Abstract
Opioid intoxications and overdose are associated with high rates of morbidity and mortality. Opioid overdose may occur in the setting of intravenous or intranasal heroin use, illicit use of diverted opioid medications, intentional or accidental misuse of prescription pain medications, or iatrogenic overdose. In this review, we focused on the epidemiology of illict opioid use in the United States and on the mechanism of action of opioid drugs. We also described the signs and symptoms, and diagnoses of intoxication and overdose. Lastly, we updated the reader about the most recent recommendations for treatment and prevention of opioid intoxications and overdose.
Keywords: illicit opioids, intoxication, treatment
Introduction
Illicit drugs are a major cause of morbidity and mortality world wide. Illicit drug intoxications may constitute life threatening medical emergencies. Therefore, early detection and rapid intervention may be life saving in many cases. Opioids are approved by the Food and Drug Administration (FDA) for treatment of pain. Heroin is an illicit synthetic opioid which has been associated with a drug addiction epidemic in the United States (US) since the 1960s. Illicit use of prescribed opioids is an emerging epidemic in the US. Illicit use of heroin and prescribed opioids can be a major risk for overdose. Opioid intoxication and overdose are life threatening emergencies that need immediate medical attention. They are a common reason for increased morbidity and mortality in this population.
In this review, we present epidemiologic facts, mechanism of action, metabolism and pharmacokinetic profile, signs and symptoms, diagnosis and treatment of illicit opioid intoxications. We review the most recent recommendations for treatment and prevention of opioid intoxications and overdose.
Epidemiology
The Drug Abuse Warning Network (DAWN) reported that the number of emergency department (ED) visits related to non medical use of prescribed opioid significantly increased (111%) between 2004 and 2008.1 The highest numbers of visits were recorded for oxycodone, hydrocodone and methadone.2 It was estimated that 1.6 million ED visits were for the misuse and abuse of all drugs in 2004 and 2 million in 2008. Among these, illicit drugs such as cocaine and heroin were involved in 1 million visits in both 2004 and 2008, whereas prescription or over the counter drugs used non-medically were involved in 0.5 million visits in 2004 and 1 million visits in 2008.3,4 The estimated number of ED visits involving nonmedical use of opioid analgesics increased from 144,600 in 2004 to 305,900 in 2008, whereas prevalence of non-medical use increased from 49.4 to 100.6 per 100,000, an increase of 104%.5,6 The non medical use of prescription opioids is a growing and deadly problem. Martyres et al5 reported in an Australian cohort of young people who died of heroin related overdose that doctor shopping in the years before heroin related death was associated with misuse of prescription drugs such as benzodiazepines and opioids. The prevalence of mortality related to prescription opioid overdose increased drastically in the US between 1999 and 2006.6,7
Mechanism of action, metabolism and pharmacokinetic profile
Opioid drugs act by binding to certain receptors in the brain which lead to specific actions based on the type of the receptor involved. There are 4 types of opioid receptors including mu, kappa, delta, and orphanin FQ nociceptin. The opioid receptors are also the binding sites for endogenous peptides which play an important role in modulating the response to pain, regulation of body temperature, respiration, endocrine and gastrointestinal activity, mood, motivation and other functions.8 Exogenous opioids may act as agonists, partial agonists or antagonists to these receptors. Most of the opioids with addictive potential are agonists at the mu receptor. Those drugs activate the mesocorticolimbic dopaminergic system through their mu agonist property leading to euphoria, positive reinforcement and drug seeking behavior. When opioid receptors are activated by an agonist (endogenous or exogenous), a cascade of intracellular changes involving second and third messenger systems is set in motion. These changes not only produce immediate changes in the responsiveness of the opioid receptor-bearing neurons but also lead to adaptive changes in other neuronal systems that interact with them. Some of these intracellular changes are related to the development of tolerance (decreased responsiveness to the same concentration of the opioid at the receptor) and altered excitability (withdrawal) when the agonist is removed after a period of receptor occupancy.9,10 The interaction between the environment and the individual may play an important role for the drug seeking behavior and elicit triggers for drug use. In other words, the neurobiologic mechanism of action of opioids may represent the interaction between the environment as a trigger for illicit drug use and the individual as the subject who would experience drug craving in response to the environmental cues.11
Opioids are classified into natural and synthetic subclasses. Morphine is a natural short acting opioid and can be detected in urine by immunoassay screening tests. Heroin (diacetalymorohine) is an illicit synthetic opioid. It is rapidly hydrolyzed to 6-monacetylmorphine (half life 3–6 minutes) as a result of spontaneous hydrolysis and hydrolysis by cholinesterase, which in turn is hydrolyzed to inactive morphine 3-glucouronide and the active morphine-6-glucouronide following intravenous administration in humans.12,13 Duration of action of heroin is usually short, but elimination of its metabolite (morphine) depends on route of administration, drug dose, body weight, time elapsed since the last dose, and inter-individual pharmacokinetics.14 Heroin is mainly excreted in the urine as free and conjugated morphine.
