Abstract
Background:
Increased protein proportions in the diet combined with energy restriction has been shown to enhance weight loss during dietary intervention. It is not known if the beneficial effect of dietary protein exists in the general population under normal living conditions without a negative energy balance.
Methods:
A total of 1834 participants (n = 443 men, n = 1391 women) were recruited from the CODING study. Participants’ dietary macronutrient compositions were determined through a Willett FFQ. Body composition variables including percent body fat (%BF), percent trunk fat (%TF), percent total lean mass (%LM), and percent trunk lean mass (%TLM) were determined using DXA. Major confounding factors including age, physical activity levels, total caloric intake, carbohydrate intake, menopausal status, smoking status and medication use were controlled for in all analyses.
Results:
Significant inverse relationships were observed between dietary protein intake (g/kg body weight/day) and weight, waist circumference, waist-to-hip ratio, BMI, %BF, and %TF (P < 0.001). Significant positive relationships were observed with %LM and %TLM (P < 0.001). Additionally, significant differences in weight (12.7 kg in men, 11.4 kg in women), BMI (4.1 BMI units in men, 4.2 units in women), and %BF (7.6% in men, 6.0% in women) were observed between low and high dietary protein consuming groups (P < 0.001). Dietary protein explained 11% of the total variation in %BF in the NL population.
Conclusion:
This study provides strong evidence that higher protein intake, even in the absence of energy restriction, is associated with a more favorable body composition in the general population.
Keywords: Protein intake, body composition, obesity, DXA
Introduction
The prevalence of obesity has been rising dramatically over the past three decades and is now a major public health concern as it affects over 300 million people globally.1 Obesity is widely recognized as a chronic disease associated with many serious health problems including type 2 diabetes, cardiovascular disease, hypertension, osteoarthritis, gall bladder disease, and some types of cancer.2,3 Due to the high prevalence of obesity and its associated health consequences, effective weight reduction and maintenance strategies are needed. The role of marconutrients (protein, fat and carbohydrate) in the diet has long been debated.4–6 Altering the levels of protein, carbohydrate, or fat is popular with those trying to find the best strategies for successful weight loss and maintenance. A great deal of attention has been focused on the modification of carbohydrate and fat intake as they are major energy contributors of the diet.7 As the efficacy of these diet plans has recently come under scrutiny, more emphasis is now being placed on high protein diets as alternative weight loss strategies.8–12
It is evident that an energy restricted diet high in protein can more effectively allow for weight loss and weight maintenance than a diet with a lower protein composition in randomized control trials and weight loss intervention studies.5,8–12 High protein diets promote a reduction in overall body fat percentage through an increase in lean body mass at the expense of fat mass in participants with similar activity levels.13,14 In addition, it has been suggested that protein may exert a greater thermogenic effect compared to carbohydrate and fat, and therefore may contribute to greater energy expenditure during rest.15–17 Most studies related to dietary protein intake and weight loss thus far have been generated from an intervention approach, in which animals or humans are fed a high protein/low carbohydrate diet, with dietary protein intake generally being greater than the recommended daily intake value of 0.86 g/kg body weight/day.18 Moreover, it is not known if dietary protein works the same way under a negative energy balance compared to an energy balanced state. At the current time, critical data are lacking regarding the beneficial effects of dietary protein on body composition in the general, non-dieting population. The objective of the current study was to investigate the relationship between dietary protein intake and body composition in the general Newfoundland population after controlling for potential confounding factors such as age, physical activity levels, gender, menopausal status, smoking status, and medication use.
Methods
Participants
A total of 1834 participants (443 men, 1391 women) were recruited from an ongoing, large scale nutrigenomics study (CODING Study: Complex Diseases in the Newfoundland Population: Environment and Genetics).19–21 All participants were from the Canadian province of Newfoundland and Labrador (NL) and were recruited via advertisements, posted flyers, and word of mouth. Inclusion criteria were as follows: 1) at least third generation Newfoundlander; 2) not pregnant at the time of the study; and 3) not having any serious metabolic, endocrine, or cardiovascular disease. Participants provided written and informed consent and the study received ethics approval from the Human Investigations Committee of the Faculty of Medicine, Memorial University of Newfoundland.
Dietary assessment
Each participant was required to complete a Willett Food Frequency Questionnaire (FFQ), a semi-quantitative method for the assessment of dietary intake patterns. The Willett FFQ is the most widely used dietary intake questionnaire for the study of nutritional information at the population level.22 For each food item listed, participants had to indicate their average use of the specified amount per week over the last year. Based on the choice selected, the amount was converted to a mean daily intake value. The daily intake for each food item consumed was entered into a meal plan using NutriBase Clinical Nutrition Manager (version 8.2.0; CyberSoft Inc, Arizona) and the total macronutrient and micronutrient intakes in kilocalorie (kcal) for each participant per day were automatically computed by the NutriBase software.
