Abstract
The increase in obesity and the aging of the population has lead to an increase in the incidence of type 2 diabetes. This has led to the development of new drugs such as thiazolidinediones (TZDs) which are Peroxisome Proliferator-Activated Receptor (PPARgamma) agonists, to treat type 2 diabetes. TZDs have recently been at the center of a controversy with regards to their cardiovascular safety. Pioglitazone is a TZD which has been shown to be effective in glycemic control by lowering insulin resistance. Pioglitazone also has beneficial effects on lipid metabolism and cardiovascular risk. The safety and efficacy of pioglitazone including its pleotropic effects are discussed at length in this article.
Keywords: Pioglitazone, safety, efficacy, cardiovascular, diabetes
Introduction
The incidence of type 2 diabetes is at epidemic proportions through out the world. Patients with diabetes have a 2–4 fold increased risk of cardiovascular disease when compared to the general population. They also have a greatly increased risk for microvascular disease. Hence medications that successfully control hyperglycemia in type 2 diabetes patients are of utmost importance. The underlying primary pathology in type 2 diabetes is insulin resistance. Drugs that address insulin resistance are effective in controlling hyperglycemia. Thiazolidinediones (TZD) are one such class of drugs that work through PPAR gamma activation. Pioglitazone is a TZD which is widely used for treating patients with type 2 diabetes. Discussed in this review are the overall safety and efficacy, cardiovascular safety and other pleotropic effects of pioglitazone.
Pioglitazone safety review
Cardiovascular safety
There has been much discussion about the cardiovascular safety of TZDs over the last few years since the findings of a meta-analysis of 42 trials, in which Nissen et al compared the risk for MI associated with rosiglitazone with that of placebo or other antihyperglycemic agents. Rosiglitazone was associated with a significant 43% increased risk for MI (P = 0.03).1 Since then several studies have shown some risk of increased myocardial infarction associated with rosiglitazone use.
The PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) study was the first randomized, double-blind outcome study in patients with type 2 diabetes managed with diet and/or oral blood glucose-lowering drugs and/or insulin who had a history of macrovascular disease, assessing the effect of pioglitazone on the secondary prevention of macrovascular events.2 A total of 5238 patients were randomized with the cohort of patients, a typical type 2 diabetic population at high risk of further macrovascular events. The average time of observation was 34.5 months. Treatment with pioglitazone reduced the secondary endpoint of combined all-cause mortality, non-fatal myocardial infarction, and stroke by 16%.2 However the primary outcome composite consisting of death, myocardi a l infarction, stroke, acute coronary syndrome, leg amputation or coronary/leg vascularization was not statistically less although a declining trend was seen. Another subgroup analysis from PROactive demonstrated that pioglitazone reduced the risk of recurrent stroke significantly in high-risk patients with T2D.3 However the criticism for PROactive was that the choice of its primary composite end-point, which included peripheral vascular disease was a physician driven rather than disease-driven outcome. In a meta-analysis of 94 trials that excluded the PROactive trial pioglitazone was associated with a reduced all-cause mortality with no relevant effect on coronary events.4
The CHICAGO study (Carotid Intima-Media Thickness in Atherosclerosis Using Pioglitazone) tested the hypothesis that pioglitazone would have a beneficial effect for reducing CIMT progression, compared with glimepiride.5 Treatment with pioglitazone produced improvement in several parameters, such as systolic blood pressure and lipid levels, including a 14% increase in HDL cholesterol, and reduced CIMT progression, compared with glimepiride. However, only the beneficial effect on HDL cholesterol predicted its beneficial effect for reducing CIMT progression. Data from the CHICAGO study indicate that the progression of carotid artery intima-media thickness, a marker of atherosclerosis and a surrogate end point for cardiovascular disease, was slowed more with pioglitazone than glimepiride in a racially diverse population of men and women with diabetes mellitus type 2.5
The PERISCOPE Trial (Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation) compared the effects of an insulin sensitizer pioglitazone, with an insulin secretagogue, glimepiride, on the progression of coronary atherosclerosis in patients with type 2 diabetes.6 A total of 543 patients underwent coronary intravascular ultrasonography and were randomized to receive glimepiride, or pioglitazone, for 18 months with titration to maximum dosage, if tolerated. Atherosclerosis progression was measured by repeat intravascular ultrasonography examination in 360 patients at study completion. In patients with type 2 diabetes and coronary artery disease, treatment with pioglitazone resulted in a significantly lower rate of progression of coronary atherosclerosis compared with glimepiride.
