods
A literature search in PubMed®, Cochrane, and the Health Technology Assessment (HTA) reports published by the National Institute for Health Research HTA Programme in the United Kingdom for peer-reviewed English-language articles published prior to February 2012 was performed using the following search terms: “([Bevacizumab] OR [Avastin]) AND ([non-small cell lung cancer] OR [NSCLC]) AND ([cost] OR [econ*] OR [burden] OR [finan*])”, where * represents a wildcard. The titles and abstracts of articles identified in the search were independently reviewed by both authors. Further reviews of full-text articles and manual searches of the bibliography in the articles identified above led to the final selection of eight publications in the study. These eight studies were then classified into two categories: cost analysis and cost-effectiveness/cost-utility analysis (CEA/CUA).
Tables 1 and 2 summarize the study characteristics and key findings of studies in the cost analysis and CEA/CUA category, respectively. All cost estimates are reported in 2011 USD. For studies reporting costs in USD, the estimates were normalized to 2011 dollars using the medical care services component of the consumer price index if costs in that study were not already reported in 2011 USD.10 For studies reporting costs as currency in other currencies, the estimates were converted to 2011 USD by first applying the local consumer price index to normalize the costs to 2011 and then using the purchasing power parity index to convert the local currency to USD.11 For studies that did not specify the year of cost reporting, the authors assumed the year of publication to be the reference year of cost reporting.
Table 1.
Cost analysis for BEV in the treatment of NSCLC
| Author/country | Approach | Cost type and study perspective | Time frame and reference year for cost | Data sources | Study population | Setting, BEV dosing, and intervention | Resultsa | Conclusion | Comment and sensitivity analyses |
|---|---|---|---|---|---|---|---|---|---|
| Isla et alb,12 Europe (Spain) |
Simple calculation: (quantity of use) × (unit cost) | Direct medical cost; Payer (Spanish national health care system) | A complete course of treatment (eg, first-line, BSC); 2009€ | Unit cost: from Spanish Health Costs Database eSalud and Spanish Database of Medicine; Health Utilization: Delphi panel |
|
First-line BEV dose: 7.5–15 mg/kg | First-line BCP mean total cost = $29,897; First-line PC mean total cost = $19,678; Other first-line regimens < $13,000 | The cost of more recently approved targeted anticancer treatments (eg, BEV, PEM) is higher than that of older anticancer pharmacotherapies |
|
| Bischoff et ald,13 Europe (Italy and Germany) |
Simple calculation: (quantity of use) × (unit cost) | Direct medical cost; Payer (national health service) | Monthly cost 2009€ | Dosage and administration: literatures; Unit cost: Italian Medicines Agency, Official Pharmacists Price Schedule in Germany |
|
First-line BEV dose (7.5 mg/kg), two comparisons:
|
Monthly cost-saving:
|
From a budget perspective, BEV should be considered as a preferred targeted treatment of choice for advanced nonsquamous NSCLC |
|
| Stanisic et ald,14 Europe (France, Germany, Italy, and Spain) |
Simple calculation: (number of nonworking days) × (labor cost) | Indirect costs (productivity loss); Societal | 1 and 1.5 year; 2009€ |
|
Patients with nonsquamous mNSCLC, PS 0–1, age < 55 years | First-line BEV dose: 7.5 mg/kg in one trial and 15 mg/kg in another trial; BCG versus CG or BCP versus CP | Mean cost saving per PF patient returned to work at year 1: France: $24,631 Germany: $26,082 Italy: $20,765 Spain: $16,702 |
Longer PFS associated with BEV-based treatment can result in substantial cost savings in PF patients with mNSCLC |
|
Notes:
Dollars in 2011 USD;
sponsored by a pharmacy company that does not produce either bevacizumab or pemetrexed;
limited to patients with nonsquamous histology for regimens containing BEV or PEM;
sponsored by a pharmacy company that does produce Bevacizumab.