Body composition measurement
Dual Energy X-Ray Absorptiometry (DXA; Lunar Prodigy; GE Medical Systems, Madison, WI) was used for the measurement of percent total body fat (%BF), percent trunk fat (%TF), percent total lean body mass (%LM), and percent trunk lean body mass (%TLM). In addition to the measurements taken by DXA, anthropometric measurements were taken relating to the participants’ weight, height, waist and hip circumference. From these measurements, the participants’ BMI and waist-to-hip ratios were calculated. All measurements were performed in the morning following a 12 h fast. For a more detailed description of body composition measurements, see our previously published papers.19–21,23
Serum measurements
Blood samples were taken from all subjects in the morning, following a 12-hour fasting period. Serum was stored at −80 °C for subsequent analyses. Serum concentrations of glucose, triacylglycerols (TG), total cholesterol, and HDL cholesterol were performed on an Lx20 analyzer (Beckman Coulter Inc., CA, USA) using Synchron reagents. LDL cholesterol was calculated using the following formula: (total cholesterol)—(HDL cholesterol)—(TG/2.2) which is reliable in the absence of severe hyperlipidemia.
Demographics and other covariates
Information regarding the participants’ lifestyles was collected through a self-administered screening questionnaire. The questions were related to demographics (age, gender, family origin), disease status, smoking status and medication use. A separate questionnaire was administered to women with relation to their menopausal status. Physical activity was measured using the ARIC-Baecke Questionnaire, which consists of a Work Index, Sports Index, and Leisure Time Activity Index.24 All responses from this questionnaire were scored based on a five point scale with the exception of the name of the participant’s main occupation and the type of sports played. Three levels of physical activity (low, medium and high) were defined for occupation25 and sports.26 Physical activity was then measured via assessment of the number of hours spent doing the activity per week, the number of months spent doing the activity per year and the assigned exertion level. The Work, Sports, and Leisure Time Activity Indices were added together to give an estimate of total physical activity.
Statistical analysis
All data are reported as mean (SD) unless otherwise indicated. Participants with daily macronutrient intakes falling outside the range of ±3SDs were considered outliers and therefore excluded from further analyses to account for possible errors associated with over- or under-reporting of food intake on the FFQ (n = 22). This left a sample size of 1812 participants (n = 437 men, 1375 women). Differences in physical characteristics between men and women, along with differences in dietary macronutrient intakes, were assessed using one-way ANOVA. Pearson correlation analyses were initially conducted to assess the linear relationship between dietary protein intake (g/kg body weight/day) and various body composition markers (weight, BMI, waist circumference, waist-to-hip ratio, %BF, %TF, %LM, %TLM).
To overcome the possible influence of various confounding factors including age, physical activity levels, total caloric intake, carbohydrate intake (g/kg body weight/day), menopausal status, smoking status, and medication use, partial correlation analyses were performed controlling for these factors. As a number of participants failed to complete a physical activity level questionnaire (n = 310), only 1502 participants (364 men, 1138 women) were included in these analyses. There were no significant differences in any physical characteristic between those that completed the physical activity questionnaire and those that did not. All men and women were also subdivided into groups according to smoking status (smokers or non-smokers) and medication use (medication users or non-medication users). Women were further subdivided according to their menopausal status. Women who had undergone a hysterectomy (n = 22) were excluded from this analysis leaving 631 pre- menopausal and 485 post-menopausal women.
To further explore the relationship between dietary protein and body composition, participants were divided into tertiles according to protein intake (g/kg/day) as follows: low (bottom 33.3%), medium (middle 33.3%) and high (top 33.3%) protein consumers. For men, the range of dietary protein intake for the low, medium and high protein consumers was 0.22–0.81 g/kg/day, 0.82–1.25 g/kg/day, and 1.26–3.99 g/kg/day, respectively. For women, the ranges were 0.09–0.95 g/kg/day, 0.96–1.37 g/kg/day, and 1.38–3.66 g/kg/day, respectively. ANCOVA analyses were used to analyze the differences in body weight and body composition variables (weight, BMI, waist circumference, %BF, %TF, %LM, %TLM) among the three protein consuming groups. Using this model, each respective body composition measurement was included as the dependent variable and the protein consuming group included as a fixed factor. Covariates included in the model were age, physical activity level, and total caloric intake.
All statistical analyses were performed using SPSS, version 16.0 (SPSS Inc, Chicago). All tests were two-sided and a P value < 0.05 was considered to be statistically significant.
Results
Physical, biochemical, and dietary characteristics of the study participants
Participants’ physical and biochemical characteristics as well as macronutrient intakes are summarized in Table 1. Men and women differed significantly with relation to all body composition variables and physical markers. Participants’ ages ranged from 19 to 84 y, and men were 3.3 y younger than women. Participants’ BMIs ranged from 16.0 to 54.3 kg/m2 and %BF ranged from 4.6 to 59.9%. Men were heavier and taller compared to women and had significantly greater BMIs, waist-to-hip ratios, %LM, and %TLM but had significantly reduced %BF and %TF compared to the female participants (Table 1). Men had higher fasting glucose compared to women but lower total cholesterol and HDL cholesterol. Circulating levels of TG were also higher in men and no significant difference was evident in LDL cholesterol between genders. With respect to diet composition, female participants consumed a statistically higher proportion of protein and carbohydrate per day than male participants. There were no significant differences in overall caloric intake or fat intake between men and women.
Table 1.