The risk of developing congestive heart failure or worsening of present heart failure is a constant feature of the thiazolidinediones. The PROactive studies as well as other studies show increased risk for congestive heart failure with pioglitazone.7 However the risk is small and some some large studies show non significant increases in heart failure risk.8
Pioglitazone’s cardiovascular effects may be linked in part to its effects on lipid metabolism. The PROactive and CHICAGO studies as well as other studies show that pioglitazone significantly lowers triglycerides (11%–15%) and increases HDL(9%–14%)(5–9). Even though pioglitazone increases LDL (5%–7%), the quality of LDL may be altered so as to be less artherogenic. Pioglitazone improves insulin resistance in T2DM in association with mobilization of fat and toxic lipid metabolites out of muscle.10
Mechanisms by which pioglitazone may mediate its cardiovascular effects
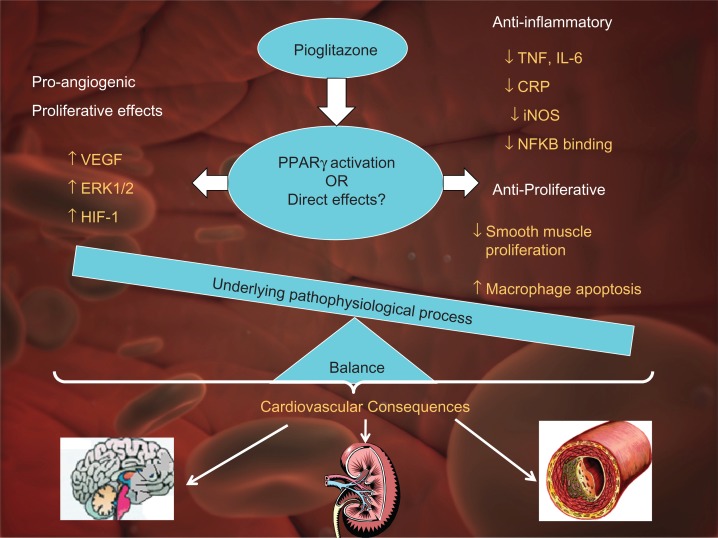
Taken together, animal and human data suggest that pioglitazone may be beneficial in terms of improving cardiovascular outcomes. However the mechanisms attributed to these cardiovascular effects are controversial. PPAR-γ agonists have widespread effects involving, inflammation, atherosclerosis, obesity and diabetes (Fig. 1).11
Figure 1.
Mechanisms underlying cardiovascular effects of pioglitazone.
Note: The cardiovascular effects of pioglitazone may be due in part to PPAR activation and in part due to direct effects. The cardiovascular outcome is dependant upon the balance between proangiogenic and anti-inflammatory, anti-angiogenic effects, interacting with the underlying pathophysiological process.
Abbreviations: ERK; Extracellular signal regulated kinase, HIF; Hypoxia inducible factor-1, VEGF; Vascular endothelial growth factor, NFKB; Nuclear factor Kappa-B.
Anti-angiogenic, anti-proliferative and anti-inflammatory effects
Thiazolidinediones have been shown to decrease post angioplasty neointimal hyperplasia in both animals and humans12–15 PPAR-γ ligands have been shown to inhibit and stimulate angiogenesis. Pioglitazone has been shown to have anti-proliferative effects in humans, decreasing in-stent neointimal proliferation.16 Pioglitazone inhibits the effects of inflammation such as decreasing bFGF in obese non-diabetes patients.17,18 Pioglitazone decreases urinary TGF-beta1 excretion in diabetes and obese non-diabetes patients.19 Pioglitazone decreases inflammatory responses in adipose tissue/cells induced by monocytes/macrophages by acting on either or both cell types. Another study demonstrated that activation of PPARgamma and PPAR beta/delta by pioglitazone in neurons triggers diverse neuroprotective mechanisms.20 A recent study showed that pioglitazone decreases urinary TGF-beta1 excretion in type 2 diabetics, which may be partly contributed to its direct reno-protection.20 Thus a review of the literature suggests that pioglitazone may have vasculoprotective effects in several organs such as heart, kidney and brain (Fig. 1).
Pro-angiogenic and proliferative effects of pioglitazone
There is however contradictory evidence that suggests that pioglitazone also has proangiogenic and proliferative effects. Diabetic mice with induced unilateral hind limb ischemia, when treated with pioglitazone showed normalization of VEGF, up-regulation of eNOS activity, and partial restoration of blood flow recovery.21 In mice treated with pioglitazone, VEGR-receptor-2 positive endothelial progenitor cells (EPCs) were up-regulated and migratory capacity was increased. In vivo angiogenesis was increased two-fold.22
Most study designs do not distinguish between direct effects and indirect effects of pioglitazone and the end outcome result is likely a sum of the different factors that contribute to the pathological process (Fig. 1).
