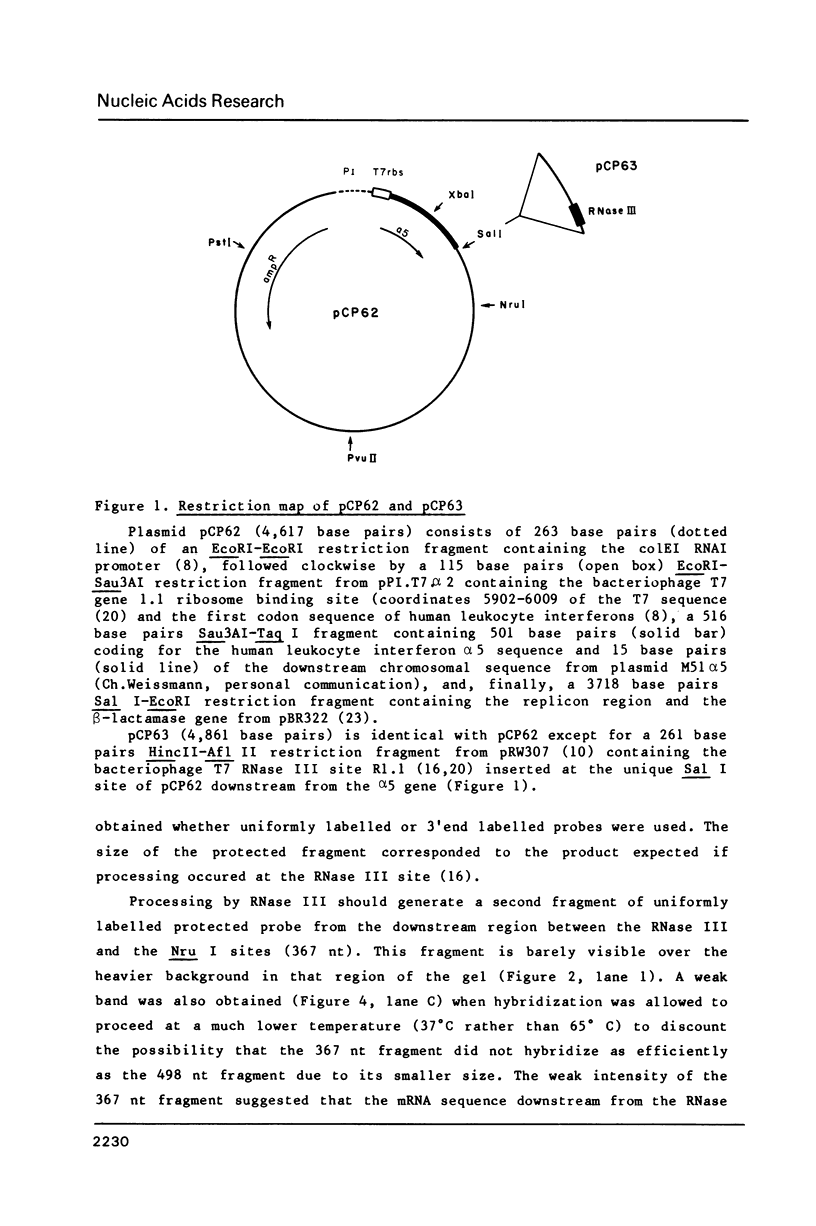
Abstract
We report that processing at a cloned bacteriophage T7 RNase III site results in strong stabilization of the mRNA relative to the full-length transcript. In contrast, processing by RNase III of the bacteriophage lambda int transcript leads to rapid degradation of the messenger. It is proposed that the mode of cleavage within the RNase III site determines mRNA stability. Single cleavage leaves part of the phage T7 RNase III site in a folded structure at the generated 3' end and stabilizes the upstream mRNA whereas double cleavage at the lambda int site removes the folded structure and accelerates degradation. In addition, the processed transcript is as active a messenger as the unprocessed one and can direct protein synthesis for longer times. This increased efficiency is accompanied by a proportional (3-4 fold) increase in protein levels. In contrast, processing at the lambda int site reduces Int synthesis. Thus, processing may either stabilize mRNA and stimulate gene expression or destabilize a messenger and prevent protein synthesis. The end result appears to be determined by the mode of cleavage within the RNase III site.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
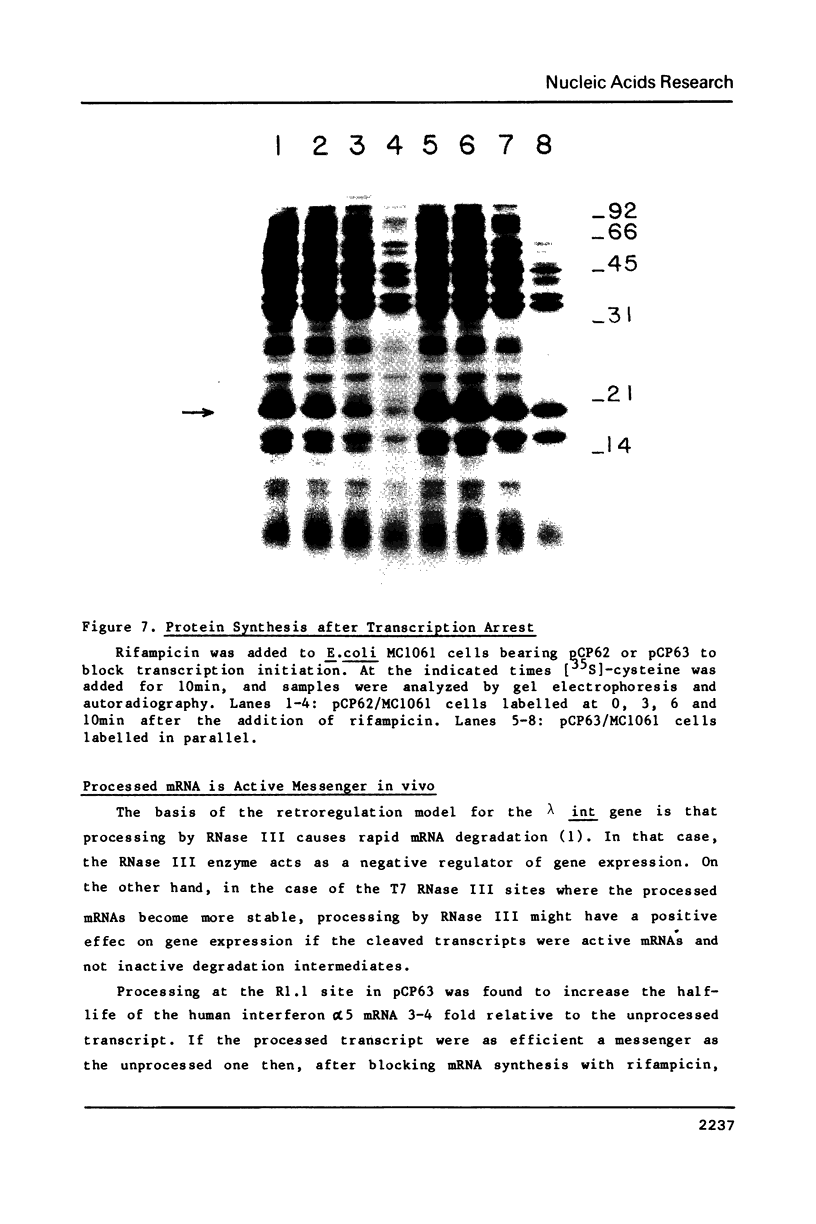
- Brosius J., Cate R. L., Perlmutter A. P. Precise location of two promoters for the beta-lactamase gene of pBR322. S1 mapping of ribonucleic acid isolated from Escherichia coli or synthesized in vitro. J Biol Chem. 1982 Aug 10;257(15):9205–9210. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenheimer P., Apirion D. Processing of procaryotic ribonucleic acid. Microbiol Rev. 1981 Dec;45(4):502–541. doi: 10.1128/mr.45.4.502-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros G., Montañez C., Hernandez T., Court D. Posttranscriptional control of bacteriophage lambda gene expression from a site distal to the gene. Proc Natl Acad Sci U S A. 1982 Jan;79(2):238–242. doi: 10.1073/pnas.79.