Physical, biochemical, and dietary intake characteristics of participants (n = 1812).a
| Variables | Men (n = 437) | Women (n = 1375) |
|---|---|---|
| Age (yrs) | 41.0 (14.0) | 44.3 (12.1)b |
| Weight (kg) | 84.8 (14.1) | 68.9 (13.9)b |
| Height (cm) | 175.7 (6.4) | 162.1 (5.9)b |
| BMI (m/kg2) | 27.5 (4.4) | 26.2 (5.2)b |
| Waist (cm) | 97.4 (12.7) | 90.0 (14.2)b |
| Waist-to-hip ratio | 0.97 (0.07) | 0.89 (0.07)b |
| Total body fat (%) | 25.3 (7.4) | 37.5 (7.5)b |
| Trunk fat (%) | 30.2 (8.7) | 38.7 (8.8)b |
| Total lean body mass (%) | 74.7 (7.4) | 62.5 (7.5)b |
| Trunk lean body mass (%) | 69.8 (8.7) | 61.3 (8.8)b |
| Glucose (mmol/L) | 5.3 (1.0) | 5.1 (0.9)b |
| Cholesterol (mmol/L) | 5.04 (1.10) | 5.17 (1.03)b |
| LDL cholesterol (mmol/L) | 3.14 (0.88) | 3.10 (0.89) |
| HDL cholesterol (mmol/L) | 1.21 (0.28) | 1.55 (0.38)b |
| Triacylglycerol (mmol/L) | 1.50 (1.03) | 1.12 (0.67)b |
| Caloric intake (kcal/kg/day) | 26.9 (12.7) | 28.2 (13.4) |
| Protein intake (g/kg/day) | 1.18 (0.79) | 1.30 (0.84)b |
| Carbohydrate intake (g/kg/day) | 3.80 (1.99) | 4.11 (2.17)b |
| Fat intake (g/kg/day) | 0.72 (0.39) | 0.73 (0.37) |
| % Calories from protein | 17.0 (4.7) | 17.9 (4.6)b |
| % Calories from carbohydrate | 55.2 (9.4) | 56.4 (9.0)b |
| % Calories from fat | 24.4 (8.3) | 23.7 (7.8) |
Data presented as mean (SD). Differences between men and women assessed using one-way ANOVA.
P < 0.05.
Dietary protein intake and body composition according to gender
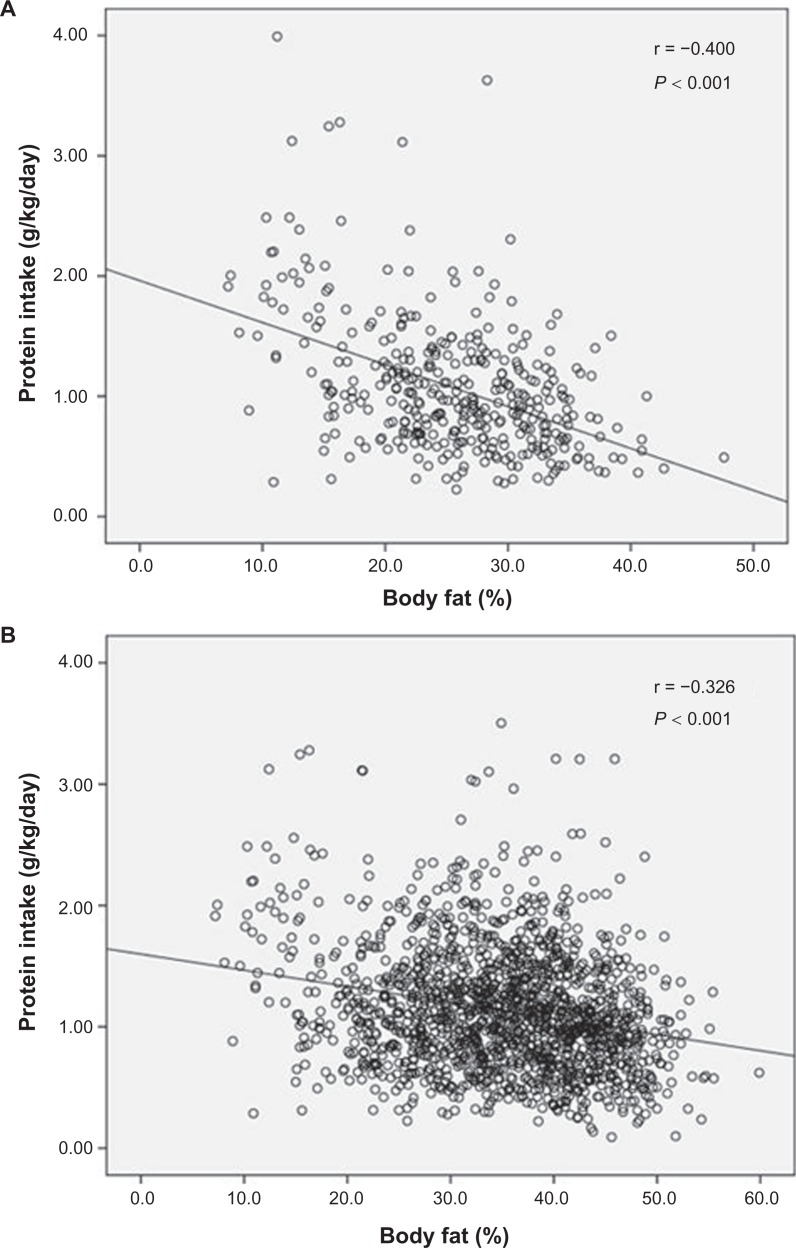
Pearson correlation analyses were conducted to assess the degree of linear relationship between dietary protein intake, measured with respect to body weight (g/kg/day), and various body composition variables. There was a significant negative relationship between dietary protein intake and weight, BMI, waist circumference, waist-to-hip ratio, %BF, and %TF (P < 0.001). Figure 1 shows the inverse relationship between dietary protein and %BF for both men and women. In addition, dietary protein intake was positively correlated with %LM and %TLM (P < 0.001). These relationships were not gender specific, as they were evident in both male and female participants.
