Abstract
Tumors require blood supply to survive, grow, and metastasize. This involves the process of angiogenesis signaling for new blood vessel growth into a growing tumor mass. Understanding the mechanism of the angiogenic signaling pathway and neovascularization for breast cancer cell proliferation and growth would help to develop molecular interventions and achieve disease free survival. Our hypothesis is that the surviving cancer cell(s) after radiotherapy can initiate angiogenic signaling pathway in the neighboring endothelial cells resulting in neovascularization for breast cancer cell growth. The angiogenic signaling pathway is initiated by angiogenic factors, VEGF and FGF-2, through activation of a transcriptional regulator NF-κB, which in turn is triggered by therapeutic doses of radiation exposure Human breast adenocarcinoma cells (MCF-7 cells) were exposed to Cesium-137 (137Cs) γ rays to a total dose of 2 Gy at a dose rate of 1.03 Gy/min. The results of mobility shift assay showed that radiation at clinical doses (2 Gy) could induce NF-κB DNA-binding activity. Then, we examined the communication of angiogenic signals from irradiated MCF-7 cells to vascular endothelial cells. At the protein level, the western blot showed induction of angiogenic factors VEGF and FGF-2 in MCF-7 cells irradiated with 2 Gy. Inhibition of NF-κB activation attenuated VEGF and FGF-2 levels. These factors are secreted into the medium. The levels of VEGF and FGF-2 in the extra cellular medium were both increased, after 2 Gy exposures. We also observed corresponding expression of VEGFR2 and FGFR1 in non-irradiated endothelial cells that were co-cultured with irradiated MCF-7 cells. In support of this, in vitro tube formation assays provided evidence that irradiated MCF-7 cells transmit signals to potentiate cultured non-irradiated endothelial cells to form tube networks, which is the hallmark of neovascularization. Inhibition of NF-κB activation attenuated irradiated MCF-7-induced tube network formation. The data provide evidence that the radiation exposure is responsible for tumor growth and maintenance by inducing an angiogenic signaling pathway through activation of NF-κB.
Keywords: breast cancer, angiogenic factors, NF-κB activation, neovascularization
Introduction
Tumors originally develop at locations of the body not directly linked to blood vessels, but experiments have shown that tumors are unable to grow beyond a few millimeters without blood vessels because the oxygen diffusion limit from a capillary to the cells is in the range of 100–200 μm. However, new blood vessels eventually can be generated to feed the tumor cells to continue growing. Under normal conditions, the endothelium of an adult is quiescent, but once tumor-induced blood vessels are activated and proliferating, endothelial cells may divide up to 50 times more frequently than those of the normal tissue of origin. During tumor growth, the angiogenic growth factors are released from the tumor cells and bind to their receptors on the endothelial cells of bystander blood vessels. The ligand-binding receptors then send a signal to the nuclei, inducing endothelial cells to produce enzymes which can break down the membrane surrounding existing blood vessels. Thus, new endothelial cells are allowed to proliferate and migrate towards the tumor. More than 25 angiogenic peptides have been discovered and sequenced. However, only a few have been tested for their expression in human breast cancer. Vascular endothelial growth factor (VEGF) is expressed in a variety of breast cancer types, and appears to be the only angiogenic factor expressed throughout the breast tumor life cycle.1–4 Basic fibroblast growth factors (FGF-2) has gained more and more attention for its ability to promote endothelial cell proliferation and for its role in the physical organization of endothelial cells into tube-like structures. Such characteristics indicate FGF-2 may play a part in promoting angiogenesis, the growth of new blood vessels from the pre-existing vasculature. Many studies have investigated the important role of VEGF and FGF-2 and angiogenesis in breast cancer, but many questions still remain.1–6
Radiotherapy is commonly used to treat local tumors by directly killing tumor cells. But recently, it has been demonstrated that growth factors such as VEGF and FGF-2 were induced by tumor cells subsequent to radiation exposure. All these evidences clearly point out that low-LET radiation could both inhibit apoptosis and enhance radio-resistance in both tumor cells and vascular endothelial cells by inducing angiogenesis growth factors.7,8 On the other hand, studies from our laboratory and others have previously demonstrated that, among several transcription factors evaluated in mammalian cells, Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is the one that is significantly activated in irradiated cells.9–12 Consistently NF-κB has been shown to be involved in the transactivation of VEGF gene by binding to the promoter region.13 Shibata et al showed that inhibition of NF-κB activity decreased VEGF mRNA abundance in MCF-7 breast cancer cells.14 Collectively, these findings suggested that radiation-triggered NF-κB activation might be responsible for the increased expression of angiogenic growth factors, leading to angiogenesis and supporting tumor re-growth. This hypothesis is supported by Okamoto et al15 who found that inhibitors of NF-κB (Cerivastatin, a hydroxymethylglutaryl CoA reductase inhibitor; pyrrolidinedithiocarbamate; or curcumin) completely prevented up-regulation of VEGF mRNA in endothelial cells. In pancreatic cancer, inhibiting NF-κB activation by forced expression of its inhibitor, IκB mutant significantly reduced in vivo expression of VEGF and, hence, decreased neo-plastic angiogenesis.16 Although the relationship between NF-κB and FGF-2 is poorly understood, there is still some evidence that NF-κB activation is associated with FGF-2 production, and involved in the FGF-2-mediated biological activity. For example, decrease of NF-κB activity by treatment with phosphorothioate antisense oligodeoxy-nucleotides against NF-κB can inhibit the basic level of fibroblast growth factor, and hence can inhibit FGF-2-mediated angiogenesis in vitro and in vivo.17,18 Understanding the mechanism of the angiogenic signaling pathway and neovascularization for breast cancer cell proliferation and growth would help to develop molecular interventions and achieve disease free survival.
Material and Methods
Cell culture
Estrogen receptor positive human adenocarcinoma (MCF-7) breast cancer cells (obtained from American Type Culture Collection, Bethesda, MD) were maintained as monolayer cultures by weekly serial passage in 100 mm tissue culture plates in Dulbecco’s Modified Eagle medium (DMEM, Life Tech., Grand Island, NY) supplemented with 44 mM sodium bi-carbonate, 2 mM L-glutamine, 10.2 I.U/mL penicillin, 10.2 μg/mL streptomycin, 10 mM HEPES and 10% heat-inactivated fetal bovine serum (JRH Tech., Lenexa, KS) in a 95% air/5% CO2 humidified incubator. Cells were serum-starved by incubating in 2% serum containing complete growth medium for at least 16 h. Cell viability was monitored by the trypan blue dye exclusion method. When we co-cultured MCF-7 cells with BAEC cells, they shared same medium, MCDB-131 medium, so we adapted the MCF-7 cells into complete MCDB-131 medium for 3–4 passages. MCF-7 cells grew well in MCDB medium, and the doubling time (about 20 hours) became shorter than in DMEM medium (about 29 hours).