Abbreviations: BCG, BEV + cisplatin + gemcitabine (CG); BCP, BEV + carboplatin + paclitaxel (CP); BEV, bevacizumab; BSA, body surface area; BSC, best supportive care; CP, carboplatin/paclitaxel; mNSCLC, metastatic non-small cell lung cancer; NSCLC, non-small cell lung cancer; PC, PEM + cisplatin; PEM, pemetrexed; PF, progression free; PFS, progression-free survival; PS, performance status.
Table 2.
Cost-effectiveness/utility analysis of BEV in the treatment of NSCLC
| Author/country | Approach | Cost type and study perspective | Time frame and reference year for cost | Data sources | Study population | Setting, dosing, and intervention | Resultsa | Conclusion | Comment and sensitivity analyses |
|---|---|---|---|---|---|---|---|---|---|
| Klein et alb,15 USA |
Modeling, semi-Markov model, no discounting |
|
|
|
Advanced NSCLC, either nonsquamous or all histology |
|
|
PC may be considered cost-effective when compared with commonly used regimens for first-line chemotherapy for advanced NSCLC, particularly in nonsquamous NSCLC |
|
| Giuliani et alc,16 Italy |
Modeling Markov model, costs and outcomes discounted at 3.5% |
|
|
|
Advanced nonsquamous NSCLC; BW 71 kg, BSA 1.8 m2 |
|
|
BEV-based therapy is cost-effective compared with PEM-based therapy in the treatment of advanced nonsquamous NSCLC |
|
| Goulart and Ramsey USA | Modeling Markov model, costs and outcomes discounted at 3% |
|
|
|
Advanced nonsquamous NSCLC, ECOG PS 0 or 1 |
|
|
BEV does not appear to be cost-effective when added to chemotherapy in patients with advanced NSCLC |
|
| Ahn et alc,18 Asia (Korea and Taiwan) |
Modeling, Markov model, cost and outcome discounted at 5% (Korea) and 3% (Taiwan) |
|
|
|
Advanced nonsquamous NSCLC, BW 56.9 kg (Korea), 60 kg (Taiwan), BSA 1.62 m2 |
|
|
BCG is cost-effective when compared with PC for patients with advanced NSCLC in Korea and Taiwan |
|
| Klein et alb,19 USA |
Modeling Semi-Markov, cost and outcome discounted at 3% |
|
|
|
Advanced NSCLC patients who have completed first-line platinum double chemotherapy without progression |
|
|
PEM may be considered cost-effective when compared with other agents for maintenance therapy in advanced NSCLC, particularly in patients with nonsquamous cell histology |
|
Notes:
Dollars in 2011 USD;
either some authors were employees of a company contracted with the pharmacy company that produce drug PEM or sponsored by the pharmacy company that produces drug PEM;
sponsored by the pharmacy company that produces drug BEV;
by currency conversion rate.
Abbreviations: AE, adverse event; ASP, average sales price; BCG, BEV + cisplatin + gemcitabine (CG); BCP, BEV + carboplatin + paclitaxol (CP); BSA, body surface area; BSC, best supportive care; BW, bodyweight; CEA, cost-effectiveness analysis; Er, erlotinib; ICER, incremental cost-effectiveness ratio; ICUR, incremental cost-utility ratio; LY, life year; NSCLC, non-small cell lung cancer; PC, premetrexed/cisplatin; PD, progressive disease; PEM, pemetrexed; PS, performance status; QALY, quality adjusted LY; RCT, randomized controlled trial; USD, US dollars.
