Abstract
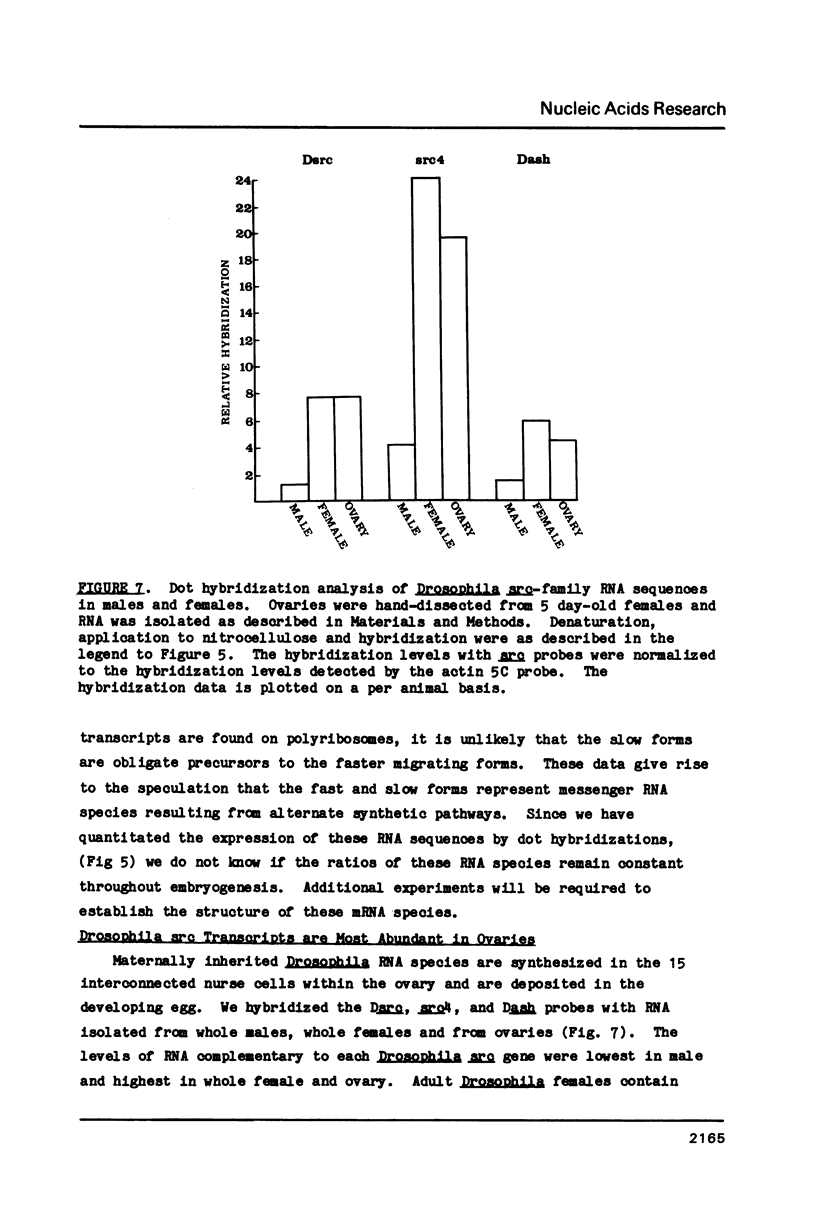
The Drosophila genome contains three major sequences related to the v-src gene. Previously published molecular studies have confirmed the structural homology between v-src and two of the Drosophila sequences. We have sequenced a portion of the third v-src-related Drosophila gene and found that it also shares structural homology with vertebrate and Drosophila src-family genes. RNA sequences from each of the src genes are present in pre-blastoderm embryos indicating that they are of maternal origin. As embryogenesis proceeds, the levels of each of the src RNA sequences decline. The pre-blastoderm src gene transcripts contain poly(A) and are present on polyribosomes suggesting that they are functional mRNAs. Since the Drosophila src transcripts were maternally inherited, we also investigated their distribution in adult females. The majority of the src transcripts in adult females were contained in ovaries. Only low levels of the transcripts were detected in males. These results strongly suggest that an abundant supply of src protein is required during early embryogenesis, perhaps at the time of cellularization of the blastoderm nuclei.