Primary Bovine aortic endothelial cells (BAEC; Clonetics/BioWhittaker, San Diego, CA) were cultured in MCDB-131 medium (Sigma, St. Louis, MO) containing 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, California) enriched with 250 ng/mL basic fibroblast growth factor (Pepro Tech, Rocky Hill, NJ), 1 mg/mL epidermal growth factor (PeproTech, Rocky Hill, NJ), 1 mg/mL hydrocortisone (Sigma, St. Louis, MO), 100 U/mL penicillin, and 100 mg/mL streptomycin (Mediatech, Herndon, VA). For all the experiments the cells were incubated in MCDB-131 medium with reduced serum concentration (2% FBS) for at least 16 h unless otherwise specified. Sterile 200 μL Gelatin solution (0.2%, 1 g gelatin to 500 mL endotoxin-free water, autoclave for 30 minutes to keep gelatin solubilize) was used for coating 100 mm cell culture plates (80 μL/well for 6-well plates) for the growth of endothelial cells, and the gelatin solution was left in the plates/wells at room-temperature for 2 hours. Cells at passages 3 were used to start the culture, cell passages 4–8 were used for all the experiments.
Exposure to ionizing radiation
For the selected doses of low Linear energy transfer (low-LET) radiation exposures, the cells were removed from 37 °C incubator and exposed to a clinically relevant dose (2 Gy) of 137Cs γ-rays at a dose rate of 1.03 Gy/min (Atomic Energy of Canada Ltd. Gamma Cell-40 Irradiator) at room temperature (22 °C). Immediately after exposures, the cultures were returned to the 37 °C incubator and harvested at selected time points specified for each experiment. Mock-irradiated control cells (0 Gy) were treated identically, except the cells were kept outside the exposure chamber.
Electrophoretic Mobility Shift Analysis (EMSA)
Nuclear proteins were extracted according to the method described in our earlier publication.27 The cell pellet was resuspended in 500 μL of cold buffer A (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES] –KOH pH 7.9 at 4 °C, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol (DTT), 1 mM of phenylmethylsulfonylfluoride (PMSF) and 1 mM of protease inhibitors (PI, Sigma, St. Louis, MO) followed by centrifugation for 10 seconds at 15,000 rpm in a microfuge centrifuge (Eppendorf, Houston, TX) at 4 °C. The cells were then lysed by incubating for 10 minutes at 4 °C with 50 μL of cold buffer A containing 0.1% NP-40, followed by centrifugation for 10 seconds at 15,000 rpm in the microfuge centrifuge at 4 °C. The nuclear pellet obtained after centrifugation was then resuspened in 20 μL of buffer B (20 mM HEPES– KOH pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM of PMSF and 1 mM of PI) and incubated on ice for 10 minutes for high salt extraction. The samples were centrifuged again for 10 minutes at 15,000 rpm in a microfuge centrifuge at 4 °C. The clear supernatant was transferred to pre-chilled tubes and diluted with 30 μL of buffer C (20 mM HEPES–KOH, pH 7.9, 50 mM KCl, 0.2 mM EDTA and 20% glycerol). The total nuclear protein concentration in each sample was determined using the bicinchonic acid (BCA) method following the manufacturer’s protocol (Pierce, Rockford, IL). The color intensity was measured with an ELISA plate reader (Dynatech MR 5000, Chantilly, VA).
For EMSA analysis, a double-stranded oligonucleotide (5′ -AGT TGA GGG GAC TTT CCC AGG C- 3′) (Promega, Madison, WI) containing a tandem repeat of the consensus sequence 5 -GGG GAC TTT CC-3 was end-labeled with [γ-32P] ATP using T4 polynucleotide kinase. A push column device (Stratagene, La Jolla, CA) was used to separate the free unbound radioisotope from the bound probe. We first equilibrated a push column by adding 75 μL of STE (10 mM Tris-7.5, 1 mM EDTA, 100 mM NaCl) and forced the buffer to go through with a 10 cc disposable syringe (BD, Franklin Lakes, NJ) until one drop came out from the tip of the column. Then a beta shield device was used to mount the column. The reaction mix mentioned above, was added into the column, and gently pushed through the column. Seventy-five microliter of fresh STE was added to the top of the column and we repeated the steps mentioned above. The enriched probe separated from free nucleotide was collected in a 1.5 mL Eppendorf tube. Two microliters of the elution was added to four microliters of scintillation cocktail (PerkinElmer, Waltham, Massachusetts) and the specific activity determined. The binding reaction was performed by mixing nuclear extract (2 μg of total protein), 0.1 ng of poly (dI-dC), and 0.5 ng of [γ-32P] ATP labeled (111 TBq/mmol) NF-κB-specific oligonucleotide probe (Amersham, Arlington Heights, IL) in binding buffer containing 10 mM Tris-Cl, pH 7.5, 100 mM NaCl, 1 mM DTT, 1 mM EDTA, and 20% (v/v) glycerol. All samples were incubated at room temperature (22 °C) for 20 min and subsequently electrophoresed at 100 V through non-denaturating 6% polyacrylamide gel in Tris-glycine buffer (25 mM Tris, 0.19 M glycine, and 1 mM EDTA, pH 8.3). The gels were then dried and autoradiographed at 70 °C with intensifying screens on hyperfilm (GE Health-care, Piscataway, NJ). After scanning the films using Epson Perfection V750, the total amount of NF-κB activation was determined by quantitative analysis using Adobe Photoshop image analysis software (Adobe, San Jose, CA).
Co-culture system
For indirect co-culture, MCF-7 cells were cultured in the apical compartments of cell culture inserts (Pore size: 0.45 μm, growth area: 4.2 cm2, Falcon-BD Bioscience, Franklin Lakes, NJ) with endothelial cells in the basal compartment of 6-well plates (growth area: 9.6 cm2). Because MCF-7 cells have shorter doubling time than endothelial cells, different numbers of MCF-7 (2 × 105 cells) and endothelial cells (5 × 104 cells) were seeded to achieve equal numbers of each cell type at the treatment time. The semipermeable membrane of the insert allows the diffusion of secreted factors but prevents the cells from moving from one chamber to the other. There was no cell contact between the two sides of the two chambers. Complete MCDB medium with 10% FBS were shared by both the MCF-7 and endothelial cells. Sixteen hours after both kinds of cells were seeded, the inserts containing MCF-7 cells were placed on a separate 6-well plate (exposure plate) containing MCDB medium with reduced serum (2% FBS), after two hours, the MCF-7 cells were exposed to selected doses of radiation or sham-exposed to radiation. Immediately after exposure, the inserts were taken out of the exposure plate and placed on the 6-well plate containing BAEC cells (bottom chamber). After 16 hours, the expression of angiogenic growth factor receptors was measured in the co-cultured endothelial cells.