Results
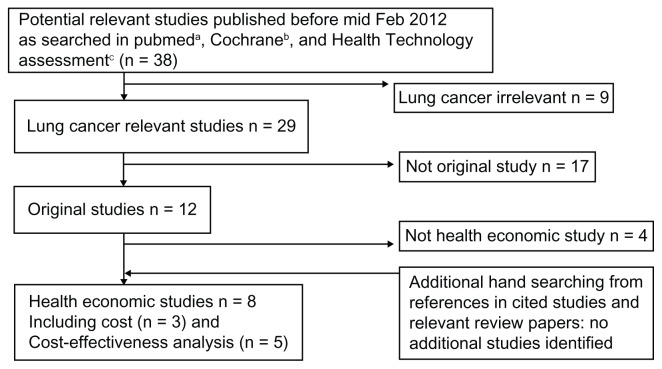
Figure 1 depicts the flow chart of the literature search process. As mentioned earlier, the search identified eight publications that examined various economic aspects of BEV in the treatment of NSCLC, including three cost studies12–14 and five publications of CEA/CUA.15–19
Figure 1.
Flowchart of literature searching.
Notes: ahttp://www.ncbi.nih.gov/sites/entrez?db=pubmed; bhttp://www.thecochranelibrary.com/view/0/index/html; chttp://www.hta.ac.uk/searchmonos.html.
Cost analysis
Table 1 lists the characteristics of the three cost studies that estimated the costs of BEV in the first-line treatment of non-squamous metastatic or advanced NSCLC. All were European studies; two studies reported direct medical costs, and one reported indirect costs. Costs in all three studies were calculated as the sum of the product of the quantity of resource utilization and its associated unit cost across various items of resources.
The two studies reporting direct medical costs reached different conclusions regarding whether BEV-containing regimen was cost saving when compared with the new third-generation chemotherapy that contains pemetrexed (PEM). A closer examination of these two studies indicates that they differed in many ways, from sponsorship and the country or countries of the study, to elements included in the calculation of direct medical costs. The comparison between BEV- and PEM-containing regimens in Isla et al12 was based on a treatment patterns study that included many first- and second-line chemotherapy regimens, as well as best supportive care, for NSCLC patients with different performance status, whereas Bischoff et al13 focused only on two regimens in their comparisons: BEV in combination with cisplatin and gemcitabine (BCG) versus PEM plus cisplatin (PC). Both studies compared BEV-containing regimens with PC; in one study, the mean cost of chemotherapy involving BEV was US$10,000 higher than that of PC, whereas in the other study BEV-containing regimen was associated with monthly cost savings in the range of US$550–US$1180.
Stanisic et al estimated the indirect cost of adding BEV to regimens commonly used to treat metastatic NSCLC, such as cisplatin plus gemcitabine (CG) or carboplatin and paclitaxel (CP). The authors hypothesized that the increasing duration in progression-free survival (PFS) for patients treated with BEV-containing regimens could transform into productivity gain (ie, reduction in indirect costs).14 The authors then applied the human capital approach to estimate indirect costs by combining the above clinical benefit with labor market data and concluded that adding BEV to standard chemotherapy regimens for metastatic NSCLC was associated with productivity gain in the range between US$16,700 and US$26,000 at year 1 and between US$30,000 and US$46,900 at year 1.5 among patients who were in the labor market prior to their cancer diagnosis and were able to return to work after completing their treatment.
CEA/CUA
Table 2 describes the five CEA/CUA studies that included BEV in the treatment of NSCLC.15–19 Four studies considered BEV-containing chemotherapy regimen as first-line treatment for patients with advanced NSCLC, especially those with non-squamous histology; another study explored BEV as maintenance therapy for advanced NSCLC after patients had completed first-line double platinum chemotherapy without experiencing disease progression. All five studies were modeling studies employing either Markov or semi- Markov models.