Full text
PDF





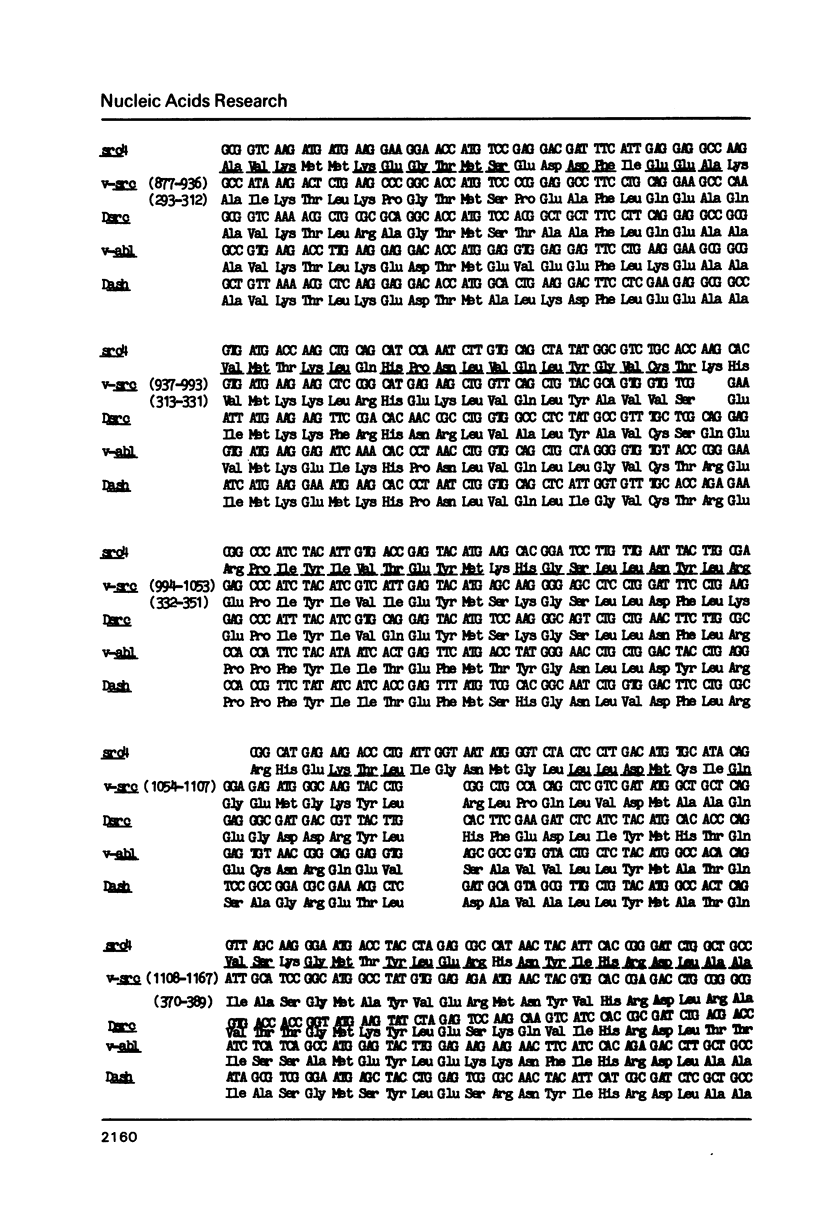









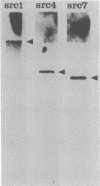
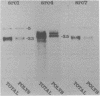
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur C. G., Weide C. M., Vincent W. S., 3rd, Goldstein E. S. mRNA sequence diversity during early embryogenesis in Drosophila melanogaster. Exp Cell Res. 1979 Jun;121(1):87–94. doi: 10.1016/0014-4827(79)90447-6. [DOI] [PubMed] [Google Scholar]
- Barker W. C., Dayhoff M. O. Viral src gene products are related to the catalytic chain of mammalian cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 May;79(9):2836–2839. doi: 10.1073/pnas.79.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Boshes R. A. Drosophila polyribosomes. The characterization of two populations by cell fractionation and isotopic labeling with nucleic acid and protein precursors. J Cell Biol. 1970 Sep;46(3):477–490. doi: 10.1083/jcb.46.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard P. A., Brandhorst B. P. Patterns of protein synthesis and metabolism during sea urchin embryogenesis. Dev Biol. 1983 Mar;96(1):74–83. doi: 10.1016/0012-1606(83)90312-3. [DOI] [PubMed] [Google Scholar]
- Colot H. V., Rosbash M. Behavior of individual maternal pA+ RNAs during embryogenesis of Xenopus laevis. Dev Biol. 1982 Nov;94(1):79–86. doi: 10.1016/0012-1606(82)90070-7. [DOI] [PubMed] [Google Scholar]
- Craig E. A., McCarthy B. J., Wadsworth S. C. Sequence organization of two recombinant plasmids containing genes for the major heat shock-induced protein of D. melanogaster. Cell. 1979 Mar;16(3):575–588. doi: 10.1016/0092-8674(79)90031-x. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980 Sep 18;287(5779):198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Scolnick E. M., Koller R., Dhar R. ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature. 1983 Dec 15;306(5944):707–709. doi: 10.1038/306707a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Dawid I. B. Use of a cloned library for the study of abundant poly(A)+RNA during Xenopus laevis development. Dev Biol. 1980 May;76(2):449–464. doi: 10.1016/0012-1606(80)90393-0. [DOI] [PubMed] [Google Scholar]
- Fullilove S. L., Jacobson A. G. Nuclear elongation and cytokinesis in Drosophila montana. Dev Biol. 1971 Dec;26(4):560–577. doi: 10.1016/0012-1606(71)90141-2. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature. 1983 Dec 15;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- Hoffman-Falk H., Einat P., Shilo B. Z., Hoffmann F. M. Drosophila melanogaster DNA clones homologous to vertebrate oncogenes: evidence for a common ancestor to the src and abl cellular genes. Cell. 1983 Feb;32(2):589–598. doi: 10.1016/0092-8674(83)90478-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann F. M., Fresco L. D., Hoffman-Falk H., Shilo B. Z. Nucleotide sequences of the Drosophila src and abl homologs: conservation and variability in the src family oncogenes. Cell. 1983 Dec;35(2 Pt 1):393–401. doi: 10.1016/0092-8674(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Hough-Evans B. R., Wold B. J., Ernst S. G., Britten R. J., Davidson E. H. Appearance and persistence of maternal RNA sequences in sea urchin development. Dev Biol. 1977 Oct 1;60(1):258–277. doi: 10.1016/0012-1606(77)90123-3. [DOI] [PubMed] [Google Scholar]