Concentration of the proteins in the medium
To enrich the proteins in the conditioned medium, the disposable Centrifugal Filter Devices containing anisotropic membranes (Millipore, Billerica, MA) were used. To concentrate VEGF and FGF-2 in the medium, filter devices containing the molecular weight cut off of 30,000 kDa (YM-10) and 10,000 kDa (YM-3), respectively were used. The conditioned medium after treatment or exposures were added to the pre-chilled sample reservoirs of the concentrator without damaging the membrane and centrifuged at 3,000 × g for 6 hours at 4 °C. The solvents and the low molecular weight solutes (<30 kDa for YM-30 and <10 kDa for YM-10) passing through the membrane were collected into the filtrate vial. Since the molecular weight of VEGF and FGF-2 are 43 kDa and 18 kDa, respectively, the macro-solute retained in the reservoir above the filter membrane were collected (as the concentrated medium) for further assays. The concentrated samples were recovered from the sample reservoir by placing the retentive vial over the sample reservoir, inverted the device, and centrifuged for 3 minutes at 2,000 × g at 4 °C. The samples concentrated using these centrifugal filter devices provided up to 100-fold sample enrichment with minimal loss of solute (by adsorption) into the device components.
Western blot
Cytoplasmic proteins from treated or exposed MCF-7 cells were extracted following the method described in our earlier publication.27 Culture medium was carefully removed from the cells and washed twice with cold 1 × PBS. The cells were lysed by adding 100 μL of cold RIPA buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) containing a cocktail of protease and phosphatase inhibitors [1 mM of protease inhibitor, (PI; Sigma) and 1 mM of Phenylmethanesulfonyl fluoride (PMSF, Sigma)] to each 100 mm plates and scraped using cell scraper. The lysates were transferred to pre-chilled microcentrifuge tubes and centrifuged at 15,000 × g for 15 minutes at 4 °C, and then transferred the clear supernatant to a new pre-chilled 1.5 mL Eppendorf tube for further analysis. Protein concentration was measured using the Bicinchonic acid (BCA) method as described in the manufacturer’s protocol (Pierce, Rockford, IL). Equal amount of protein samples from the cytoplasmic extract or from the concentrated medium (50 or 100 μg) were added with 1 × sample treatment buffer (125 mM Tris-Cl pH 6.8, 4% SDS, 20% glycerol and 10% 2-mercaptoethonol) and incubated at 96 °C for 10 min. The samples were then subjected to 10% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis. Separated proteins fractions were transferred to Hybond PVDF membrane (Amersham, Piscataway, NJ) by electro-transfer. The membrane was incubated in 1 × PBST (TBE; 10 mM Tris-Cl, pH 8.0, 150 mM NaCl; 0.05% Tween-20) containing 5% non-fat dry milk for 1 h to block non-specific binding sites. The blots were then incubated with anti-VEGF or anti-FGF-2 (1:1,000 dilution; Abcam, Cambridge, MA) for overnight at 4 °C. After the membrane was washed with 1 × PBST, the blots were then incubated with 1:10,000 dilution of horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, CA) for 1 h at room temperature. The specific proteins were visualized via enzyme-linked chemiluminescence using the enhanced chemiluminescence (ECL) reagent (Amersham, Piscataway, NJ). The membranes were then exposed to BioMax ML film (Eastman Kodak, Rochester, NY). To monitor equal loading, the samples were probed with monoclonal antibodies for β-actin (Santa Cruz Biotechnology). The autoradiograms were scanned (Epson Perfection V750) and the protein expression was quantified using Adobe Photoshop Image-J software (Adobe, San Jose, CA).
To determine the expression of reciprocal receptor expression in the endothelial cells that were co-cultured with MCF-7 cells, Vascular endothelial growth factor receptor (VEGFR2) and fibroblast growth factors receptor (FGFR1) were examined. Western blotting was performed as described above using anti-VEGFR2 and anti-FGFR1 antibodies (1:1,000 dilution; Abcam, Cambridge, MA) as primary antibodies and a 1:10,000 dilution of horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, CA).
Tube formation assay in 3D matrigel matrix
To determine indirect effect of radiation on tubule formation, co-culture system were used. First, MCF-7 cells (4 × 104 cells/well) in complete MCDB medium (10% FBS) were seeded in the compartment of the cell culture inserts, which were in 24-well exposure plates overnight. Four hours before radiation exposure, the complete MCDB medium were replaced by plain MCDB medium in the MCF-7 cells, then were either mock irradiated or exposed to radiation. After radiation exposure, another 24-well plate was coated with 300 μL of Matrigel per well to the growth surface and incubate coated surface for 1 h at 37 °C to allow the gel to solidify. Endothelial cells (1 × 104 cells/well) in plain MCDB medium were then seeded in coated 24-well plate in the presence or absence of angiogenesis inducers (VEGF, 50 ng/mL) or inhibitors (NF-κB inhibitor, 10 μM). After 3 hours, cell culture inserts with MCF-7 cells were transferred to the endothelial cells plates. The tube formations were scored 8–10 hours later. Three random fields were viewed in triplicate wells for each test condition under high power Nikon ECLIPSE TE 2000-U microscope linked to a computer with Adobe Photoshop 7 software. Quantification of tubule formation was carried out by counting the number of tubule junction in the total area covered by tubules in each field using image analysis software Photoshop version 7.
Statistical design and methodologies
The proposed experiments were performed a minimum of three times in two sets. Data was expressed as mean ± SD. Statistical analyses were performed using paired and unpaired Student’s t-test to determine significant changes between test samples and controls. Paired data was used with repeated measures of analysis of variance. Statistical significance was defined as P < 0.05, P < 0.01.