The metabolism of the short acting synthetic opioids (eg, oxycodone and hydrocodone) is different from heroin. After a dose of conventional oral oxycodone, peak plasma levels of the drug are attained in approximately one hour; in contrast, after a dose of OxyContin (an oral continuous release formulation), peak plasma levels of oxycodone occur in about three hours. Oxycodone is metabolized to α and β oxycodol; oxymorphone, then α and β oxymorphol and noroxymorphone; and noroxycodone, then α and β noroxycodol and noroxymorphone (N-desmethyloxycodone). Unlike morphine and hydromorphone, oxycodone is metabolized by the cytochrome P450 enzyme system in the liver, making it vulnerable to drug interactions. Some people are fast metabolizers resulting in reduced analgesic effect but increased adverse effects, while others are slow metabolisers resulting in increased toxicity without improved analgesia. Unlike morphine and hydromorphone, oxycodone is metabolized by the cytochrome P450 enzyme system in the liver, making it vulnerable to drug interactions. Oxycodone and its metabolites are mainly excreted in the urine and sweat.15–17
Methadone is a long acting synthetic opioid. It is extensively metabolized in the body mainly in the liver but also by intestinal cytochrome P450 3A4 enzymes. The main metabolite of methadone (2-ethylidene-1,5-dimethyl 1-3,3-diphenylpyrrolidine; EDDP) is inactive. In addition to methadone nine metabolites including EDDP, have been identified in urine and three in feces.18–20
Heroin has been the main source of illicitly used opioids for decades. Sniffing, smoking and IV administration are common routes for illicit heroin use. Recently a new epidemic of non medical use of prescribed opioids emerged in the US. New routes of administration of prescribed opioids have been reported in order to achieve euphoria by illicit users. These routes include chewing, crushing and IV use of controlled release oxycodone (oxycontin), and licking of fentanyl patches and also oral ingestion of prescribed opioids for non medical use.
Signs and symptoms of intoxication, overdose and withdrawal
Cardinal signs of opioid intoxication and overdose include a reduced level of consciousness which may range from drowsiness to a stuporous state to a coma. Other cardinal signs include pinpoint pupils and a depressed respiratory rate. Cyanosis, hypotension, bradycardia, and hypothermia may also be present. Death is usually from respiratory depression.13 Some case reports describe non fatal heroin overdoses associated with significant morbidity.21 The most commonly reported signs and symptoms of overdose morbidity are pulmonary conditions such as edema and pneumonia22–25 and muscular complications such as rhadomyolysis26,27 from prolonged pressure on muscles during coma and renal failure from lysis of muscle tissue.21 Cardiovascular28–30 and cognitive impairment have been reported.31,32 Warner Smith et al21 also report overdose related morbidity including peripheral neuropathy, vomiting, temporal paralysis of limbs, chest infection and seizures.
Opioid withdrawal is a syndrome related to sudden discontinuation of opioids after prolonged period of use. Short acting opioids such as heroin usually exhibit signs and symptoms of withdrawal within 8–12 hours after the last dose. If untreated, it reaches a peak within 36–72 hours and usually subsides substantially within 5 days. For long acting opioids like methadone, withdrawal may reach a peak between 5–6 days, and the syndrome will not usually subside for 14–21 days. The signs and symptoms of opioid withdrawal may be classified as objective and subjective.33 Objective signs include vomiting, lacrimation, rhinorrhea, pupillary dilatation, piloerection, sweating, diarrhea, yawning, fever, elevated pulse and blood pressure. Subjective symptoms may include dysphoric mood, insomnia, muscle aches and cramps, abdominal pain and colic. The Clinical Opiate Withdrawal Scale (COWS) is usually used to classify the severity of opioid withdrawal based on the generated score.34
Diagnosis of intoxication and overdose