Hypoglycemia
Patients receiving pioglitazone in combination with insulin or oral hypoglycemic agents may be at risk for hypoglycemia, and a reduction in the dose of the concomitant agent may be necessary. A recent observational study from the U.K. studying the incidence of hypoglycemia in patients using different oral anti-diabetic drugs concluded that the incidence rate of hypoglycemia was 50%–100% less in pioglitazone treated patients as opposed to those who were on nateglinide or repaglinide.23 An interesting observation was that hypoglycemia was more common in women on TZDs (pioglitazone and rosiglitazone) than men on similar drugs.23 Pioglitazone monotherapy was associated with much less hypoglycemia than glyburide treated patients with newly diagnosed type 2 diabetes (24.3% in glyburide group vs. 4.4% in pioglitazone group, P = 0.0001).24 Also, pioglitazone treated patients had significantly less hypoglycemia when used as an add on therapy to sulfonylurea or metformin, as compared to insulin glargine.25 In combination with insulin the rates of hypoglycemia are higher than insulin use alone, as shown in the post hoc analysis of PROACTIVE study (42.1% in the combination group vs. 29% in the insulin alone group, P < 0.001).26
Edema
Pioglitazone treatment is associated with edema and appears to be dose related.27 Peripheral edema occurs in 4 to 6 percent of patients treated with TZDs (vs. 1 to 2% in control group) and more frequently in patients with history of heart failure.28 Hence it should be used with caution in patients with edema. In the post-marketing experience, reports of initiation or worsening of edema have been received (www.fda.gov). Pioglitazone was associated with higher rates of heart failure and edema in type 2 diabetics with mild cardiac disease, as compared to glyburide.29 Thus, pioglitazone should be used with caution in patients at risk for heart failure and monitored for signs and symptoms.
Weight gain
Pioglitazone causes dose-dependent and time-dependent weight gain alone and in combination with other hypoglycemic agents. The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation.27,30
Hematologic
Pioglitazone may cause decreases in hemoglobin and hematocrit, possibly without clinical consequence.31 Across all clinical studies, mean hemoglobin values declined by 2% to 4% in patients treated with pioglitazone (www.fda.gov). These changes primarily occurred within the first 4 to 12 weeks of therapy and remained relatively constant thereafter. These changes may be related to increased plasma volume and have rarely been associated with any significant hematologic clinical effects.
Hepatic effects
There was no evidence of pioglitazone-induced hepatotoxicity or elevation of ALT levels in the pre-approval clinical studies (US and worldwide) (www.fda.gov). The liver toxicity associated with troglitazone is likely not a class effect.32 In a review of 13 double blind studies, pioglitazone was associated with ALT elevation (3 × ULN) in 0.26% of 1526 patients as opposed to 1.91% of 2510 patients receiving troglitazone.33 ALT levels 10 × ULN was not observed in the pioglitazone group. However, 2 cases of hepatotoxicity have been reported with pioglitazone, which resolved after discontinuation of pioglitazone.34,35 In a randomized, double blind, hepatic safety study of pioglitazone in 411 patients for 3 years, 3 people had ALT > 3 × ULN compared to 9 people in a similar group on glimepiride. 36 The study concluded that pioglitazone has similar hepatic safety profile as glimepiride in long-term use in poorly controlled diabetics.36 Also, pioglitazone was not associated with liver impairment when used as a add on therapy with other oral hypoglycemic agents.37 The FDA recommends that patients treated with pioglitazone undergo periodic monitoring of liver enzymes. Therapy with pioglitazone should not be initiated if the patient exhibits clinical evidence of active liver disease or the ALT levels exceed 2.5 times the upper limit of normal.
Macular edema
It is unknown whether or not there is a causal relationship between pioglitazone and macular edema. Concern for TZD associated macular edema has risen due to multiple case reports.38,39 A case report of macular edema being resolved with systemic furosemide suggests that thiazolidinediones may exacerbate macular edema.40 In a larger study of 292 patients, Shen et al found no association of rosiglitazone with macular edema.41 The largest cross-sectional study to date did not show an association between thiazolidinedione exposure and macular edema in patients with type 2 diabetes.42 In conclusion, TZDs do not appear to be associated with macular edema in the largest study to-date. Further studies are needed to confirm this lack of effect. Patients with diabetes should have regular eye exams, as recommended by ADA.43
Pioglitazone’s effect on bone
Thiazolidinediones have been associated with low bone density and increased fracture risk.44,45 A 4y observational study in older diabetic population showed that pioglitazone (and rosiglitazone) lowered bone density at trochanter, spine and whole body compared to those not taking TZDs.45 Separately pioglitazone has been shown to increase peripheral fractures in women compared to control groups (1.9 fracture vs. 1.1 per 100 patient years), but not in men, in the PROactive trial. Similar findings have been reported in other trials.6,46,47 There is insufficient data to suggest that pioglitazone increases risk of hip or spine fracture.
Drug interactions
In vivo drug-drug interaction studies have suggested that pioglitazone may be a weak inducer of CYP 450 isoform 3 A4 substrate. An enzyme inhibitor of CYP2C8 (such as gemfibrozil) may significantly increase the AUC of pioglitazone and an enzyme inducer of CYP2C8 (such as rifampin) may significantly decrease the AUC of pioglitazone. Therefore, if an inhibitor or inducer of CYP2C8 is started or stopped during treatment with pioglitazone, changes in diabetes treatment may be needed based on clinical response.
Special populations
Pioglitazone is labeled as Category C for pregnancy. It is not known whether pioglitazone is secreted in human milk. Since many drugs are excreted in human milk, pioglitazone should not be administered to a breastfeeding woman. Safety and effectiveness of pioglitazone in pediatric patients have not been established. No adjustment is necessary for renal impairment.