Results
Clinical doses of radiation induce NF-κB DNA binding activity in MCF-7 cells
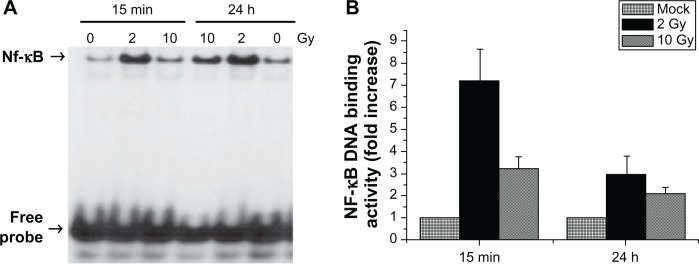
Since the radiation-induced cascade of events that result in angiogenic signaling pathway and neovascularization is proposed to be initiated by the activation and the nuclear translocation of NF-κB, we first examined the DNA-binding activity of NF-κB in the nuclear extracts of cells exposed to low-LET radiation. MCF-7 cells were exposed to 137Cs gamma radiation to a total dose of 2 Gy and 10 Gy. Irradiated cells were incubated for 15 minutes and 24 h. NF-κB DNA-binding activity in the nuclear extracts were examined by EMSA. There was a persistent activation of NF-κB after radiation exposure. The activation was found to increase after 2 Gy and 10 Gy at both 15 minutes and 24 h, compared to mock irradiated control (Fig. 1A).
Figure 1.
(A) Representative autoradiogram of the gel demonstrating the induction of NF-κB DNA-binding activity in MCF-7 cells after radiation exposure. Equal amounts of protein (2 μg) from nuclear extracts obtained from cells harvested at 15 minutes and 24 h after the exposure were analyzed by EMSA. NF-κB-specific DNA binding is shown by arrow head. Corresponding increase or decrease in free probe levels indicates the equal loading. (B) Quantitative analysis of NF-κB DNA-binding activity in MCF-7 cells exposed to 2 Gy and 10 Gy.
Notes: The increase in stimulation is presented by calculating the ratio of the optical densities of the induced level of NF-κB activity to that of the constitutive level (0 Gy) at each incubation time from three independent experiments (mean ± SD, **P < 0.01).
Radiation induced angiogenic growth factors in MCF-7 cells
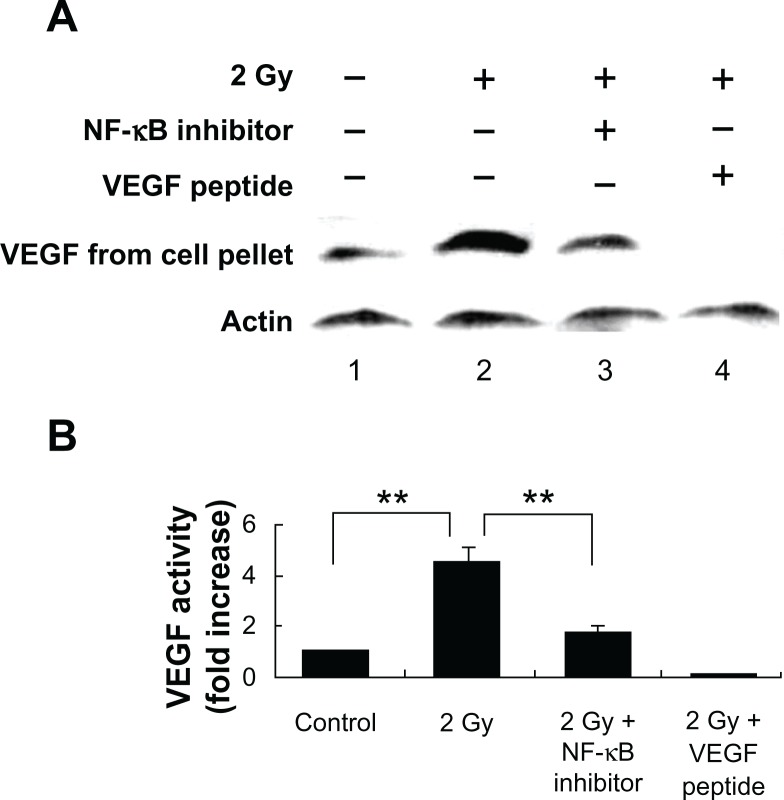
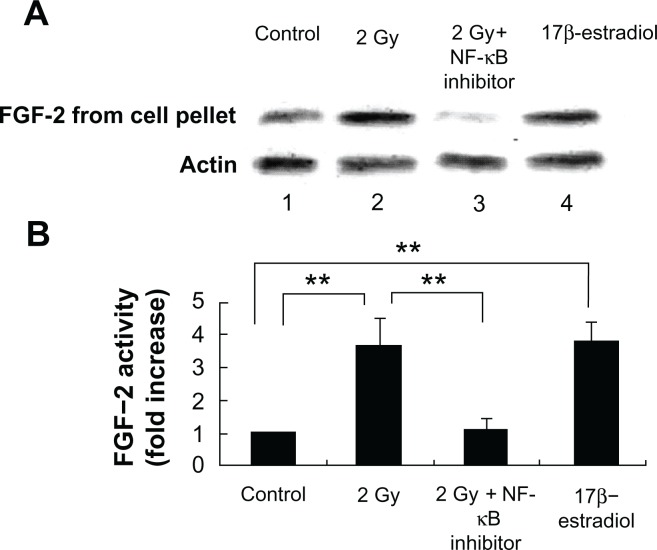
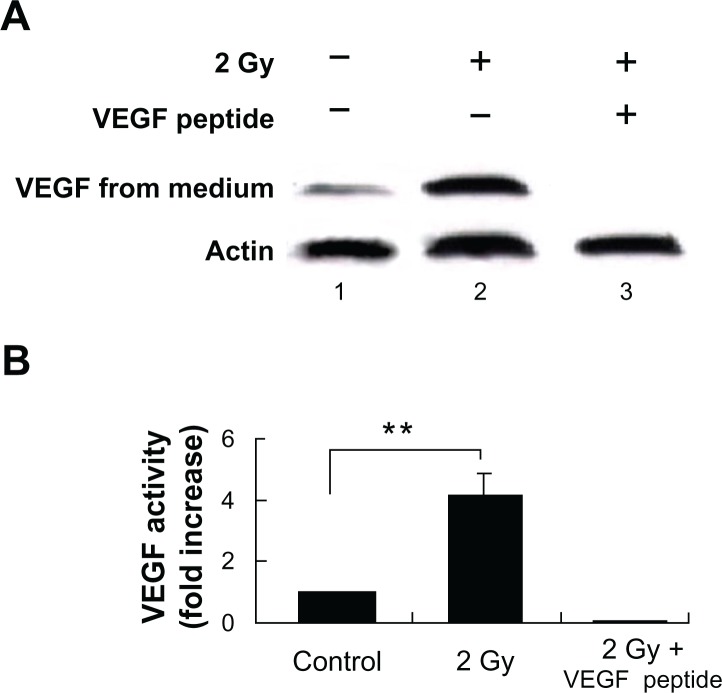
Tumors require a blood supply to survive, grow, and metastasize, which involves the process of angiogenesis signaling for new blood vessel growth into a growing tumor mass. Secreted angiogenic growth factors function as the mitogens for micro- and macrovascular endothelial cells. Angiogenic growth factors-responsive endothelial cells will then sprout out from arteries, veins, and lymphatics. To test the hypothesis that radiation exposure could enhance the expression and secretion of angiogenic factors from tumors cells, we used western blot to quantitatively measure the VEGF and FGF-2 in the cytoplasm and incubation medium of MCF-7 cells. Our results showed that the VEGF and FGF-2 extracted from the cytoplasm of the MCF-7 cells increased by 4.5 ± 0.6-fold and 3.7 ± 0.8-fold, respectively, at 16 h after 2 Gy exposure (Figs. 3 and 5), and the angiogenic factors VEGF ligand and FGF-2 secreted by MCF-7 increased by 3.4 ± 0.7-fold and 2.6 ± 0.9-fold separately at 16 h after 2 Gy exposure (Figs. 2 and 4). VEGF peptide (1 μg/mL) co-incubated with VEGF antibody during the western blot was used as a negative control to ensure the specificity of VEGF antibody. We also incubated MCF-7 cells with 17β-estradiol (1 μM) for 6 hours to induce FGF-2 expression as a positive control to ensure the specificity of FGF-2 antibody. To demonstrate whether this radiation triggered inter-cellular bystander communication responsible for tumor cell proliferation and growth by inducing the onset of angiogenic signaling pathway is mediated through NF-κB activation, we incubated the NF-κB inhibitor isohelenin (10 μM) with the MCF-7 cells one hour before radiation exposure. Our results showed that isohelenin treatment decreased the expression and secretion of VEGF and FGF-2 (Figs. 3 and 5).