Opioid drugs have a wide biodistribution and can be identified in virtually all parts of the body and in all body fluids. The drugs are typically metabolized by the liver, producing metabolites that are often longer lasting and detectable at higher levels than the parent drug. Several laboratory tests can identify the presence of opioids or their metabolites in the blood, urine, hair or saliva. Inexpensive immunoassay screening tests are usually used to detect the presence of opiates (derivatives of the opium poppy) or their metabolites in the blood, urine, hair or saliva. The presence of opioids or their metabolites may be detected in the blood up to 3–12 hours, in urine up to 1–3 days, in hair up to 7–90 days, and in saliva up to 3–24 hours. Gas chromatography/ mass spectrometry is a more expensive laboratory test that is available for confirmation of the results and/or detection of synthetic opioids not usually included in screening immunoassays.35
Treatment and prevention of intoxication and overdose
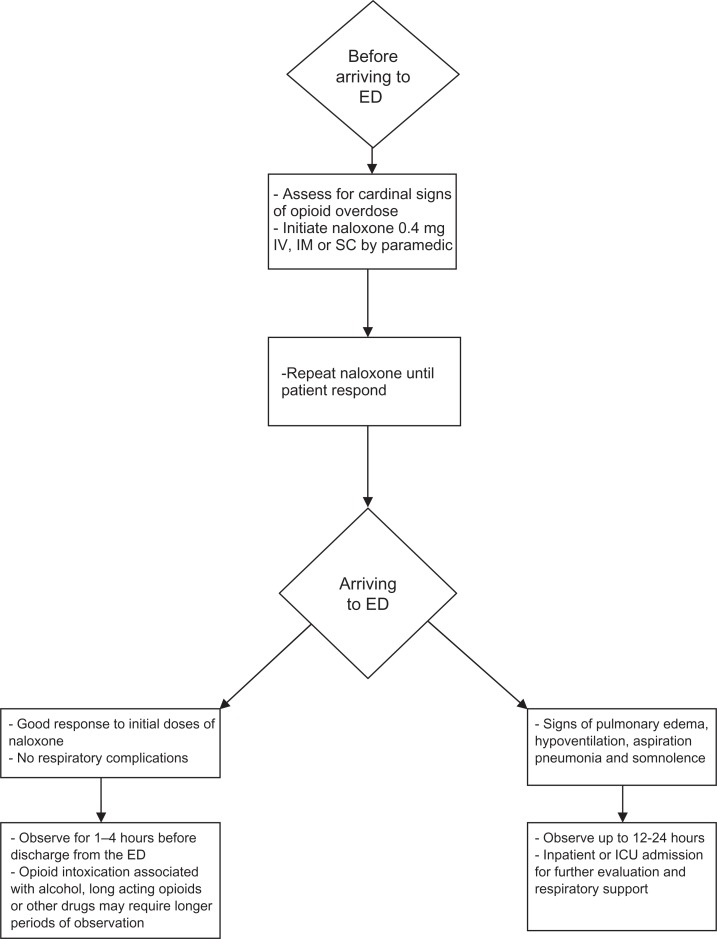
Naloxone is the standard treatment for opioid overdose (Fig. 1). It is a short acting mu receptor antagonist. Its active metabolite, 6-alpha-naloxol has a much longer half-life than naloxone.36 It is usually given intravenously (IV), subcutaneously (SC) or by intramuscular injection (IM). Some reports indicate that the IM administration may prolong the effect of naloxone.37 It is usually administered by paramedics before transferring the patient to the ED. There is evidence that it antagonizes the respiratory depressant effect of morphine up to six hours.38 The starting dose is usually 0.4 mg IV/SC/IM. It can be repeated until the patient responds. Some studies reported a total dose range between 2–6 mg depending on the half-life of the opioid involved in the overdose.39–42 Other factors may also be associated with the need for higher doses of naloxone for resuscitating overdose patients such as concomitant use of alcohol with opioids.43
Figure 1.
Management of acute opioid intoxication and overdose.
There have been controversies about the suggested post opioid overdose period of observation. Several factors need to be considered for this observation period. Opioids with short duration of action such as heroin may need a short period of observation44–46 while the long acting opioids such as methadone may need a longer period of observation.47,48 Response to initial doses of naloxone also plays an important factor in determining the duration of observation and the need for an inpatient admission. Signs of pulmonary edema, hypoventilation, aspiration pneumonia and somnolence may warrant longer periods of observation up to 12–24 hours and in some cases an inpatient or intensive care unit (ICU) admission for further evaluation and respiratory support. Patients who overdose on short acting opioids (eg, IV heroin) with good response to initial doses of naloxone before arriving to the ED and no respiratory complications may need to be observed for 1–4 hours before discharge from the ED.38 Boyd et al39 reported recently that in their study population, allowing presumed heroin overdose patients to sign out after pre-hospital care with naloxone appears to be safe. When transported to an ED, and if no adverse events related to heroin use are evident on arrival, a 1-h observation period after naloxone administration seems to be adequate for recurrent heroin toxicity. In an older study, Smith et al36 found on retrospective review that complications after an IV heroin overdose are relatively few in number and usually evident on or soon after presentation of the patient in the ED. They reported that in most cases, there is no evidence to support 12 to 24 hours of observation or hospitalization for patients who are awake, alert, and who lack evidence of pulmonary complications after a brief observation period of two to four hours. Opioid intoxication associated with alcohol or other drugs may require longer periods of observation. Patients who do not fully regain consciousness or are confused or otherwise mentally incompetent should not be allowed to leave or sign out of the hospital against medical advice.