Common adverse effects
Adverse effects of pioglitazone occurring in at least 5% of patients include upper respiratory tract infection, headache, sinusitis, myalgia, tooth disorder, aggravation of diabetes mellitus, and pharyngitis (www.fda.gov). A postmarketing safety study of pioglitazone from 2008 showed that malaise/lassitude and nausea/vomiting were the most frequently reported adverse reactions.48 Other adverse reactions included dizziness, headache/migraine, diarrhea, weight gain and abnormal liver function tests.48
Pioglitazone Efficacy
Prevention of type 2 diabetes
TZDs have been shown to prevent the onset of type 2 diabetes, which appears to be a class effect.49–51 Most recent data from Defronzo et al has shown that use of pioglitazone 45 mg reduced the incidence of type 2 diabetes by around 62%.51 Separately rosiglitzone and troglitazone have also been shown to be effective in prevention of type 2 diabetes.49,50 It is plausible that this effect is mediated by preservation of beta cell function as shown in studies of pioglitazone and troglitazone in the prevention of type 2 diabetes in insulin resistance Hispanic women.49,50
Treatment of type 2 diabetes
Pioglitazone is approved to be used as monotherapy or in combination with metformin, sulfonylurea or insulin. It has been shown to be moderately effective in achieving glycemic control in placebo-controlled studies of patients with type 2 diabetes, either as monotherapy or in combination with metformin, sulfonylurea or insulin.30,52–56 Scherbaum et al showed that pioglitazone, both 15 and 30 mg/day, in addition to dietary control, was associated with significant reductions (vs. placebo) in mean levels of both glycosylated haemoglobin (HbA1C) and fasting blood glucose. HbA1C was reduced by 0.92% and 1.05%, respectively, and fasting blood glucose was reduced by 34.3 and 36.0 mg/dl, respectively, compared with the control group. Pioglitazone also significantly reduced postprandial blood glucose levels at all visits (−163 and—165 mg/dl/hour, respectively). 52 A study by Einhorn et al showed that patients receiving pioglitazone 30 mg + metformin had statistically significant mean decreases in HbA1c (−0.83%) and fasting plasma glucose levels (−37.7 mg/dL) compared with placebo + metformin.54 Adding pioglitazone (15 mg or 30 mg) to a stable insulin regimen resulted in a mean decrease in HbA1c of −1.0 and −1.3, respectively.56
Nonalcoholic steatohepatitis (NASH)
NASH is an increasingly frequent liver disease in patients with overweight/obesity, diabetes, hypertension and dyslipidemia.57,58 Although most of patients with NASH have a mild hepatic course, significant proportions do progress to cirrhosis, hepatocellular carcinoma, and endstage liver disease.58–60 Thiazolidinediones have multiple insulin-sensitizing actions and counteract insulin resistance, which is an important part of pathogenesis of NASH. Few randomized, controlled trials with either pioglitazone or rosiglitazone have been reported, with a variable effect on liver histology, mostly improving steatosis, but one study reported a marginal improvement in fibrosis.61–65 In conclusion, although TZDs improve hepatic steatosis, the ensuing injury i.e. NASH, does not appear to be affected.
Treatment of polycystic ovary syndrome
Therapy with pioglitazone, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women.66,67 As a result, these patients may be at an increased risk for pregnancy while taking pioglitazone. Thus, adequate contraception in premenopausal women should be recommended.
Treatment of diabetic nephropathy
Small studies have suggested that TZDs may have protective effects on the kidney. A recent systematic review and meta-analysis comparing the use of TZDs (pioglitazone and rosiglitazone) to placebo or other anti-diabetic agents concluded that thiazolidinedione treatment was associated with a significant decrease in urinary albumin excretion.68 Limitations of this study included significant heterogeneity across included studies in several subgroup analyses and unavailable patient-level data. In a small 1-year open labeled randomized controlled trial of 34 normoalbuminuric patients with type 2 diabetes, rosiglitazone appeared to exert nephroprotective effects beyond glycemic control.69 Future clinical trials looking into hard renal outcomes should be conducted to further delineate the potential benefits of thiazolidinediones on diabetic nephropathy.
Pioglitazone and nontraditional risk factors
Nontraditional cardiovascular risk factors are increasingly being recognized as novel targets to reduce CVD.70 Pioglitazone has been shown to decrease a variety of different mediators of inflammation, including C reactive protein, vascular endothelial growth factor and others.71 Treatment with pioglitazone in subjects with type 2 diabetes and metabolic syndrome for 12 months provided a significant decrease in Lp(a) concentration despite a substantial neutrality of rosiglitazone plus metformin combination.72 Thus pioglitazone appears to have affect both the traditional as nontraditional risk factors of CVD.