Figure 3.
Radiation-induced intracellular VEGF expression in MCF-7 cells is mediated through NF-κB pathway. (A) Immunoblot showing radiation-induced VEGF expression in the cell lysate extracted from MCF-7 cells 16 h after exposure. MCF-7 cells were either mock irradiated (lane 1) or exposed to 2 Gy in the presence (lane 3) or absence (lane 2) of NF-κB inhibitor isohelenin (10 μM) added one hour before radiation exposure. Immunogen peptide of VEGF antibody (1 μg/mL) was co-incubated with VEGF antibody to ensure the specificity of VEGF detection (lane 4). The expression of VEGF was determined in the cell lysate extracted from MCF-7 cells. β-Actin was used as a loading control. (B) Densitometric quantitation of three independent experiments.
Note: **P < 0.01.
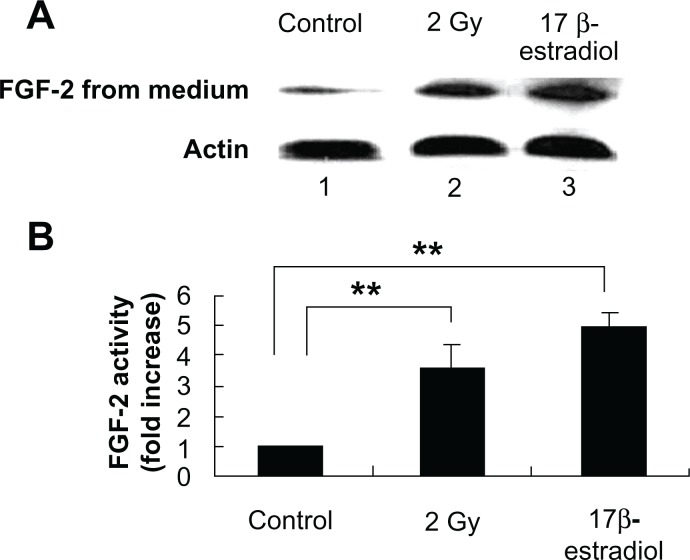
Figure 5.
Radiation-induced intracellular FGF expression in MCF-7 cells is mediated through NF-κB pathway. (A) Immunoblot showing radiation-induced FGF expression in the cell lysate extracted from MCF-7 cells 16 h after exposure. MCF-7 cells were either mock irradiated (lane 1) or exposed to 2 Gy in the presence (lane 3) or absence (lane 2) of NF-κB inhibitor isohelenin (10 μM) added one hour before radiation exposure. 17β-estradiol (1 μM) was incubated with MCF-7 cells for 6 hours to induce FGF-2 expression as a positive control (lane 4). The expression of VEGF was determined in the cell lysate extracted from MCF-7 cells. β-Actin was used as a loading control. (B) Densitometric quantitation of three independent experiments.
Note: **P < 0.01.
Figure 2.
Radiation-induced soluble VEGF expression in the culture supernatant of MCF-7 cells. (A) Immunoblot showing radiation-induced soluble VEGF expression in the culture supernatant. MCF-7 cells were either mock irradiated (lane 1) or exposed to 2 Gy in the presence (lane 3) or absence (lane 2) of VEGF blocking peptide (1 μg/mL). The expression of VEGF was determined in the culture supernatant. β-Actin was used as a loading control. (B) Densitometric quantitation of three independent experiments.
Note: **P < 0.01.
Figure 4.
Radiation-induced soluble FGF expression in the culture supernatant of MCF-7 cells. (A) Immunoblot showing radiation-induced soluble FGF expression in the culture supernatant. MCF-7 cells were either mock irradiated (lane 1) or exposed to 2 Gy (lane 2). 17β-estradiol (1 μM) was incubated with MCF-7 cells for 6 hours to induce FGF-2 as a positive control (lane 3). The expression of VEGF was determined in the culture supernatant. β-Actin was used as a loading control. (B) Densitometric quantitation of three independent experiments.
Note: **P < 0.01.
Radiation can induce the expression of VEGF and FGF receptors on the bystander non-irradiated endothelial cells
The interaction between angiogenic growth factors and angiogenic growth factor receptors can lead to multiple downstream effects, including tumor angiogenesis. Different angiogenic factors bind to their specific receptors, playing distinct roles in the angiogenesis. VEGFR2 is the most important VEGF receptor in tumor angiogenesis, and mediates the majority of VEGF effects. The results from other labs have shown that FGFR1 were expressed for angiogenesis in the aortic endothelial cells upon FGF-2 stimulation.19,20 Therefore, we focused our studies on VEGFR2 and FGFR1. We co-cultured the irradiated MCF-7 cells with the non-irradiated BAEC for 16 h. Co-culture of irradiated MCF-7 cells with 2 Gy exposures could significantly increase the expression levels of VEGFR2 and FGFR1 in BAEC by 2.9 ± 0.9-fold and 3.8 ± 0.3-fold separately. Treatment of BAECs directly with 50 ng/mL VEGF or 0.1 μM PMA for 10 h was used as positive control (Figs. 6 and 7).