Some states developed harm reduction programs to reduce the growing rate of overdose mortality. Since the 1990s the Chicago Recovery Alliance program developed anti-overdose kits of naloxone injections to be administered by opiate addicted individuals in case of accidental opioid overdose. Those kits are distributed through needle exchange programs. This program has led to at least 1,000 successful overdose reversals in Chicago city since 2001. Other states developed similar programs to fight the growing incidents of drug overdose.49 There has been controversy about whether it is good public health policy to allow distribution and use of naloxone without medical supervision. On the other hand, those programs could be live saving for many individuals with opioid addiction.49
Naloxone injection may induce opioid withdrawal which may require treatment post overdose. Opioid withdrawal is usually not life threatening but may trigger opioid use and relapse. Ambulatory opioid detoxification is the standard treatment for opioid withdrawal. Substitution with a long acting mu opioid receptor agonist such as buprenorphine50 or methadone51 with gradual taper over a few days may block withdrawal signs and symptoms and minimize the patient’s suffering. Symptomatic treatment with an alpha 2 receptor agonist such as clonidine or lofexidine decreases noradrenergic activity and reduces some signs and symptoms of opioid withdrawal. However, several studies reported that this option is not as effective as substitution with mu receptor agonists in reducing the severity of the withdrawal syndrome.52–54
Some studies report that non-fatal illicit drug overdose significantly predicted subsequent drug overdose.55–57 Therefore prevention of future opioid overdose in high risk patients may reduce mortality and morbidity in this population. Several studies reported that methadone maintenance treatment (MMT) can reduce the risk of overdose and mortality in this population.58–62 Brugal et al63 recently reported that the life expectancy of their cohort of heroin users in MMT increased by 21 years during the period of the study. Factors contributing to increased life expectancy included a reduced incidence of acquired immune deficiency syndrome (AIDS) and reduction of death related to drug overdose. Caplehorn et al64 performed a meta-analysis to study the relationship between being in MMT and the risk of drug related mortality. They found that MMT reduces the risk of overall mortality in this population by 25%, primarily due to reduction of the risk of accidental overdose (heroin in particular). Conversely, dropping out of MMT increases the risk of drug overdose and mortality. Langendam et al65 reported that in their cohort of drug users, participation in harm-reduction MMT reduced the risk of overdose death, whereas leaving treatment was associated with increased risk. On the other hand, several studies have reported an increased risk of fatal drug overdose during the first few weeks of initiating MMT.65–69 Therefore the induction period for MMT may constitute high risk for drug related overdose. Illicit use of central nervous depressants such as benzodiazepines or alcohol during this period may increase the risk for overdose and mortality.5 Increased patient monitoring and education during the induction phase of MMT may attenuate the risk of non opioid drug-related mortality and morbidity. Buprenorphine maintenance treatment (BMT) may be an alternative to MMT for certain high risk patients. Buprenorphine has an improved safety profile compared to methadone due to its pharmacokinetic and pharmacodynamic properties.50 It is a partial agonist of the mu opiate receptor and has a ceiling effect which may reduce the risk for drug overdose.50 It is also safer than methadone as regards its cardiotoxic effect.70 Bell et al71 reported that the risk of overdose death during the 9-month period of their study was significantly lower for patients receiving BMT compared to patients on MMT. In another study, Bell et al72 reported that BMT was associated with less mortality during the induction phase of treatment but shorter retention in treatment compared to MMT. Overall, one may understand from these data that opioid maintenance treatment may improve the risk of illicit opioid overdose and should be considered as a preventive measure for high risk patients.
A new interest in using a sustained release naltrexone implant as prophylaxis against heroin overdose has gained some popularity in Australia.69,73,74 Hulse et al73 found a lower rate of hospitalization for accidental overdose in patients receiving the implant treatment. Ngo et al74 reported that naltrexone implants, but not methadone maintenance, have long-term benefits in reducing opioid related hospital morbidity. However, long-lasting and increased nonopioid drug–related morbidity following naltrexone implantation is particularly concerning. They attributed the nonopioid drug related morbidity to the switching of the drug of choice in those patients to nonopioid drugs. The switch of the drug of choice is probably due to the lack of the expected pleasurable effects upon using opioids. Also, there are some reports75,76 about the increased risk of drug overdose following naltrexone treatment due to decreased opioid tolerance after treatment. More studies are needed to confirm the safety and efficacy of naltrexone implant for prevention of opioid overdose.
In addition to psychopharmacologic intervention for the prevention of illicit opioid overdose, psychotherapeutic intervention is also important. ED and primary care visits represent an opportunity for educational interventions and referral for substance use disorder (SUD) treatment. Brief interventions may be useful for primary care and ED settings but intensive behavioral treatments are preferred for long term management. Brief motivational interviewing (MI) has been implemented in primary care and proved to be an effective intervention for patients with alcohol disorders.77 Recent reports about implementing MI by ED staff and offering referral to SUD treatment programs for patients with alcohol related injuries seem promising.78–81 There are limited data about using MI for patients presenting to ED due to opioid intoxications or overdose. Early intervention after reviving those patients may decrease the burden on ED and prevent future episodes of illicit opioid intoxications and overdose. There is a need to study whether MI and offering referral to SUD treatment by ED staff is effective in preventing future illicit opioid intoxications and overdose.