Figure 1.
Relationship between body fat percentage (%BF) and dietary protein intake (g/kg/day) among Newfoundland men (A; n = 437) and women (B; n = 1375). A. r = −0.400, P < 0.001; B. r = −0.326, P < 0.001.
After controlling for confounding factors including age, physical activity level, and total caloric intake, the relationship between dietary protein intake and all of the measured body composition markers remained significant (Table 2). This was also true of the positive association between dietary protein and %LM as well as %TLM (P < 0.001), regardless of gender. Based on the coefficient of determination value, R2, dietary protein alone explained approximately 11% of the variance in %BF and 16% of the variation in body weight in our cohort.
Table 2.
Partial correlations between dietary protein intake (g/kg/day) and body composition variables for Newfoundland men and women (n = 1502).a
| Variables |
Men (n = 364)
|
Women (n = 1138)
|
|---|---|---|
| r | r | |
| Weight (kg) | −0.399b | −0.396b |
| BMI (m/kg2) | −0.349b | −0.355b |
| Waist (cm) | −0.393b | −0.367b |
| Waist-to-hip ratio | −0.167b | −0.127b |
| Total body fat (%) | −0.332b | −0.314b |
| Trunk fat (%) | −0.350b | −0.301b |
| Total lean body mass (%) | 0.332b | 0.314b |
| Trunk lean body mass (%) | 0.350b | 0.301b |
Controlled for age, physical activity levels, and total caloric intake.
P < 0.001.
Dietary protein intake and body composition in pre- and post-menopausal women
We also sought to explore the relationship between dietary protein and menopausal status in women (Table 3). Significant negative correlations were found between dietary protein (g/kg/day) and weight, BMI, waist circumference, waist-to-hip ratio, %BF, and %TF in both pre- and post-menopausal women (P < 0.001). A significant positive correlation was also evident between dietary protein and %LM as well as %TLM regardless of menopausal status. When taking into account potential confounding factors such as age, physical activity levels, and total caloric intake, the correlation coefficients were even greater in all measures.
Table 3.
Partial correlations between dietary protein intake (g/kg/day) and body composition variables for Newfoundland women based on menopausal status (n = 1116).a
| Variables |
Pre-menopausal (n = 631)
|
Post-menopausal (n = 485)
|
|---|---|---|
| r | r | |
| Weight (kg) | −0.376b | −0.384b |
| BMI (m/kg2) | −0.330b | −0.347b |
| Waist (cm) | −0.361b | −0.351b |
| Waist-to-hip ratio | −0.135b | −0.116b |
| Total body fat (%) | −0.318b | −0.286b |
| Trunk fat (%) | −0.290b | −0.304b |
| Total lean body mass (%) | 0.318b | 0.286b |
| Trunk lean body mass (%) | 0.290b | 0.304b |
Controlled for age, physical activity level, and total caloric intake.
P < 0.001.
Dietary protein intake and body composition based on smoking status and medication use
To further investigate the influence of additional covariates, male and female participants were subdivided based on smoking status (Table 4) and medication use (Table 5). For each subsample, partial correlation analyses were conducted controlling for confounding factors including age, physical activity levels, and total caloric intake. We found that dietary protein intake was negatively correlated with all body composition markers (weight, BMI, waist circumference, waist-to-hip ratio, %BF, and %TF) regardless of smoking status and medication use, however the degree of association was different between males and females as well as smokers vs. non-smokers, and medication users vs. non-medication users. A significant positive relationship was also found between dietary protein intake and %LM as well as %TLM.
Table 4.
Partial correlations between dietary protein intake (g/kg/day) and body composition variables based on smoking status (n = 1502).a
| Variables |
Men
|
Women
|
||
|---|---|---|---|---|
| Non-smoking (n = 323) | Smoking (n = 41) | Non-Smoking (n = 1002) | Smoking (n = 136) | |
|
|
|
|||
| r | r | r | r | |
| Weight (kg) | −0.406b | −0.562b | −0.416b | −0.326b |
| BMI (kg/m2) | −0.347b | −0.509b | −0.374b | −0.299b |
| Waist (cm) | −0.397b | −0.597b | −0.371b | −0.377b |
| Waist-to-hip ratio | −0.157b | −0.439b | −0.121b | −0.196b |
| %BF | −0.336b | −0.462b | −0.338b | −0.220b |
| %TF | −0.351b | −0.511b | −0.320b | −0.218b |
| %LM | 0.336b | 0.462b | 0.338b | 0.220b |
| %TLM | 0.351b | 0.511b | 0.320b | 0.218b |
Controlled for age, physical activity level, and total caloric intake.