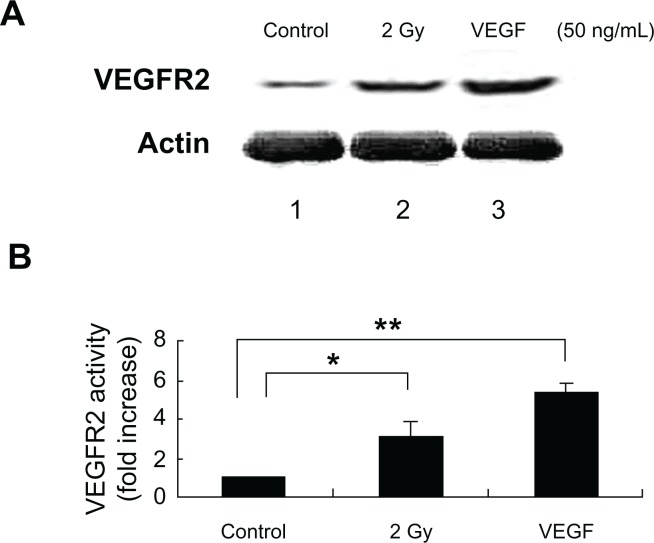
Figure 6.
Irradiated MCF-7 cells trigger VEGFR2 expression in the bystander non-irradiated endothelial cells. (A) Immunoblot showing VEGFR2 expression in the cell lysate extracted from BAEC at 16 h after co-culture with non-irradiated (lane 1) or irradiated MCF-7 cells with 2 Gy exposure (lane 2). Treatment of BAECs for 10 h with VEGF (50 ng/mL) was used as positive control (lane 3). The expression of VEGFR2 was determined in the cell lysate extracted from BAEC cells. β-Actin was used as a loading control. (B) Densitometric quantitation of three independent experiments.
Notes: *P < 0.05; **P < 0.01.
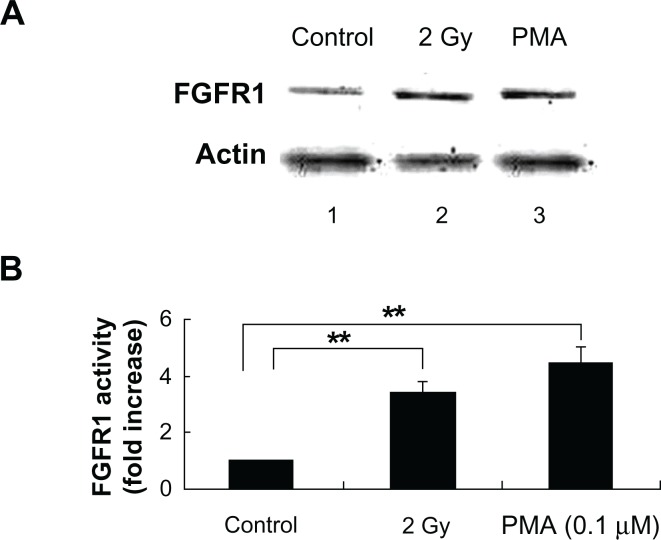
Figure 7.
Irradiated MCF-7 cells trigger FGFR1 expression in the bystander non-irradiated endothelial cells. (A) Immunoblot showing FGFR1 expression in the cell lysate extracted from BAEC at 16 h after co-culture with non-irradiated (lane 1) and irradiated MCF-7 cells with 2 Gy exposure (lane 2). PMA (0.1 μM), as a positive control, was directly treated with BAEC for 10 h (lane 3). The expression of FGFR1 was determined in the cell lysate extracted from BAEC cells. β-Actin was used as a loading control. (B) Densitometric quantitation of three independent experiments.
Note: **P < 0.01.
NF-κB impacted the radiation induced tube formation of BAEC
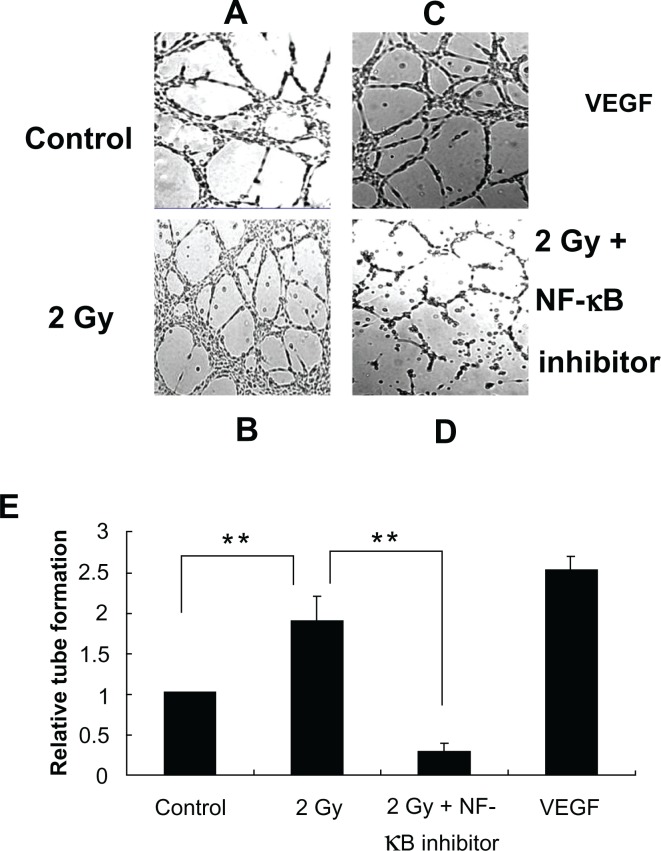
An important example of angiogenesis is the generation of new blood vessels to feed a tumor. Hence, in vitro tube formation assays will provide better understanding of these processes on cellular level. To determine indirect effects of radiation on BAEC tube formation, co-culture system of BAECs and MCF-7 cells were used. From the results, we found more tube formation of BAECs incubated with irradiated MCF-7 cells compared with the BAEC incubated with non-irradiated MCF-7 cells, which suggested that irradiated MCF-7 cells transmit signals to potentiate cultured non-irradiated endothelial cells to form tube networks. To test if NF-κB was involved in this process, NF-κB inhibitor (10 μM) was added to the MCF-7 cells one hour before radiation exposure. The results confirmed our hypothesis by showing that NF-κB inhibitor decreased the number of tube junctions in the total area covered by BAECs. Treatment of BAEC cells with 50 ng/mL VEGF for 10 h was used as a positive control (Fig. 8). This mechanism may be responsible for the stimulation of the proliferative response, tumor-cell re-growth and subsequent tumor relapse at the treatment site.
Figure 8.