Conclusions and Future Directions
There are a number of different intoxicants that may be seen in the ED with different presentations and interventions. Opioid intoxications and overdose in particular can be life threatening. Naloxone is the standard treatment for acute opioid overdose and can be life saving if it is given soon enough to revive individuals with opioid overdose. Opioid maintenance treatment may prevent opioid overdose and decrease the risk of mortality for high risk patients. The induction phase of MMT warrants frequent monitoring and education for high risk patients to limit the risk of drug overdose during the first few weeks. Buprenorphine treatment may offer a better safety profile compared to methadone. The naltrexone implant is promising for the prevention of overdose mortality for some patients with opioid dependence but more research is needed to confirm its safety and efficacy. Motivational interviewing and referral to SUD treatment by ED staff are nonpharmacologic interventions for individuals who survive opioid overdose.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Substance Abuse and Mental Health Services Administration Drug Abuse Warning Network, 2007: national estimates of drug-related emergency department visits. Available at http://dawninfo.samhsa.gov/files/ed2007/dawn2k7ed.pdf. Accessed June 10, 2010. [PubMed]
- 2.Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004–2008. Centers for Disease Control and Prevention (CDC) MMWR Morb Mortal Wkly Rep. 2010 Jun 18;59(23):705–9. [PubMed] [Google Scholar]
- 3.Dormitzer C. Summary of drug abuse “rates” in the United States. Available at http://www.fda.gov/ohrms/dockets/ac/08/slides/2008-4356s1-04-fdacorepresentations.ppt. Accessed June 10, 2010.
- 4.Substance Abuse and Mental Health Services Administration . Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009. Results from the 2008 National Survey on Drug Use and Health: national findings. HHS publication no. SMA 09-4434. Available at http://www.oas.samhsa.gov/nsduh/2k8nsduh/2k8results.cfm. Accessed June 10, 2010. [Google Scholar]
- 5.Martyres RF, Clode D, Burns JM. Seeking drugs or seeking help? Escalating “doctor shopping” by young heroin users before fatal overdose. Med J Aust. 2004 Mar 1;180(5):211–4. doi: 10.5694/j.1326-5377.2004.tb05887.x. [DOI] [PubMed] [Google Scholar]
- 6.Warner M, Chen LJ, Makuc DM. Hyattsville, MD: National Center for Health Statistics; 2009. Increase in fatal poisonings involving opioid analgesics in the United States, 1999–2006. NCHS data brief, no 22. [PubMed] [Google Scholar]
- 7.Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Safety. 2006;15:618–27. doi: 10.1002/pds.1276. [DOI] [PubMed] [Google Scholar]
- 8.Vaccarino AL, Kastin AJ. Endogenous opiates: 2000. Peptides. 2001 Dec;22(12):2257–328. doi: 10.1016/s0196-9781(01)00566-6. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001 Feb;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 10.Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001 Summer;10(3):201–17. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- 11.Fareed A, Vayalapalli S, Casarella J, Amar R, Drexler K. Heroin Anti-Craving Medications, a Systematic Review. Am J Drug Alcohol Abuse. 2010 Nov;36(6):332–41. doi: 10.3109/00952990.2010.505991. [DOI] [PubMed] [Google Scholar]
- 12.Darke S, Zador D. Fatal heroin ‘overdose’: a review. Addiction. 1996 Dec;91(12):1765–72. doi: 10.1046/j.1360-0443.1996.911217652.x. [DOI] [PubMed] [Google Scholar]
- 13.Goodman, Gilman . The Pharmacologic Basis of Therapeutics. 8th ed. New York and Oxford Pergamon Press; 1991. [Google Scholar]
- 14.Aderjan R, Hoemann S, Schmitt G, Skopp G. Morphine and morphine glucuronides in serum of heroin consumers and in heroin-related deaths determined by HPLC with native fluorescence detection. Journal of Analytical Toxicology. 1995;19:163–8. doi: 10.1093/jat/19.3.163. [DOI] [PubMed] [Google Scholar]
- 15.Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther. 2006 May;79(5):461–79. doi: 10.1016/j.clpt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 16.AHFS Drug Information. Mar, 2008. Oxycodone (28:08.08)—382132. American Society of Health-System Pharmacists. http://www.ashp.org/mngrphs/ahfs/a382132.htm. Retrieved 2009-03-27.