Four of the five studies included the comparison of BEV-containing regimens and PEM-containing, as both BEV and PEM are newly available therapies for NSCLC. Because, to date, there is no head-to-head trial comparing regimens, including these two new agents, indirect comparison was performed in these four studies. As in the review of the above cost studies, conflicting findings were observed in this present review of the CEA/CUA studies. Using BEV dose of 15 mg/kg as the base of comparison, Klein and colleagues reported that BEV alone or in combination with other chemotherapy agents is no more cost-effective than PEM, either as first-line treatment or maintenance therapy.15,19 On the contrary, Guiliani et al16 and Ahn et al18 both concluded that BCG (using BEV 7.5 mg/kg) is cost-effective when compared with PC. The study by Goulart and Ramsey explored the cost-effectiveness of adding BEV to more conventional third-generation chemotherapy (CP) using data from a pivotal Eastern Cooperative Oncology Group (ECOG) clinical trial and concluded that BCP (using BEV 15 mg/kg) was not cost effective compared with CP.17
Discussion
In this review of economic evaluation of BEV in the treatment of NSCLC, inconsistent findings were found on the cost saving or cost effectiveness of BEV-containing regimens across studies. The authors’ attempts to reconcile the differences across studies were hindered by numerous factors, such as the dose of BEV administered, variations in treatment pattern by countries, comparators of choice, elements included in the calculation of costs, type of costs (direct and/or indirect costs) reported, and the study timeframe.
A noticeable difference in the two cost studies that report opposite findings regarding whether BEV is cost saving is the dosage of BEV, with a lower dose (7.5 mg/kg) observed in the study that concludes lower costs of BEV-containing regimens than PEM-containing regimens.13 The same observation applied to the cost-effectiveness analyses as well. In the two studies where the dosage of BEV was included in sensitivity analyses,15,17 the incremental cost-effectiveness ratio (ICER) associated with BEV administrated at 7.5 mg/kg was approximately half of that estimated based on BEV dosed at 15 mg/kg (US$163,839 versus US$318,386 per life year in Goulart and Ramsey17 and US$136,814 versus US$359,302 per life year in Klein et al15). While it is not surprising that a lower dosage of BEV would be associated with more favorable economic benefit of BEV-containing regimens, it should be noted that the dosage of BEV in the initial clinical trial which led to the FDA approval was 15 mg/kg. The ECOG 4599 trial demonstrated that patients treated with BEV 15 mg/kg in combination with CP had significantly higher PFS and overall survival than those treated with CP.6
In a subsequent trial known as the AVAiL trial, the investigators compared BEV at 7.5 or 15 mg/kg in combination with CG alone.20,21 The trial showed improved PFS (hazard ratio 0.82, P = 0.03) in the comparison of low-dose BEV plus CG versus CG alone, but no statistically significant difference in survival between low-dose and high-dose (15 mg/kg) BEV-containing regimens.20,21 However, the investigators cautioned that although the treatment effect of BEV at either dose relative to placebo appeared to be similar, the trial was not powered to directly compare these two doses of BEV. Although BEV dosed at 7.5 mg/kg is considered off-label use, as the dosage differs from that approved by the FDA, the low-dose BEV is not against the current National Comprehensive Cancer Network guideline for NSCLC, in which the guideline states that “bevacizumab + chemotherapy or chemotherapy alone is indicated in performance status 0–1 patients with advanced or recurrent NSCLC.”22 Future trials with adequate power to compare the efficacy of BEV administered at high versus low dose will solidify cost-effectiveness based upon low-dose BEV had the trial demonstrated equal efficacy between these two doses. This is important because this current review reveals that the choice of BEV dose was highly correlated with the sponsorship, with lower dose used in studies sponsored by the company manufacturing BEV and higher dose in those sponsored by the company manufacturing PEM.
None of the economic studies employed a societal perspective, despite recommendations from various textbooks or good practice guidelines of economic evaluations.23,24 Theoretically, one could proximate estimates from societal perspective by combining the estimates of indirect costs by Stanisic et al14 with estimates of direct medical costs. However, researchers interested in applying this approach need to exercise extreme caution and not use the estimates of indirect cost reported in that study without making further adjustment. The estimated US$16,700–US$26,000 cost savings associated with BEV-containing regimen was the mean cost for the group of patients who were under age 55 and eligible to return to work, a highly selected subgroup. That is, these estimates do not represent the average cost savings, but only reflect conditional means. This present study’s re- calculation using the clinical and labor market information provided in that paper suggested that the average cost-saving would be in the range of US$240–US$420.