Angiogenic tube network formation assay. BAECs co-cultured with irradiated MCF-7 cells in the presence (Panel D) or absence (Panel B) of NF-κB inhibitor or co-cultured with non-irradiated MCF-7 cells (Panel A) in Matrigel for 10 h. BAECs incubated with VEGF as a positive control (Panel C). Densitometric quantitation of three independent experiments (**P < 0.01) (Panel E).
Discussion
Our findings suggested that radiation-triggered NF-κB activation might be responsible for the increased expression of angiogenic growth factors, leading to angiogenesis and supporting tumor re-growth. In our study, MCF-7 cells when exposed to 2 Gy induced angiogenic factors VEGF and FGF-2. Increased levels of VEGF and FGF-2 observed in the supernatant from irradiated MCF-7 cells showed that these factors were synthesized and subsequently secreted into the medium by MCF-7 cells. Inhibition of the expression of VEGF and FGF-2 by incubating with NF-κB inhibitor isohelenin demonstrated the onset of angiogenic signaling pathway is through NF-κB activation.
The presence of soluble VEGF and FGF-2 into the medium clearly suggested that these mediators secreted by MCF-7 cells may be involved in stimulating the nearby cells to express complementary surface receptors. The interaction between angiogenic growth factors released by tumor cells and their corresponding receptors on the bystander vascular endothelial cells in the nearby blood vessels may co-operatively lead to multiple downstream signal pathways to induce tumor angiogenesis. Results from Martin et al have demonstrated that IL8 can stimulate VEGF expression and the autocrine activation of VEGFR2 in endothelial cells by activating NF-κB signaling pathway.21 Studies also have shown that ionizing radiation up-regulated VEGF and basic fibroblast growth factor in human prostate cancer cells (PC3 cells) and, in response to these growth factors, there was an increase in VEGFR2 expression in endothelial cells.22 Similarly, we have observed an increase in endothelial response in terms of receptor expression when co-cultured with breast cancer cells. When MCF-7 cells are irradiated, the concordant expression of both VEGFR2 and FGFR1 was significantly increased in non-irradiated co-cultured aortic endothelial cells. Our study is the first time to show the angiogenic effect of radiation on breast cancer cells.
An important example of angiogenesis is therefore the generation of new blood vessels to feed a tumor. Hence, in vitro tube formation assays will provide better understanding of these processes on a cellular and molecular level. In our study, the in vitro tube formation induced by radiation exposure observed through the co-culture system is attenuated by blocking NF-κB activation. NF-κB induced by radiation in the MCF-7 cells caused an increase in the non-irradiated co-cultured endothelium cell capillary-like tube formation. NF-κB inhibitor added in the MCF-7 cells decreased the tube formation of BAEC and the tumor angiogenesis. The result was supported by the finding that the supernatant from transfected adenoid cystic carcinoma cell lines (inhibited NF-κB activity by stable expression of a phosphorylation-defective IκB mutant) decreased endothelial cell tube formation.23 There is also evidence showing the direct effect of NF-κB on angiogenesis that about 50% tube formation was inhibited by human umbilical endothelial cells with direct treatment of NF-κB antisense oligo.18 Compared to the results from other labs mentioned above, we used the transwell co-culture system, which can avoid gap-junction communication, and allow us to focus studies on mechanisms underlying medium-mediated bystander effects. In accordance with previous published work and our recent research, it demonstrated that regulation of growth factors through the NF-κB signaling pathway induced by radiation exposure is mediated at the transcription level, which then affects protein expression. NF-κB signal blockage might be involved in angiogenesis inhibition through down-regulation of growth factor expression in MCF-7 cells.
Since our results suggested NF-κB-involved sprouting angiogenesis is also one of the most important factors for tumor recurrence after radiotherapy treatment for original cancer, the tumor vasculature is a main target for anticancer therapy not only for original cancer, but also for preventing tumor recurrence. Antiangiogenic therapy used to prevent the formation of the blood vessels would constrain the tumor. Lots of antiangiogenic chemicals and reagents are currently being explored in both clinical and preclinical settings. At present, three main groups of inhibiting angiogenesis drugs approved for clinical use and experimentation are: secreted VEGF inhibitors, tyrosine kinase inhibitors (mainly VEGFR inhibitors) and anti-angiogenic drugs with complicated mechanism.13 The first antiangiogenic drug approved for metastatic colorectal cancer therapy, non-small cell lung carcinoma therapy and metastatic breast carcinoma therapy with different standard chemotherapies called bevacizumab (Avastin®, developed by Genentech/Roche) is a humanized monoclonal antibody against VEGF-A. Unfortunately, bevacizumab cannot completely cure cancer and prevent tumor recurrence, and its effects against tumor are relatively modest.24 For metastatic breast cancer, bevacizumab could only delay tumor growth, but cannot decrease the chance of tumor recurrence and enhance the disease-free survival of patients. Side effects of bevacizumab include the toxicity to the cardiovascular and gastrointestinal systems.24 Many clinical trials and laboratory research are working on other antiangiogenic compounds that do not target VEGF, which is more effective and tolerable antiangiogenic drugs. Now, clinical trials are investigating small molecule inhibitors to the receptors of VEGF, such as sunitinib and sorafenib, but the findings are not inspiring.25 Selective inhibitors of cycloxygenase-2, particularly celecoxib (Celebrex®, developed by Pfizer), were also investigated for their anti-tumor and anti-angiogenic properties. But now, there is a main problem that still remains. The drugs mentioned above have modest effects on original breast cancer treatment, but none of them are significantly effective for breast cancer recurrence. Now the high anti-endothelial and anti-tumor efficacy of triple combination therapy including radiation therapy, chemotherapy (like pemetrexed), and VEGFR inhibition (SU5416, a receptor tyrosine kinase inhibitor of VEGFR2) are getting more and more attention to cause the apoptosis of both human endothelial cells and tumor cells to increase of survival and prevent tumor recurrence in patients.26
In a word, tumor recurrence is a complex process regulated by lots of growth factors, enzymes, and signaling pathways. To achieve the goal of breast cancer recurrence-free, if we not only focus on the direct anti-angiogenesis therapy, but also on the up-regulator of angiogenic factors, maybe the results can be effective and potent. Since results from other labs and our project all showed that NF-κB was involved in the up-regulation of VEGF and FGF-2 activity induced by radiation exposure, inhibition of NF-κB activity could be a new approach to develop potential therapy techniques to target tumor recurrence after radiation therapy. This will also strengthen the rationale for the use of NF-κB inhibitors to prevent or overcome radio-resistance in breast cancer, enable alternative therapeutic modalities and help to achieve a prolonged disease-free survival.