- 17.Package insert Oxycontin. Stamford, CT: Purdue Pharma L.P.; 2007-11-05. http://www.purduepharma.com/PI/Prescription/Oxycontin.pdf. Retrieved 2009-03-23. [Google Scholar]
- 18.Inturrisi CE, Colburn WA, Kaiko RF, et al. Pharmacokinetics and pharmacodynamics of methadone in patients with chronic pain. Clin Pharmacol Ther. 1987;41:392–401. doi: 10.1038/clpt.1987.47. [DOI] [PubMed] [Google Scholar]
- 19.Änggàrd E, Gunne LM, Homstrand J, et al. Disposition of methadone in methadone maintenance. Clin Pharmacol Ther. 1975;17:258–66. doi: 10.1002/cpt1975173258. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan HR, Due SL. Urinary metabolites of dl-methadone in maintenance subjects. J Med Chem. 1973;16:909–13. doi: 10.1021/jm00266a009. [DOI] [PubMed] [Google Scholar]
- 21.Warner-Smith M, Darke S, Day C. Morbidity associated with non-fatal heroin overdose. Addiction. 2002 Aug;97(8):963–7. doi: 10.1046/j.1360-0443.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- 22.Duberstein JL, Kaufman DM. A clinical study of an epidemic of heroin intoxication and heroin-induced pulmonary edema. American Journal of Medicine. 1971;51:704–14. doi: 10.1016/0002-9343(71)90298-1. [DOI] [PubMed] [Google Scholar]
- 23.Schachter EN, Basta W. Bronchiectasis following heroin overdose. Chest. 1973;63:363–6. doi: 10.1378/chest.63.3.363. [DOI] [PubMed] [Google Scholar]
- 24.Neaderthal RL, Calabro JJ. Treating heroin overdose. American Family Physician. 1975;11:141–5. [PubMed] [Google Scholar]
- 25.Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Academic Emergency Medicine. 1996;3:660–7. doi: 10.1111/j.1553-2712.1996.tb03487.x. [DOI] [PubMed] [Google Scholar]
- 26.Gans J, Stam J, Witingaarden GK. Rhabdomyolysis and concomitant neurological lesions after intravenous heroin abuse. Journal of Neurology, Neurosurgery and Psychiatry. 1985;48:1057–9. doi: 10.1136/jnnp.48.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, Yang G, Ger J, Tsai W, Deng J. Severerhabdomyolisis mimicking transverse myelitis in a heroin addict. Journal of Toxicology: Clinical Toxicology. 1995;33:591–5. doi: 10.3109/15563659509010614. [DOI] [PubMed] [Google Scholar]
- 28.Crowe A, Howse M, Bell G, Henry J. Substance abuse and the kidney. Quarterly Journal of Medicine. 2000;93:147–52. doi: 10.1093/qjmed/93.3.147. [DOI] [PubMed] [Google Scholar]
- 29.Brust JC, Richter RW. Stroke associated with addiction to heroin. Journal of Neurology, Neurosurgery and Psychiatry. 1976;39:194–9. doi: 10.1136/jnnp.39.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart. 2000;83:627–33. doi: 10.1136/heart.83.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction. 2000;95:687–95. doi: 10.1046/j.1360-0443.2000.9556874.x. [DOI] [PubMed] [Google Scholar]
- 32.Fareed A, Casarella J, Amar R, Drexler K. Dose dependent cognitive impairment in an elder methadone maintained patient. J Addict Med. 2009 Jun;3(2):109–10. doi: 10.1097/ADM.0b013e3181898a9c. [DOI] [PubMed] [Google Scholar]
- 33.Galanter M, kleber HD. Textbood of Substance Abuse Treatment. American Psychiatric publishing; 2010. p. 269. [Google Scholar]
- 34.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003 Apr-Jun;35(2):253–9. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 35.Galanter M, kleber HD. Textbood of Substance Abuse Treatment. American Psychiatric publishing; 2010. p. 588. [Google Scholar]
- 36.Smith DA, Leake L, Loflin JR, Yealy DM. Is admission after intravenous heroin overdose necessary? Ann Emerg Med. 1992 Nov;21(11):1326–30. doi: 10.1016/s0196-0644(05)81896-7. [DOI] [PubMed] [Google Scholar]
- 37.Longnecker BE, 6razis P, Eggers GWN. Naloxone antagonism of morphine induced respiratory depression. 4nesth 4nalg. 1973;52:447–52. [PubMed] [Google Scholar]
- 38.Konieczko KM, Jones JG, Barrowcliffe MP, et al. Antagonism of morphineinduced respiratory depression with nalrnefene. BrJ, 4nesth. 1988;61:318–23. doi: 10.1093/bja/61.3.318. [DOI] [PubMed] [Google Scholar]
- 39.Boyd JJ, Kuisma MJ, Alaspää AO, Vuori E, Repo JV, Randell TT. Recurrent opioid toxicity after pre-hospital care of presumed heroin overdose patients. Acta Anaesthesiol Scand. 2006 Nov;50(10):1266–70. doi: 10.1111/j.1399-6576.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- 40.Watson WA, Steele MT, Muelleman RL, Rush MD. Opioid toxicity recurrence after an initial response to naloxone. J Toxicol Clin Toxicol. 1998;36:11–7. doi: 10.3109/15563659809162577. [DOI] [PubMed] [Google Scholar]
- 41.Christenson J, Etherington J, Grafstein E, et al. Early discharge of patients with presumed opioid overdose: development of a clinical prediction rule. Acad Emerg Med. 2000;7:1110–8. doi: 10.1111/j.1553-2712.2000.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 42.Seidler D, Stuhlinger GH, Fischer C, et al. After antagonization of acute opiate overdose: a survey at hospitals in Vienna. Addiction. 1996;91:1479–87. doi: 10.1046/j.1360-0443.1996.911014797.x. [DOI] [PubMed] [Google Scholar]
- 43.Cantwell K, Dietze P, Flander L. The relationship between naloxone dose and key patient variables in the treatment of non-fatal heroin overdose in the prehospitalsetting. Resuscitation. 2005;65:315–9. doi: 10.1016/j.resuscitation.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Vilke GM, Sloane C, Smith AM, Chan TC. Assessment for deaths in out-of-hospital heroin overdose patients treated with naloxone who refuse transport. Acad Emerg Med. 2003;10:893–6. doi: 10.1111/j.1553-2712.2003.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 45.Osterwalder JJ. Patients intoxicated with heroin or heroin mixtures: how long should they be monitored? Eur J Emerg Med. 1995;2:97–101. doi: 10.1097/00063110-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Osterwalder JJ. Naloxone for intoxications with intravenous heroin and heroin mixtures—harmless or hazardous? A prospective clinical study. J Toxicol Clin Toxicol. 1996;34:409–16. doi: 10.3109/15563659609013811. [DOI] [PubMed] [Google Scholar]
- 47.Frand UI, Shim CS. Methadone-induced pulmonary edema. Ann Intern Med. 1972;76:975. doi: 10.7326/0003-4819-76-6-975. [DOI] [PubMed] [Google Scholar]
- 48.Bradberry JC, Raebel MA. Continuous infusion of naloxone in the treatment of narcotic overdose. Drug Intefl Clin Pharm. 1981;15:945–50. doi: 10.1177/106002808101501205. [DOI] [PubMed] [Google Scholar]
- 49.http://www.time.com/time/health/article/0,8599,1901794,00.html.