- Isnenghi E., Cassada R., Smith K., Denich K., Radnia K., von Ehrenstein G. Maternal effects and temperature-sensitive period of mutations affecting embryogenesis in Caenorhabditis elegans. Dev Biol. 1983 Aug;98(2):465–480. doi: 10.1016/0012-1606(83)90376-7. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Smith M. J. Sequence complexity in the maternal RNA of the starfish Pisaster ochraceus (Brandt). Dev Biol. 1982 Jan;89(1):56–63. doi: 10.1016/0012-1606(82)90293-7. [DOI] [PubMed] [Google Scholar]
- Lamb M. M., Laird C. D. Increase in nuclear poly(A)-containing RNA at syncytial blastoderm in Drosophila melanogaster embryos. Dev Biol. 1976 Aug;52(1):31–42. doi: 10.1016/0012-1606(76)90004-x. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Lev Z., Xin J. H., Britten R. J., Davidson E. H. Messenger RNA prevalence in sea urchin embryos measured with cloned cDNAs. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5317–5321. doi: 10.1073/pnas.77.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett J. A., Goldstein E. S. The cytoplasmic distribution and characterization of poly(A)+RNA in oocytes and embryos of Drosophilia. Dev Biol. 1977 Nov;61(1):70–78. doi: 10.1016/0012-1606(77)90342-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Perlman S., Rosbash M. Analysis of Xenopus laevis ovary and somatic cell polyadenylated RNA by molecular hybridization. Dev Biol. 1978 Mar;63(1):197–212. doi: 10.1016/0012-1606(78)90125-2. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A high speed, high capacity homology matrix: zooming through SV40 and polyoma. Nucleic Acids Res. 1982 Aug 11;10(15):4765–4782. doi: 10.1093/nar/10.15.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shilo B. Z., Weinberg R. A. DNA sequences homologous to vertebrate oncogenes are conserved in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6789–6792. doi: 10.1073/pnas.78.11.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Parmelee D. C., Wade R. D., Kumar S., Ericsson L. H., Walsh K. A., Neurath H., Long G. L., Demaille J. G., Fischer E. H. Complete amino acid sequence of the catalytic subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):848–851. doi: 10.1073/pnas.78.2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. A., Kornberg T. B., Bishop J. M. Three loci related to the src oncogene and tyrosine-specific protein kinase activity in Drosophila. Nature. 1983 Apr 28;302(5911):837–839. doi: 10.1038/302837a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbly R. J., Jarry B. Stage-specific protein synthesis during early embryogenesis in Drosophila melanogaster. EMBO J. 1983;2(8):1281–1290. doi: 10.1002/j.1460-2075.1983.tb01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., O'Connell P., Gray M. R., Rosbash M. Drosophila maternal and embryo mRNAs transcribed from a single transcription unit use alternate combinations of exons. EMBO J. 1984 May;3(5):1003–1013. doi: 10.1002/j.1460-2075.1984.tb01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., Hecht R., Carr S., Vanderslice R., Wolf N., Hirsh D. Parental effects and phenotypic characterization of mutations that affect early development in Caenorhabditis elegans. Dev Biol. 1980 Feb;74(2):446–469. doi: 10.1016/0012-1606(80)90445-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. L., Petri W., Meselson M. Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without heat shock. Cell. 1983 Apr;32(4):1161–1170. doi: 10.1016/0092-8674(83)90299-4. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Taylor S. S. Affinity labeling of the nucleotide binding site of the catalytic subunit of cAMP-dependent protein kinase using p-fluorosulfonyl-[14C]benzoyl 5'-adenosine. Identification of a modified lysine residue. J Biol Chem. 1979 Sep 10;254(17):8363–8368. [PubMed] [Google Scholar]