Footnotes
Author Contributions
Conceived and designed the experiments: SM, MN. Analyzed the data: HY. Wrote the first draft of the manuscript: HY. Contributed to the writing of the manuscript: SM, MN. Agree with the manuscript results and conclusions: SM, MN. Jointly developed the structure and arguments for the paper: HY, MN. Made critical revisions and approved final version: HY, MN. All authors reviewed and approved of the final manuscript.
Funding
This work was supported in part by National Aeronautics and Space Administration (NASA) Ground-Based Studies in Space Radiobiology, Grant NNX12-AC32G and by the Office of Sciences (BER), US Department of Energy Grant DEFG03-02ER63449.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Foekens JA, Peters HA, Grebenchtchikov N, et al. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61:5407–14. [PubMed] [Google Scholar]
- 2.Gasparini G, Toi M, Gion M, et al. Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst. 1997;89:139–47. doi: 10.1093/jnci/89.2.139. [DOI] [PubMed] [Google Scholar]
- 3.Konecny GE, Meng YG, Untch M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10:1706–16. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- 4.Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol. 2000;18:1423–31. doi: 10.1200/JCO.2000.18.7.1423. [DOI] [PubMed] [Google Scholar]
- 5.Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol Med. 2002;8:483–9. doi: 10.1016/s1471-4914(02)02394-8. [DOI] [PubMed] [Google Scholar]
- 6.Rieck PW, Gigon M, Jaroszewski J, Pleyer U, Hartmann C. Increased endothelial survival of organ-cultured corneas stored in FGF-2-supplemented serum-free medium. Invest Ophthalmol Vis Sci. 2003;44:3826–32. doi: 10.1167/iovs.02-0601. [DOI] [PubMed] [Google Scholar]
- 7.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Shi M, Guo XT, Shu MG, Li LW. Enhancing tumor radiosensitivity by intracellular delivery of survivin antagonists. Med Hypotheses. 2007;68:1056–8. doi: 10.1016/j.mehy.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991;88:691–5. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu S, Rosenzweig KR, Youmell M, Price BD. The DNA-dependent protein kinase participates in the activation of NF kappa B following DNA damage. Biochem Biophys Res Commun. 1998;247:79–83. doi: 10.1006/bbrc.1998.8741. [DOI] [PubMed] [Google Scholar]
- 11.Prasad AV, Mohan N, Chandrasekar B, Meltz ML. Induction of transcription of “immediate early genes” by low-dose ionizing radiation. Radiat Res. 1995;143:263–72. [PubMed] [Google Scholar]
- 12.Sahijdak WM, Yang CR, Zuckerman JS, Meyers M, Boothman DA. Alterations in transcription factor binding in radioresistant human melanoma cells after ionizing radiation. Radiat Res. 1994;138:S47–51. [PubMed] [Google Scholar]
- 13.Guadagni F, Ferroni P, Palmirotta R, Portarena I, Formica V, Roselli M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: an early target in anticancer therapeutic strategy. In Vivo. 2007;21:147–61. [PubMed] [Google Scholar]
- 14.Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H. Inhibition of NF-kappa B activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat. 2002 Jun;73(3):237–43. doi: 10.1023/a:1015872531675. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto T, Yamagishi S, Inagaki Y, et al. Angiogenesis induced by advanced glycation end products and its prevention by cerivastatin. FASEB J. 2002 Dec;16(14):1928–30. doi: 10.1096/fj.02-0030fje. Epub Oct 4, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Fujioka S, Sclabas GM, Schmidt C, et al. Inhibition of constitutive NF-kappa B activity by I kappa B alpha M suppresses tumorigenesis. Oncogene. 2003 Mar 6;22(9):1365–70. doi: 10.1038/sj.onc.1206323. [DOI] [PubMed] [Google Scholar]
- 17.Cenni E, Perut F, Zuntini M, et al. Inhibition of angiogenic activity of renal carcinoma by an antisense oligonucleotide targeting fibroblast growth factor-2. Anticancer Res. 2005 Mar-Apr;25(2A):1109–13. [PubMed] [Google Scholar]
- 18.Kitajima I, Unoki K, Maruyama I. Phosphorothioate oligodeoxynucleotides inhibit basic fibroblast growth factor-induced angiogenesis in vitro and in vivo. Antisense Nucleic Acid Drug Dev. 1999 Apr;9(2):233–9. doi: 10.1089/oli.1.1999.9.233. [DOI] [PubMed] [Google Scholar]
- 19.Antoine M, Wirz W, Tag CG, et al. Expression pattern of fibroblast growth factors (FGFs), their receptors and antagonists in primary endothelial cells and vascular smooth muscle cells. Growth Factors. 2005;23:87–95. doi: 10.1080/08977190500096004. [DOI] [PubMed] [Google Scholar]
- 20.Holmqvist K, Welsh M, Lu L. A role of the protein Cbl in FGF-2-induced angiogenesis in murine brain endothelial cells. Cell Signal. 2005;17:1433–8. doi: 10.1016/j.cellsig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappa B through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2008 Mar 6;284(10):6038–42. doi: 10.1074/jbc.C800207200. Epub Dec 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdollahi A, Lipson KE, Han X, et al. SU5416 and SU6668 attenuate the angiogenic effects of radiation-induced tumor cell growth factor production and amplify the direct anti-endothelial action of radiation in vitro. Cancer Res. 2003;63:3755–63. [PubMed] [Google Scholar]
- 23.Zhang J, Peng B. NF-kappa B promotes iNOS and VEGF expression in salivary gland adenoid cystic carcinoma cells and enhances endothelial cell motility in vitro. Cell Prolif. 2009 Apr;42(2):150–61. doi: 10.1111/j.1365-2184.2009.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruegg C, Mutter N. Anti-angiogenic therapies in cancer: achievements and open questions. Bull Cancer. 2007;94(9):753–62. [PubMed] [Google Scholar]
- 25.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312(5777):1171–5. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 26.Bischof M, Abdollahi A, Gong P, et al. Triple combination of irradiation, chemotherapy (pemetrexed), and VEGFR inhibition (SU5416) in human endothelial and tumor cells. Int J Radiat Oncol Biol Phys. 2004;60:1220–32. doi: 10.1016/j.ijrobp.2004.07.689. [DOI] [PubMed] [Google Scholar]
- 27.Mohan S, Koyoma K, Thangasamy A, Nakano H, Glickman RD, Mohan N. Low shear stress preferentially enhances IKK activity through selective sources of ROS for persistent activation of NF-kappa B in endothelial cells. Am J Physiol Cell Physiol. 2007 Jan;292(1):C362–71. doi: 10.1152/ajpcell.00535.2005. [DOI] [PubMed] [Google Scholar]