Mechanisms of non-cardiovascular effects of pioglitazone
Renal and vascular mechanisms have been proposed for pioglitazone induced peripheral edema (Table 1). Underlying molecular mechanisms are unclear. Pioglitazone stimulates plasma renin activity and increases sodium retention and weight gain in healthy subjects, which might explain the edema seen in type 2 diabetics treated with pioglitazone.73 Renal collecting duct is a major site for increased fluid reabsorption in response to pioglitazone. Increased vascular permeability in adipose tissues may also contribute to edema formation and body weight gain74 (Table 1).
Table 1.
Non cardiac effects of pioglitazone.
Decreased osteoblast formation and increased osteoclast activity may potentially mediate pioglitazone induced bone loss (Table 1). Naturally occurring PPAR-gamma ligand reduce osteoblast formation while increasing bone adipocytes in vitro75 and in vivo.76 Pioglitazone has been shown to reduce alkaline phosphatase, marker of bone formation, after 16 week treatment of premenopausal women with PCOS.77 No changes were observed in markers of bone resoprtion in the same study. Studies with rosiglitazone have shown similar effect on reduction of bone formation markers but in addition have been associated with increased bone resorption markers.78–80
Insulin resistance and oxidative stress have been implicated as key players in the pathophysiology of non-alcoholic steatohepatitis.81 Pilot studies of TZDs, which improve insulin sensitivity, have been shown to improve clinical and histologic features NASH, primarily improving steatosis, without improvement in markers of cell injury.61–65
Discussion
As reviewed in the above sections pioglitazone has some potential cardiovascular protective effects as shown in some trials but at least it does not increase cardiovascular risk. The mechanism by which this occurs is controversial. Studies show that pioglitazone has anti-inflammatory, anti-proliferative, anti-angiogenic and plaque stabilizing properties. At the same time pioglitazone also has proangiogenic and proliferative properties in certain situations. The net effect of the vascular effects of pioglitazone is likely tissue specific and depends on the biological context of the pathophysiological process. For example, its anti-proliferative, anti-inflammatory effects effects may be beneficial in terms of decreasing post angioplasty restenosis or damage post-stroke ischemia. These mechanisms may also be beneficial in the setting of NASH. However, its proangiogenic, proliferative properties exerted through VEGF, may be beneficial in wound healing in type 2 diabetes patients who lack adequate vascular supply to chronic wounds such as ulcers. Congestive heart failure may be one of the detrimental effects of pioglitazone mediated by a stimulation of VEGF expression. It is unknown whether these are effects due to PPARgamma agonism or direct pleotrophic effects. Studies are needed to delineate these effects.
Weight gain remains one of the main issues with pioglitazone treatment. This is likely due to fluid gain as well as fat accumulation. However fat accumulation has been observed to be subcutaneous and not intra-abdominal. Moreover there may be a transfer of fat from the intra-abdominal compartment to the subcutaneous compartment. This might lead to decreased cardiovascular risk as increased visceral fat is strongly linked to adverse cardiovascular outcomes.
Newer detrimental effects on bone health and macular edema are yet to be characterized by adequately powered and designed studies. Mechanisms by which these occur are not clear at this time.
In terms of efficacy, overall reduction of insulin resistance and hyperglycemia shows that pioglitazone is clinically efficacious in type 2 diabetes.
In conclusion current evident suggests that pioglitazone has an acceptable safety profile, may have beneficial cardiovascular and pleotropic effects and is clinically efficacious in patients with type 2 diabetes.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 2.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke. 2007;38:865–73. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- 4.Mannucci E, Monami M, Lamanna C, Gensini GF, Marchionni N. Pioglitazone and cardiovascular risk. A comprehensive meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2008;10:1221–38. doi: 10.1111/j.1463-1326.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 5.Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. Jama. 2006;296:2572–81. doi: 10.1001/jama.296.21.joc60158. [DOI] [PubMed] [Google Scholar]