This present study contributes to the literature of economic evaluation of new treatments for NSCLC by reviewing cost or cost-effectiveness studies involving BEV. In a recently published systematic review for cost-effectiveness of new agents for advanced NSCLC, the authors concluded that in first-line treatment, CG was cost-effective when compared with other conventional regimens, and that PC was cost-effective when compared with CG for nonsquamous advanced NSCLC.4 The same study also reported that erlotinib was the most cost-effective regimen for second-line treatment of NSCLC. The role of BEV was not discussed in that review.
This present review identified several gaps in the current literature. First, the role of BEV in other settings (such as adjuvant or second-line) and thus its economic values remained unknown. Second, in the era of personalized medicine, the role of BEV-containing regimen for specific genetic disposition (such as those with epidermal growth factor receptor [EGFR] mutation) remained unclear when BEV-containing regimen was compared with EGFR inhibitors in this population. The cost-effectiveness of BEV could vary drastically if a predictive biomarker for BEV becomes available in the future. Lastly, there is currently no comprehensive economic evaluation (ie, include both direct and indirect costs) of BEV for NSCLC, and information available from the literature cannot be directly applied to generate estimates from the societal perspective.
Conclusion
In this review of economic evaluation of BEV in the treatment of NSCLC, it was found that the literature is inconclusive on the cost and cost-effectiveness of BEV, especially when the comparison involves PEM, another new agent for the treatment of NSCLC. With the exception of the study by Goulart and Ramsey17 commercial interests of the study sponsor appeared to play a role in the other studies. The role of BEV in other treatment settings for NSCLC was unknown. Further studies, such as clinical trials with adequate power to compare the efficacy between low-dose and high-dose BEV, potential impact of predictive biomarkers for BEV, and comprehensive economic evaluation, will strengthen the current state of knowledge on the economic value of BEV in NSCLC.
Acknowledgments
This study was partly supported by a grant from the National Science Council, Taiwan (NSC 98-2314-B-039-014-MY3) (C-R Chien), a grant from the Department of Health, Taiwan (DOH102-TD-C-111005) (C-R Chien), and funding from an NCI Challenge Grant (RC1CA145799), Agency for Healthcare Research and Quality (R01 HS018535), and The University of Chicago Cancer Research Foundation Women’s Board (YCT Shih).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12(10):933–980. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 2.Chouaid C, Atsou K, Hejblum G, Vergnenegre A. Economics of treatments for non-small cell lung cancer. Pharmacoeconomics. 2009;27(2):113–125. doi: 10.2165/00019053-200927020-00003. [DOI] [PubMed] [Google Scholar]
- 3.Vergnenègre A, Ray JA, Chouaid C, et al. Cross-market cost-effectiveness analysis of erlotinib as first-line maintenance treatment for patients with stable non-small cell lung cancer. Clinicoecon Outcomes Res. 2012;4:31–37. doi: 10.2147/CEOR.S25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bongers ML, Coupé VM, Jansma EP, Smit EF, Uyl-de Groot CA. Cost effectiveness of treatment with new agents in advanced non-small- cell lung cancer: a systematic review. Pharmacoeconomics. 2012;30(1):17–34. doi: 10.2165/11595000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Bajorin DF, Bleiberg H, et al. Clinical cancer advances 2005: major research advances in cancer treatment, prevention, and screening – a report from the American Society of Clinical Oncology. J Clin Oncol. 2006;24(1):190–205. doi: 10.1200/JCO.2005.04.8678. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Bevacizumab. Silver Spring: US Food and Drug Administration; [Accessed May 27, 2012]. [revised May 2012; cited: May 12, 2012]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125085s0238lbl.pdf. [Google Scholar]
- 8.Schrag D. The price tag on progress – chemotherapy for colorectal cancer. N Engl J Med. 2004;351(4):317–319. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 9.Shih YC, Elting LS, Pavluck AL, Stewart A, Halpern MT. Immunotherapy in the initial treatment of newly diagnosed cancer patients: utilization trend and cost projections for non-Hodgkin’s lymphoma, metastatic breast cancer, and metastatic colorectal cancer. Cancer Invest. 2010;28(1):46–53. doi: 10.3109/07357900902783187. [DOI] [PubMed] [Google Scholar]
- 10.Bureau of Labor Statistics. Consumer Price Index (All Urban Consumers, item: medical care) Washington: US Bureau of Labor Statistics; [Accessed May 27, 2012]. [cited May 18, 2012]. Available from: http://data.bls.gov/timeseries/CUUR0000SAM. [Google Scholar]
- 11.International Monetary Fund. World Economic Outlook Database. [Accessed May 27, 2012]. [cited May 18, 2012]. Available from: http://www.imf.org/external/pubs/ft/weo/2009/02/weodata/index.aspx.