- 50.Cowan A. Buprenorphine: The basic pharmacology revisited. J Addict Med. 2007 Jun;1(2):68–72. doi: 10.1097/ADM.0b013e31806c9202. [DOI] [PubMed] [Google Scholar]
- 51.Dole VP, Nyswander ME. A medical treatment for diacetyl morphine (heroin) addiction: A clinical trial with methadone hydrochloride. Journal of the American Medical Association. 1965;193:646–50. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- 52.Wilson RS, DiGeorge WS. Methadone combined with clonidine versus clonidine alone in opiate detoxification. J Subs Abuse Treat. 1993 Nov-Dec;10(6):529–354. doi: 10.1016/0740-5472(93)90056-8. [DOI] [PubMed] [Google Scholar]
- 53.Rounsaville BJ, kosten T, Kleber H. Success and failure at outpatient opioid detoxification. Evaluating the process of clonidine and methadone assisted withdrawal. J nerv Men Dis. 1985 Feb;173(2):103–10. doi: 10.1097/00005053-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 54.San L, Cami J, Peri JM, Mata R, Porta M. Efficacy of clonidine, guanfacine and methadone in the rapid detoxification of heroin addicts: A controlled clinical trial. Br J Addict. 1990 doi: 10.1111/j.1360-0443.1990.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 55.Kerr T, Fairbairn N, Tyndall M, et al. Predictors of nonfatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend. 2007;87:39–45. doi: 10.1016/j.drugalcdep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Darke S, Williamson A, Ross J, Mills KL, Havard A, Teesson M. Patterns of non-fatal heroin overdose over a 3-year period: findings from the Australian Treatment Outcome Study. J Urban Health. 2007;84:283–91. doi: 10.1007/s11524-006-9156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Beek I, Dakin A, Kimber J, Gilmour S. The Sydney supervised injecting centre: reducing harm associated with heroin overdose. Crit Public Health. 2004;14:391–406. [Google Scholar]
- 58.Gunne L, Gronbladh L. The Swedish methadone maintenance program: A controlled study. Drug Alcohol Depend. 1981;7:249–56. doi: 10.1016/0376-8716(81)90096-x. [DOI] [PubMed] [Google Scholar]
- 59.Gronbladh L, Ohlund LS, Gunne L. Mortality in heroin addiction: Impact of methadone treatment. Acta Psychiatr Scand. 1990;82:223–7. doi: 10.1111/j.1600-0447.1990.tb03057.x. [DOI] [PubMed] [Google Scholar]
- 60.Gearing FR, Schweitzer MD. An epidemiologic evaluation of long-term methadone maintenance treatment for heroin addiction. Am J Epidemiol. 1974;100:102–12. doi: 10.1093/oxfordjournals.aje.a112012. [DOI] [PubMed] [Google Scholar]
- 61.Cushman P. Ten years of methadone maintenance treatment: Some clinical observations. Am J Drug Alcohol Abuse. 1977;4:543–53. doi: 10.3109/00952997709007010. [DOI] [PubMed] [Google Scholar]
- 62.Poser W, Koc J, Ehrenreich H. Letter. Br Med J. 1995;310:463. doi: 10.1136/bmj.310.6977.463a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brugal MT, Barrio G, De LF, Regidor E, Royuela L, Suelves JM. Factors associated with non-fatal heroin overdose: assessing the effect of frequency and route of heroin administration. Addiction. 2002 Mar;97(3):319–27. doi: 10.1046/j.1360-0443.2002.00058.x. [DOI] [PubMed] [Google Scholar]
- 64.Caplehorn JR, Dalton MS, Haldar F, Petrenas AM, Nisbet JG. Methadone maintenance and addicts’ risk of fatal heroin overdose. Subst Use Misuse. 1996 Jan;31(2):177–96. doi: 10.3109/10826089609045806. [DOI] [PubMed] [Google Scholar]
- 65.Langendam MW, van Brussel GH, Coutinho RA, van Ameijden EJ. The impact of harm-reduction-based methadone treatment on mortality among heroin users. Am J Public Health. 2001 May;91(5):774–80. doi: 10.2105/ajph.91.5.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buster MC, van Brussel GH, van den Brink W. An increase in overdose mortality during the first 2 weeks after entering or re-entering methadone treatment in Amsterdam. Addiction. 2002;97:993–1001. doi: 10.1046/j.1360-0443.2002.00179.x. [DOI] [PubMed] [Google Scholar]