- 6.Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs. glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. Jama. 2008;299:1561–73. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 7.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Jama. 2007;298:1180–8. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 8.Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. Bmj. 2009;339:b4731. doi: 10.1136/bmj.b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spanheimer R, Betteridge DJ, Tan MH, Ferrannini E, Charbonnel B. Long-term lipid effects of pioglitazone by baseline anti-hyperglycemia medication therapy and statin use from the PROactive experience (PROactive 14) Am J Cardiol. 2009;104:234–9. doi: 10.1016/j.amjcard.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj M, Baig R, Suraamornkul S, et al. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 95:1916–23. doi: 10.1210/jc.2009-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pershadsingh HA. Peroxisome proliferator-activated receptor-gamma: therapeutic target for diseases beyond diabetes: quo vadis? Expert Opin Investig Drugs. 2004;13:215–28. doi: 10.1517/13543784.13.3.215. [DOI] [PubMed] [Google Scholar]
- 12.Rosmarakis ES, Falagas ME. Effect of thiazolidinedione therapy on restenosis after coronary stent implantation: a meta-analysis of randomized controlled trials. Am Heart J. 2007;154:144–50. doi: 10.1016/j.ahj.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Peroxisome Proliferator-Activated Receptor-gamma Ligands: Potential Pharmacological Agents for Targeting the Angiogenesis Signaling Cascade in Cancer. PPAR Res. 2008:431763. doi: 10.1155/2008/431763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desouza CV, Gerety M, Hamel FG. Long-term effects of a PPAR-gamma agonist, pioglitazone, on neointimal hyperplasia and endothelial regrowth in insulin resistant rats. Vascul Pharmacol. 2007;46:188–94. doi: 10.1016/j.vph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Aljada A, O’Connor L, Fu YY, Mousa SA. PPAR gamma ligands, rosiglitazone and pioglitazone, inhibit bFGF- and VEGF-mediated angiogenesis. Angiogenesis. 2008;11:361–7. doi: 10.1007/s10456-008-9118-0. [DOI] [PubMed] [Google Scholar]
- 16.Takagi T, Okura H, Kobayashi Y, et al. A prospective, multicenter, randomized trial to assess efficacy of pioglitazone on in-stent neointimal suppression in type 2 diabetes: POPPS (Prevention of In-Stent Neointimal Proliferation by Pioglitazone Study) JACC Cardiovasc Interv. 2009;2:524–31. doi: 10.1016/j.jcin.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Pfutzner A, Schondorf T, Hanefeld M, Forst T. High-Sensitivity C-Reactive Protein Predicts Cardiovascular Risk in Diabetic and Nondiabetic Patients: Effects of Insulin-Sensitizing Treatment with Pioglitazone. J Diabetes Sci Technol. 4:706–16. doi: 10.1177/193229681000400326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Permana PA, Zhang W, Wabitsch M, Fischer-Posovszky P, Duckworth WC, Reaven PD. Pioglitazone reduces inflammatory responses of human adipocytes to factors secreted by monocytes/macrophages. Am J Physiol Endocrinol Metab. 2009;296:E1076–84. doi: 10.1152/ajpendo.91013.2008. [DOI] [PubMed] [Google Scholar]
- 19.Hu YY, Ye SD, Zhao LL, Zheng M, Chen Y. Hydrochloride pioglitazone decreases urinary TGF-beta1 excretion in type 2 diabetics. Eur J Clin Invest. doi: 10.1111/j.1365-2362.2010.02302.x. [DOI] [PubMed] [Google Scholar]
- 20.Glatz T, Stock I, Nguyen-Ngoc M, et al. Peroxisome-proliferator-activated receptors gamma and peroxisome-proliferator-activated receptors beta/delta and the regulation of interleukin 1 receptor antagonist expression by pioglitazone in ischaemic brain. J Hypertens. doi: 10.1097/HJH.0b013e3283396e4e. [DOI] [PubMed] [Google Scholar]
- 21.Huang PH, Sata M, Nishimatsu H, Sumi M, Hirata Y, Nagai R. Pioglitazone ameliorates endothelial dysfunction and restores ischemia-induced angiogenesis in diabetic mice. Biomed Pharmacother. 2008;62:46–52. doi: 10.1016/j.biopha.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Gensch C, Clever YP, Werner C, Hanhoun M, Bohm M, Laufs U. The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192:67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Vlckova V, Cornelius V, Kasliwal R, Wilton L, Shakir SA. Hypoglycaemia with oral antidiabetic drugs: results from prescription-event monitoring cohorts of rosiglitazone, pioglitazone, nateglinide and repaglinide. Drug Saf. 2009;32:409–18. doi: 10.2165/00002018-200932050-00004. [DOI] [PubMed] [Google Scholar]
- 24.Jain R, Osei K, Kupfer S, Perez AT, Zhang J. Long-term safety of pioglitazone versus glyburide in patients with recently diagnosed type 2 diabetes mellitus. Pharmacotherapy. 2006;26:1388–95. doi: 10.1592/phco.26.10.1388. [DOI] [PubMed] [Google Scholar]
- 25.Meneghini LF, Traylor L, Schwartz S. Improved Glycemic Control with Insulin Glargine vs. Pioglitazone as ADD-ON Therapy to Sulfonylurea or Metformin in Patients with Uncontrolled Type 2 Diabetes. Endocr Pract. 2010 Mar;29:1–6. doi: 10.4158/EP09281.OR. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26.Charbonnel B, Defronzo R, Davidson J, et al. Pioglitazone Use in Combination with Insulin in the Prospective Pioglitazone Clinical Trial in Macrovascular Events Study (PROactive19) J Clin Endocrinol Metab. 2010 May;95(5):2163–71. doi: 10.1210/jc.2009-1974. [DOI] [PubMed] [Google Scholar]