P < 0.001.
Table 5.
Partial correlations between dietary protein intake (g/kg/day) and body composition variables based on medication use (n = 1502).a
| Variables |
Men
|
Women
|
||
|---|---|---|---|---|
| Non-medication user (n = 217) | Medication user (n = 147) | Non-medication user (n = 440) | Medication user (n = 698) | |
|
|
|
|||
| r | r | r | r | |
| Weight (kg) | −0.405b | −0.385b | −0.409b | −0.393b |
| BMI (kg/m2) | −0.348b | −0.351b | −0.347b | −0.363b |
| Waist (cm) | −0.397b | −0.388b | −0.346b | −0.382b |
| Waist-to-hip ratio | −0.161b | −0.179b | −0.047 | −0.168b |
| %BF | −0.308b | −0.385b | −0.304b | −0.330b |
| %TF | −0.331b | −0.392b | −0.269b | −0.330b |
| %LM | 0.308b | 0.385b | 0.304b | 0.330b |
| %TLM | 0.331b | 0.392b | 0.269b | 0.330b |
Controlled for age, physical activity level, and total caloric intake.
P < 0.001.
Comparison of body composition measures in low, medium and high protein consuming groups
Lastly, we wanted to investigate the relationship between low, medium, and high protein consuming groups and markers of body composition. The results presented in Table 6 demonstrate that those in the highest protein consuming group (top 33.3%) had significantly lower weight, BMI, waist circumference, %BF and %TF, and significantly higher %LM and %TLM compared to medium and low protein consumers. After correcting for confounding factors, the results remained significant in both males and females (P < 0.001). Table 7 shows the differences in body composition variables between participants in the low and high protein consuming groups as well as the percentage of total variation in body composition resulting from dietary protein intake alone. Between low and high protein groups, the percentage of total variation in weight, BMI, %BF, and %TF resulting from dietary protein reached 16.2%, 16.2%, 36.2% and 38.3% respectively for men (P < 0.05), and 18.0%, 17.4%, 17.4% and 18.7% respectively for women (P < 0.05).
Table 6.
Body composition measurements among low, medium and high dietary protein consuming participants (n = 1502).a
| Variablesb | Protein group(g/kg × day)b | Men (n = 364) | Women (n = 1138) |
|---|---|---|---|
| Weight (kg) | L | 91.0 (14.6) | 74.7 (16.0) |
| M | 85.2 (13.0) | 68.7 (11.9) | |
| H | 78.3 (12.0) | 63.3 (10.0) | |
| P value | <0.001 | <0.001 | |
| Waist (cm) | L | 103.8 (13.1) | 95.8 (15.6) |
| M | 97.9 (11.1) | 90.2 (13.3) | |
| H | 90.6 (10.1) | 84.4 (10.9) | |
| P value | <0.001 | <0.001 | |
| BMI (kg/m2) | L | 29.4 (4.5) | 28.4 (5.9) |
| M | 27.7 (4.0) | 26.1 (4.5) | |
| H | 25.3 (3.7) | 24.2 (3.9) | |
| P value | <0.001 | <0.001 | |
| %BF | L | 28.6 (6.5) | 40.4 (7.0) |
| M | 26.4 (6.3) | 37.8 (7.0) | |
| H | 21.0 (7.7) | 34.4 (7.6) | |
| P value | <0.001 | <0.001 | |
| %TF | L | 34.3 (6.7) | 41.9 (8.1) |
| M | 31.7 (7.1) | 39.1 (8.2) | |
| H | 24.8 (9.6) | 35.3 (9.0) | |
| P value | <0.001 | <0.001 | |
| %LM | L | 71.4 (6.5) | 59.6 (7.0) |
| M | 73.8 (6.3) | 62.2 (7.0) | |
| H | 79.0 (7.7) | 65.6 (7.6) | |
| P value | <0.001 | <0.001 | |
| %TLM | L | 65.7 (6.7) | 58.1 (8.1) |
| M | 68.2 (7.1) | 60.9 (8.2) | |
| H | 75.2 (9.6) | 64.7 (9.0) | |
| P value | <0.001 | <0.001 |
Data are presented as mean (SD). Significance differences between groups assessed using AN COVA. Using this model, each body composition measurement was the dependent variable and protein consuming group as the fixed factor. Covariates included in this model were age, physical activity level, and caloric intake.
%BF, body fat percentage; %TF, trunk fat percentage; %LM, % lean body mass percentage; %TLM, trunk lean mass percentage; L, low protein group (bottom 33.3%); M, medium protein group (middle 33.3%); H, high protein group (top 33.3%).
Table 7.