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Wang S., Inouye M. Cell-free synthesis of a specific lipoprotein of the Escherichia coli outer membrane directed by purified messenger RNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4149–4153. doi: 10.1073/pnas.71.10.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lozeron H. A., Anevski P. J., Apirion D. Antitermination and absence of processing of the leftward transcript of coliphage lambda in the RNAase III-deficient host. J Mol Biol. 1977 Jan 15;109(2):359–365. doi: 10.1016/s0022-2836(77)80039-9. [DOI] [PubMed] [Google Scholar]
- Lozeron H. A., Dahlberg J. E., Szybalski W. Processing of the major leftward mRNA of coliphage lambda. Virology. 1976 May;71(1):262–277. doi: 10.1016/0042-6822(76)90111-2. [DOI] [PubMed] [Google Scholar]
- Marrs B. L., Yanofsky C. Host and bacteriophage specific messenger RNA degradation in T7-infected Escherichia coli. Nat New Biol. 1971 Dec 8;234(49):168–170. doi: 10.1038/newbio234168a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Panayotatos N. DNA replication regulated by the priming promoter. Nucleic Acids Res. 1984 Mar 26;12(6):2641–2648. doi: 10.1093/nar/12.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotatos N., Truong K. Specific deletion of DNA sequences between preselected bases. Nucleic Acids Res. 1981 Nov 11;9(21):5679–5688. doi: 10.1093/nar/9.21.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cloning and localization of the in vitro functional origin of replication of bacteriophage T7 DNA. J Biol Chem. 1979 Jun 25;254(12):5555–5561. [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Dunn J. J. A nucleotide sequence from a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):822–826. doi: 10.1073/pnas.74.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. The specificity of RNase III cleavage of bacteriophage T7 early messenger RNAs. Brookhaven Symp Biol. 1975 Jul;(26):277–285. [PubMed] [Google Scholar]
- Schmeissner U., McKenney K., Rosenberg M., Court D. Removal of a terminator structure by RNA processing regulates int gene expression. J Mol Biol. 1984 Jun 15;176(1):39–53. doi: 10.1016/0022-2836(84)90381-4. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]