- 27.Basu A, Jensen MD, McCann F, Mukhopadhyay D, Joyner MJ, Rizza RA. Effects of pioglitazone versus glipizide on body fat distribution, body water content, and hemodynamics in type 2 diabetes. Diabetes Care. 2006;29:510–4. doi: 10.2337/diacare.29.03.06.dc05-2004. [DOI] [PubMed] [Google Scholar]
- 28.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 29.Giles TD, Elkayam U, Bhattacharya M, Perez A, Miller AB. Comparison of Pioglitazone vs. Glyburide in Early Heart Failure: Insights From a Randomized Controlled Study of Patients With Type 2 Diabetes and Mild Cardiac Disease. Congest Heart Fail. 2010 May 1;16(3):111–7. doi: 10.1111/j.1751-7133.2010.00154.x. [DOI] [PubMed] [Google Scholar]
- 30.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–11. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 31.Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J. Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation. Health Technol Assess. 2004;8:iii, ix–x, 1–91. doi: 10.3310/hta8130. [DOI] [PubMed] [Google Scholar]
- 32.Watkins PB, Whitcomb RW. Hepatic dysfunction associated with troglitazone. N Engl J Med. 1998;338:916–7. doi: 10.1056/NEJM199803263381314. [DOI] [PubMed] [Google Scholar]
- 33.Lebovitz HE, Kreider M, Freed MI. Evaluation of liver function in type 2 diabetic patients during clinical trials: evidence that rosiglitazone does not cause hepatic dysfunction. Diabetes Care. 2002;25:815–21. doi: 10.2337/diacare.25.5.815. [DOI] [PubMed] [Google Scholar]
- 34.May LD, Lefkowitch JH, Kram MT, Rubin DE. Mixed hepatocellular-cholestatic liver injury after pioglitazone therapy. Ann Intern Med. 2002;136:449–52. doi: 10.7326/0003-4819-136-6-200203190-00008. [DOI] [PubMed] [Google Scholar]
- 35.Maeda K. Hepatocellular injury in a patient receiving pioglitazone. Ann Intern Med. 2001;135:306. doi: 10.7326/0003-4819-135-4-200108210-00029. [DOI] [PubMed] [Google Scholar]
- 36.Tolman KG, Freston JW, Kupfer S, Perez A. Liver safety in patients with type 2 diabetes treated with pioglitazone: results from a 3-year, randomized, comparator-controlled study in the US. Drug Saf. 2009;32:787–800. doi: 10.2165/11316510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Abe M, Okada K, Maruyama T, Maruyama N, Soma M, Matsumoto K. Clinical effectiveness and safety evaluation of long-term pioglitazone treatment for erythropoietin responsiveness and insulin resistance in type 2 diabetic patients on hemodialysis. Expert Opin Pharmacother. 2010 Jul;11(10):1611–20. doi: 10.1517/14656566.2010.495119. [DOI] [PubMed] [Google Scholar]
- 38.Ryan EH, Jr, Han DP, Ramsay RC, et al. Diabetic macular edema associated with glitazone use. Retina. 2006;26:562–70. doi: 10.1097/00006982-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Colucciello M. Vision loss due to macular edema induced by rosiglitazone treatment of diabetes mellitus. Arch Ophthalmol. 2005;123:1273–5. doi: 10.1001/archopht.123.9.1273. [DOI] [PubMed] [Google Scholar]
- 40.Ciardella AP. Partial resolution of diabetic macular oedema after systemic treatment with furosemide. Br J Ophthalmol. 2004;88:1224–5. doi: 10.1136/bjo.2004.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen LQ, Child A, Weber GM, Folkman J, Aiello LP. Rosiglitazone and delayed onset of proliferative diabetic retinopathy. Arch Ophthalmol. 2008;126:793–9. doi: 10.1001/archopht.126.6.793. [DOI] [PubMed] [Google Scholar]
- 42.Ambrosius WT, Danis RP, Goff DC, Jr, et al. Lack of association between thiazolidinediones and macular edema in type 2 diabetes: the ACCORD eye substudy. Arch Ophthalmol. 2010 Mar;128(3):312–8. doi: 10.1001/archophthalmol.2009.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Standards of medical care in diabetes. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–35. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz AV, Sellmeyer DE, Vittinghoff E, et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91:3349–54. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med. 2008;168:820–5. doi: 10.1001/archinte.168.8.820. [DOI] [PubMed] [Google Scholar]
- 47.Habib ZA, Havstad SL, Wells K, Divine G, Pladevall M, Williams LK. Thiazolidinedione use and the longitudinal risk of fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010 Feb;95(2):592–600. doi: 10.1210/jc.2009-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasliwal R, Wilton LV, Shakir SA. Monitoring the safety of pioglitazone: results of a prescription-event monitoring study of 12,772 patients in England. Drug Saf. 2008;31:839–50. doi: 10.2165/00002018-200831100-00003. [DOI] [PubMed] [Google Scholar]
- 49.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 50.Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368:1096–105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 51.Defronzo RA, Banerji M, Bray GA, et al. Actos Now for the prevention of diabetes (ACT NOW) study. BMC Endocr Disord. 2009;9:17. doi: 10.1186/1472-6823-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherbaum WA, Goke B. Metabolic efficacy and safety of once-daily pioglitazone monotherapy in patients with type 2 diabetes: a double-blind, placebo-controlled study. Horm Metab Res. 2002;34:589–95. doi: 10.1055/s-2002-35421. [DOI] [PubMed] [Google Scholar]