Differences in body composition variables between low and high dietary protein consuming groups.a
| Variables | Men (n = 364) | Percentage of total variationb | Women (n = 1138) | Percentage of total variationb |
|---|---|---|---|---|
| Weight (kg) | −2.7 (1.7) | 16.2% | −11.4 (0.9) | 18.0% |
| Waist (cm) | −13.2 (1.5) | 14.6% | −11.4 (1.0) | 13.5% |
| BMI (kg/m2) | −4.1 (0.5) | 16.2% | −4.2 (0.3) | 17.4% |
| %BF | −7.6 (0.9) | 36.2% | −6.0 (0.5) | 17.4% |
| %TF | −9.5 (1.0) | 38.3% | −6.6 (0.6) | 18.7% |
| %LM | 7.6 (0.9) | 9.6% | 6.0 (0.5) | 9.1% |
| %TLM | 9.5 (1.0) | 12.6% | 6.6 (0.6) | 10.2% |
Data expressed as mean (SE).
Calculated based on Table 6 values using equation: (L-H)/H × 100%.
Effect of dietary carbohydrate intake on the relationship between protein intake and body composition
High protein diets are typically restricted in carbohydrates in various clinical weight reduction programs and as such, we cannot dismiss the possibility that the observed association with body composition is attributable to restricted carbohydrate intake as opposed to increased protein intake. To further address the effect of carbohydrate intake on the inverse relationship between dietary protein and body composition, we chose to repeat all dietary protein analyses after adjustment for carbohydrate intake. All significant associations between dietary protein intake and markers of body composition remained (P < 0.001 for all variables measured including weight, waist circumference, waist-to-hip ratio, BMI, %BF, and %TF).
Discussion
The role of macronutrients in the development of obesity has been debated. Currently, a number of popular diets exist with varying macronutrient compositions. For example, high protein/low carbohydrate diets are now becoming quite popular, and this is especially true of the recent Atkins diet.27 A number of studies have been done to evaluate the effectiveness of these diets on weight loss and maintenance with varying results.4–6,28 Although some studies have shown that energy restricted diets high in protein are more effective for weight loss and weight maintenance compared to diets with a lower protein composition,8–12 others have found no differences in weight loss as a result of varying protein intake.29 In a recent randomized controlled trial, four calorie-restricted diets varying in macronutrient composition resulted in similar weight reduction regardless of the proportion of protein, carbohydrate or fat.29 Although there is little doubt that a calorie-restricted diet will result in weight loss as a result of the negative energy balance, less is known regarding the beneficial role of dietary protein under normal living conditions, without energy restriction, in the general population. We have shown, for the first time that higher dietary protein is associated with a more favorable body composition profile in a large, non-dieting, NL cohort.
The strength of the current study lies in the fact that a number of potential confounding factors have been controlled for in all analyses. For example, menopause is an important factor related to changes in body composition as it is usually accompanied by dramatic changes in sexual hormones that can predispose women to weight gain and may affect macronutrient intake.30 Our results indicate that the protein/body composition relationship is evident in both pre- and post-menopausal women. Smoking status and medication use are potentially important covariates as well as both are known to affect appetite and body weight regulation. Almost one quarter of subjects in our cohort were self-reported smokers and over 50% reported taking medication (including multi-vitamin supplements). After separating the study sample to account for these covariates, the association between protein intake and body composition remained significant. Lastly, we controlled for both total caloric intake and physical activity levels in all analyses as body composition is largely determined by energy intake and expenditure. Even after controlling for these factors, the association between protein intake and body composition was significant. As a result, we provide a convincing argument that higher levels of dietary protein lead to lower body fat percentage.
The exact mechanisms through which dietary protein results in a more favorable body composition profile are not known. It has been suggested that the anorectic gut hormone peptide YY (PYY) likely plays a key role in the satiating effect of high protein diets as long term exposure to increased dietary protein results in elevated PYY levels as well as a reduction in hunger and weight gain.31 This suggests that alterations in the macronutrient composition of our diet may modulate the release of specific endogenous satiety factors, such as PYY, and therefore provide a potential treatment for weight gain and obesity.31 Other plausible meachanisms also exist. For example, the type of protein consumed may play a role in its satiating effects. It has been demonstrated that meals containing lactalbumin (a high-tryptophan compound found in whey or milk) suppress hunger more so than meals containing gelatin or gelatin plus trypotphan.32 As well, glycomacropeptide (GMP; a casein-derived whey protein) results in increased satiety compared to whey without GMP.33 Moreover, the response of appetite regulatory hormones, such as ghrelin, GLP-1, and cholecystokinin, to various types of dietary protein is different among normal weight, over weight and obese individuals suggesting that body composition itself may be an important factor related to the beneficial effects of dietary protein on satiety.34 In addition, a recent study has shown that a high protein meal at breakfast has greater effects on satiety compared to meals consumed at other times during the day indicating that timing may also be important.35 It is clear from these studies that the issue of dietary protein intake is a very complicated one. It is necessary to address this problem using total dietary protein intake at the population level as we did in this study, and this is likely the only practical approach at the present time.