- 67.Caplehorn JRM, Drummer OH. Mortality associated with New South Wales methadone programs in 1994: Lives lost and saved. Medical Journal of Australia. 1999;170:104–9. doi: 10.5694/j.1326-5377.1999.tb127675.x. [DOI] [PubMed] [Google Scholar]
- 68.Gibson A, Degenhardt L. Sydney: National Drug and Alcohol Research Centre; Mortality related to naltrexone in the treatment of opioid dependence: A comparative analysis. Technical report. [Google Scholar]
- 69.Tait RJ, Ngo HT, Hulse GK. Mortality in heroin users 3 years after naltrexone implant or methadone maintenance treatment. J Subst Abuse Treat. 2008 Sep;35(2):116–24. doi: 10.1016/j.jsat.2007.08.014. Epub 2007 Oct 10. [DOI] [PubMed] [Google Scholar]
- 70.Wedam EF, Bigelow GE, Johnson RE, Nuzzo PA, Haigney MC. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007 Dec 10;167(22):2469–75. doi: 10.1001/archinte.167.22.2469. [DOI] [PubMed] [Google Scholar]
- 71.Bell JR, Butler B, Lawrance A, Batey R, Salmelainen P. Comparing overdose mortality associated with methadone and buprenorphine treatment. Drug Alcohol Depend. 2009 Sep 1;(1–2):104. 73–7. doi: 10.1016/j.drugalcdep.2009.03.020. Epub 2009 May 13. [DOI] [PubMed] [Google Scholar]
- 72.Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009 Jul;104(7):1193–200. doi: 10.1111/j.1360-0443.2009.02627.x. [DOI] [PubMed] [Google Scholar]
- 73.Hulse GK, Tait RJ, Comer SD, Sullivan MA, Jacobs IG, Arnold-Reed D. Reducing hospital presentations for opioid overdose in patients treated with sustained release naltrexone implants. Drug Alcohol Depend. 2005 Sep 1;79(3):351–7. doi: 10.1016/j.drugalcdep.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ngo HT, Tait RJ, Hulse GK. Comparing drug-related hospital morbidity following heroin dependence treatment with methadone maintenance or naltrexone implantation. Arch Gen Psychiatry. 2008 Apr;65(4):457–65. doi: 10.1001/archpsyc.65.4.457. [DOI] [PubMed] [Google Scholar]
- 75.Gibson AE, Degenhardt LJ, Hall WD. Opioid overdose deaths can occur in patients with naltrexone implants. Med J Aust. 2007;186(3):152–3. doi: 10.5694/j.1326-5377.2007.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 76.Miotto K, McCann MJ, Rawson RA, Frosch D, Ling W. Overdose, suicide attempts and death among a cohort of naltrexone-treated opioid addicts. Drug Alcohol Depend. 1997;45(1–2):131–4. doi: 10.1016/s0376-8716(97)01348-3. [DOI] [PubMed] [Google Scholar]
- 77.Kaner EF, Beyer F, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007 Apr 18;2:CD004148. doi: 10.1002/14651858.CD004148.pub3. Review. [DOI] [PubMed] [Google Scholar]
- 78.Walton MA, Goldstein AL, Chermack ST, et al. Brief alcohol intervention in the emergency department: moderators of effectiveness. J Stud Alcohol Drugs. 2008 Jul;69(4):550–60. doi: 10.15288/jsad.2008.69.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bazargan-Hejazi S, Bing E, Bazargan M, et al. Evaluation of a brief intervention in an inner-city emergency department. Ann Emerg Med. 2005 Jul;46(1):67–76. doi: 10.1016/j.annemergmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 80.Bernstein E, Bernstein J, Feldman J, Academic ED. SBIRT Research Collaborative. The impact of screening, brief intervention, and referral for treatment on emergency department patients’ alcohol use. Ann Emerg Med. 2007 Dec;50(6):699–710. 710. e1–6. doi: 10.1016/j.annemergmed.2007.06.486. Epub 2007 Sep 17. [DOI] [PubMed] [Google Scholar]
- 81.Bernstein E, Bernstein J, Feldman J, Academic ED. SBIRT Research Collaborative. The impact of screening, brief intervention and referral for treatment in emergency department patients’ alcohol use: a 3-, 6- and 12-month follow-up. Alcohol Alcohol. 2010 Nov-Dec;45(6):514–9. doi: 10.1093/alcalc/agq058. Epub 2010 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]