- 12.Isla D, González-Rojas N, Nieves D, Brosa M, Finnern HW. Treatment patterns, use of resources, and costs of advanced non-small-cell lung cancer patients in Spain: results from a Delphi panel. Clin Transl Oncol. 2011;13(7):460–471. doi: 10.1007/s12094-011-0683-0. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff HG, Heigener DF, Walzer S, Nuijten M. Costs of bevacizumab and pemetrexed for advanced non-squamous NSCLC in Italy and Germany. Lung Cancer. 2010;69( Suppl 1):S18–S23. doi: 10.1016/S0169-5002(10)70134-3. [DOI] [PubMed] [Google Scholar]
- 14.Stanisic S, Bischoff HG, Heigener DF, et al. Societal cost savings through bevacizumab-based treatment in non-small cell lung cancer (NSCLC) Lung Cancer. 2010;69( Suppl 1):S24–S30. doi: 10.1016/S0169-5002(10)70135-5. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Muehlenbein C, Liepa AM, Babineaux S, Wielage R, Schwartzberg L. Cost-effectiveness of pemetrexed plus cisplatin as first-line therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2009;4(11):1404–1414. doi: 10.1097/JTO.0b013e3181ba31e0. [DOI] [PubMed] [Google Scholar]
- 16.Giuliani G, Grossi F, de Marinis F, Walzer S. Cost-effectiveness analysis of bevacizumab versus pemetrexed for advanced non-squamous NSCLC in Italy. Lung Cancer. 2010;69( Suppl 1):S11–S17. doi: 10.1016/S0169-5002(10)70133-1. [DOI] [PubMed] [Google Scholar]
- 17.Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health. 2011;14(6):836–845. doi: 10.1016/j.jval.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Ahn MJ, Tsai CM, Hsia TC, et al. Cost-effectiveness of bevacizumab-based therapy versus cisplatin plus pemetrexed for the first-line treatment of advanced non-squamous NSCLC in Korea and Taiwan. Asia Pac J Clin Oncol. 2011;7( Suppl 2):S22–S33. doi: 10.1111/j.1743-7563.2011.01399.x. [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Wielage R, Muehlenbein C, et al. Cost-effectiveness of pemetrexed as first-line maintenance therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2010;5(8):1263–1272. doi: 10.1097/JTO.0b013e3181e15d16. [DOI] [PubMed] [Google Scholar]
- 20.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 21.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21(9):1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.http://www.nccn.org/index.asp. Non-small cell lung cancer v2.2012. Fort Washington: National Comprehensive Cancer Network; [Accessed December 17, 2011]. NCCN clinical practice guidelines in oncology. [cited December 17, 2011]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (free registration required) [Google Scholar]
- 23.Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health. 2003;6(1):9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 24.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. New York: Oxford University Press; 2005. [Google Scholar]