The recommended dietary allowance for protein in Canada is 46 g/day for women and 56 g/day for men or 0.86 g/kg/day.18 These recommendations are in place to prevent protein deficiency as a result of nitrogen loss from the body due to natural cell death or through normal metabolic processes such as ammonia detoxification and urea production. As such, these values represent the minimum daily protein requirement to maintain normal physiological function. So far, there is little data available regarding the relationship between dietary protein and %BF in a population with adequate protein intake. Our results suggest that increasing dietary protein to exceed the minimum requirements may be a plausible way to achieve a desirable body composition profile.
Although we provide convincing results regarding the beneficial effect of protein in the diet, our study is not without limitations. The use of the Willett FFQ for the assessment of dietary intake patterns is one potential limitation. FFQs may have inherent measurement error or bias from under-reporting or possible over-reporting of some food items, however, this questionnaire has been repeatedly validated in the literature. It is the most frequently used questionnaire for the assessment of dietary intake at the population level and has been successfully used in a number of studies in the Canadian population.36–38 Thus, we considered the Willett FFQ to be a reasonable method for the assessment of dietary intake patterns in this population-based study. Furthermore, it would be beneficial to divide our cohort according to specific medications used, however due to the vast number of medications consumed by our subjects (including vitamins and supplements) it is just not feasible to divide them into treatment groups. Doing this would decrease the sample size of each group drastically and reduce statistical power.
We have shown, for the first time that dietary protein is inversely associated with the whole spectrum of body composition measurements determined by DXA (%BF and %TF) as well as conventional obesity markers such as weight, BMI, waist circumference, and waist-to-hip ratio in a free-living, non-dieting population. In addition, we found a significant difference in weight and BMI between low and high protein consuming groups. Moreover, this favorable association between dietary protein and body composition was independent of age, gender, physical activity levels, total caloric intake, carbohydrate intake, smoking status, medication use and menopausal status at the population level. In our cohort, dietary protein was able to explain 11% of the variation in %BF in participants. This suggests that it may be possible to achieve a more favorable body composition by increasing the amount of protein in the diet, even in the absence of energy restriction. Therefore, an adequate increase in dietary protein is a potential recommendation for the general population to improve overall body composition.
Acknowledgments
We would like to thank all of the volunteers who participated in the present study. We would also like to thank the following people for their contributions to the collection of data: Hongwei Zhang, Jeffery Power, Laura Nugent, Jordan Stone, Anne Gregory, Meghan Greene, Lindsay Barbour, Kelda Ghaney, and Naghmeh Soltani. Additionally, we would like to thank Dr. Ahmed El-Sohemy for his help with data analysis.
KG was responsible for the data analysis, writing of the manuscript, and assisting in the collection of data. JS aided with data collection, data analysis and editing of the manuscript. ER and SV aided in editing the manuscript and provided excellent comments. GS was responsible for the study design and the revision of the manuscript and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors had a personal or financial conflict of interest. GS holds the position of chair of pediatric genetics, which is supported by Novartis Pharmaceuticals. This study is supported in part by the Canadian Foundation for Innovation (CFI), the Canadian Institute for Health Research (operating grant: OOP-77984 to Guang Sun), and the Newfoundland and Labrador Centre for Applied Health Research (NLCAHR).
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.World Health Organization . Fact Sheet. 2003. Obesity and Overweight. [Google Scholar]
- 2.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 3.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 4.Eisenstein J, Roberts SB, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev. 2002;60:189–200. doi: 10.1301/00296640260184264. [DOI] [PubMed] [Google Scholar]
- 5.Noakes M, Keogh JB, Foster PR, Clifton PM. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr. 2005;81:1298–306. doi: 10.1093/ajcn/81.6.1298. [DOI] [PubMed] [Google Scholar]
- 6.Schoeller DA, Buchholz AC. Energetics of obesity and weight control: does diet composition matter? J Am Diet Assoc. 2005;105:S24–8. doi: 10.1016/j.jada.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 7.St. Jeor ST, Howard BV, Prewitt TE, Bovee V, Bazzarre T, Eckel RH. Dietary protein and weight reduction: a statement for healthcare professionals from the Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2001;104:1869–74. doi: 10.1161/hc4001.096152. [DOI] [PubMed] [Google Scholar]
- 8.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity. 2007;15:421–9. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 9.Weigle DS, Breen PA, Matthys CC, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–8. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Meckling KA, Sherfey R. A randomized trial of a hypocaloric high-protein diet, with and without exercise, on weight loss, fitness, and markers of the Metabolic Syndrome in overweight and obese women. Appl Physiol Nutr Metab. 2007;32:743–52. doi: 10.1139/H07-059. [DOI] [PubMed] [Google Scholar]
- 11.Layman DK, Evans EM, Erickson D, et al. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J Nutr. 2009;139:514–21. doi: 10.3945/jn.108.099440. [DOI] [PubMed] [Google Scholar]
- 12.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–10. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- 13.Skov AR, Toubro S, Rønn B, Holm L, Astrup A. Randomized trial on protein vs. carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999;23:528–36. doi: 10.1038/sj.ijo.0800867. [DOI] [PubMed] [Google Scholar]
- 14.Layman DK, Boileau RA, Erickson DJ, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–7. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 15.Crovetti R, Porrini M, Santangelo A, Testolin G. The influence of thermic effect of food on satiety. Eur J Clin Nutr. 1998;52:482–8. doi: 10.1038/sj.ejcn.1600578. [DOI] [PubMed] [Google Scholar]
- 16.Halton TL, Hu FB. The Effects of High Protein Diets on Thermogenesis, Satiety and Weight Loss: A Critical Review. J Am Coll Nutr. 2004;23:373–85. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 17.Johnston CS, Day CS, Swan PD. Postprandial thermogenesis is increased 100% on a high protein, low fat diet versus a high-carbohydrate, low fat diet in healthy, young women. J Am Coll Nutr. 2002;21:55–61. doi: 10.1080/07315724.2002.10719194. [DOI] [PubMed] [Google Scholar]
- 18.Health Canada Eating Well with Canada’s Food Guide: A Resource for Educators and Communicators. Version current 5 Feb 2007. Internet: http://www.hc-sc.gc.ca/fn-an/pubs/res-educat/res-educat_2_e.html (accessed 6 April 2009).
- 19.Sun G, French CR, Martin GR, et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr. 2005;81:74–8. doi: 10.1093/ajcn/81.1.74. [DOI] [PubMed] [Google Scholar]
- 20.Sun G, Vasdev S, Martin GR, Gadag V, Zhang H. Altered calcium homeostasis is correlated with abnormalities of fasting serum glucose, insulin resistance, and beta-cell function in the Newfoundland population. Diabetes. 2005;54:3336–9. doi: 10.2337/diabetes.54.11.3336. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AP, Shea JL, Sun G. comparison of the classification of obesity by BMI vs. dual-energy X-ray absorptiometry in the newfoundland population. Obesity. 2009. Epub ahead of print. [DOI] [PubMed]
- 22.Michels KB, Willett WC. Self-administered semiquantitative food frequency questionnaires: patterns, predictors, and interpretation of omitted items. Epidemiology. 2009;20:295–301. doi: 10.1097/EDE.0b013e3181931515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randell EW, Mathews M, Gadag V, Zhang H, Sun G. Relationship between serum magnesium values, lipids and anthropometric risk factors. Atherosclerosis. 2008;196:413–9. doi: 10.1016/j.atherosclerosis.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Baecke JAH, Burema J, Frijters JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 25.Nederlandse Voedingsmiddelentabel. 32nd ed. ‘s-Gravenhage: Voorhichtingsbureau voor de Voeding; 1979. [Google Scholar]
- 26.Durnin JVGA, Passmore R. Energy, work and leisure. 1st ed. Heinemann Educational Books Ltd; London: 1967. [Google Scholar]
- 27.Buchholz AC, Schoeller DA. Is a calorie a calorie? Am J Clin Nutr. 2004;79(5):899S–906S. doi: 10.1093/ajcn/79.5.899S. [DOI] [PubMed] [Google Scholar]
- 28.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 29.Sacks FM, Bray GA, Carey VJ, et al. Comparison of Weight-Loss Diets with Different Compositions of Fat, Protein, and Carbohydrates. N Engl J Med. 2009;360(9):859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovejoy JC. The influence of sex hormones on obesity across the female life span. J Womens Health. 1998;7:1247–56. doi: 10.1089/jwh.1998.7.1247. [DOI] [PubMed] [Google Scholar]
- 31.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–33. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, et al. Effects of complete whey-protein breakfasts versus whey without GMP-breakfasts on energy intake and satiety. Appetite. 2009;52(2):388–95. doi: 10.1016/j.appet.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Burton-Freedom BM. Glycomacropeptide (GMP) is not critical to whey-induced satiety, but may have a unique role in energy intake regulation through cholecystokinin (CCK) Physiol Behav. 2008;93(1–2):379–87. doi: 10.1016/j.physbeh.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91(8):2913–9. doi: 10.1210/jc.2006-0609. [DOI] [PubMed] [Google Scholar]
- 35.Leidy HJ, Bossingham MJ, Mattes RD, Campbell WW. Increased dietary protein consumed at breakfast leads to an initial and sustained feeling of fullness during energy restriction compared to other meal times. Br J Nutr. 2009;101(6):798–803. doi: 10.1017/s0007114508051532. [DOI] [PubMed] [Google Scholar]
- 36.Eny KM, Wolever TM, Fontaine-Bisson B, El-Sohemy A. Genetic variant in the glucose transporter type 2 is associated with higher intakes of sugars in two distinct populations. Physiol Genomics. 2008;33:355–60. doi: 10.1152/physiolgenomics.00148.2007. [DOI] [PubMed] [Google Scholar]
- 37.Fontaine-Bisson B, Wolever TM, Connelly PW, Corey PN, El-Sohemy A. NF-kappaB —94Ins/Del ATTG polymorphism modifies the association between dietary polyunsaturated fatty acids and HDL-cholesterol in two distinct populations. Atherosclerosis. 2009;204:465–70. doi: 10.1016/j.atherosclerosis.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 38.Paradis AM, Godin G, Lemieux S, Pérusse L, Vohl MC. Eating behaviours of non-obese individuals with and without familial history of obesity. Br J Nutr. 2009;101(7):1103–09. doi: 10.1017/S0007114508055645. [DOI] [PubMed] [Google Scholar]