- 53.Rosenblatt S, Miskin B, Glazer NB, Prince MJ, Robertson KE. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron Artery Dis. 2001;12:413–23. doi: 10.1097/00019501-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Einhorn D, Rendell M, Rosenzweig J, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled study. The Pioglitazone 027 Study Group. Clin Ther. 2000;22:1395–409. doi: 10.1016/s0149-2918(00)83039-8. [DOI] [PubMed] [Google Scholar]
- 55.Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001;111:10–7. doi: 10.1016/s0002-9343(01)00713-6. [DOI] [PubMed] [Google Scholar]
- 56.Rosenstock J, Einhorn D, Hershon K, Glazer NB, Yu S. Efficacy and safety of pioglitazone in type 2 diabetes: a randomised, placebo-controlled study in patients receiving stable insulin therapy. Int J Clin Pract. 2002;56:251–7. [PubMed] [Google Scholar]
- 57.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 58.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 59.Ratziu V, Poynard T. Assessing the outcome of nonalcoholic steatohepatitis? It’s time to get serious. Hepatology. 2006;44:802–5. doi: 10.1002/hep.21391. [DOI] [PubMed] [Google Scholar]
- 60.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 61.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 62.Sanyal AJ, Mofrad PS, Contos MJ, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–15. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 63.Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–10. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 64.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–84. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 65.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010 May 6;362(18):1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ota H, Goto T, Yoshioka T, Ohyama N. Successful pregnancies treated with pioglitazone in infertile patients with polycystic ovary syndrome. Fertil Steril. 2008;90:709–13. doi: 10.1016/j.fertnstert.2007.01.117. [DOI] [PubMed] [Google Scholar]
- 67.Ghazeeri G, Kutteh WH, Bryer-Ash M, Haas D, Ke RW. Effect of rosiglitazone on spontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome. Fertil Steril. 2003;79:562–6. doi: 10.1016/s0015-0282(02)04843-4. [DOI] [PubMed] [Google Scholar]
- 68.Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN, Lasaridis AN. Effect of Thiazolidinediones on Albuminuria and Proteinuria in Diabetes: A Metaanalysis. Am J Kidney Dis. 2010 May;55(5):835–47. doi: 10.1053/j.ajkd.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 69.Petrica L, Petrica M, Vlad A, et al. Nephro- and neuroprotective effects of rosiglitazone versus glimepiride in normoalbuminuric patients with type 2 diabetes mellitus: a randomized controlled trial. Wien Klin Wochenschr. 2009;121:765–75. doi: 10.1007/s00508-009-1279-3. [DOI] [PubMed] [Google Scholar]
- 70.Fonseca V, Desouza C, Asnani S, Jialal I. Nontraditional risk factors for cardiovascular disease in diabetes. Endocr Rev. 2004;25:153–75. doi: 10.1210/er.2002-0034. [DOI] [PubMed] [Google Scholar]
- 71.Aljada A, Shah KA, Mousa SA. Peroxisome proliferator-activated receptor agonists: do they increase cardiovascular risk? PPAR Res. 2009:460764. doi: 10.1155/2009/460764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Derosa G, D’Angelo A, Ragonesi PD, et al. Metformin-pioglitazone and metformin-rosiglitazone effects on non-conventional cardiovascular risk factors plasma level in type 2 diabetic patients with metabolic syndrome. J Clin Pharm Ther. 2006;31:375–83. doi: 10.1111/j.1365-2710.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 73.Zanchi A, Chiolero A, Maillard M, Nussberger J, Brunner HR, Burnier M. Effects of the peroxisomal proliferator-activated receptor-gamma agonist pioglitazone on renal and hormonal responses to salt in healthy men. J Clin Endocrinol Metab. 2004;89:1140–5. doi: 10.1210/jc.2003-031526. [DOI] [PubMed] [Google Scholar]
- 74.Yang T, Soodvilai S. Renal and vascular mechanisms of thiazolidinedioneinduced fluid retention. PPAR Res. 2008:943614. doi: 10.1155/2008/943614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–84. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 76.Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int. 2008;19:129–37. doi: 10.1007/s00198-007-0477-y. [DOI] [PubMed] [Google Scholar]
- 77.Glintborg D, Andersen M, Hagen C, Heickendorff L, Hermann AP. Association of pioglitazone treatment with decreased bone mineral density in obese premenopausal patients with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2008;93:1696–701. doi: 10.1210/jc.2007-2249. [DOI] [PubMed] [Google Scholar]
- 78.Grey A, Bolland M, Gamble G, et al. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–10. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 79.Berberoglu Z, Gursoy A, Bayraktar N, et al. Rosiglitazone decreases serum bone-specific alkaline phosphatase activity in postmenopausal diabetic women. J Clin Endocrinol Metab. 2007;92:3523–30. doi: 10.1210/jc.2007-0431. [DOI] [PubMed] [Google Scholar]
- 80.Zinman B, Haffner SM, Herman WH, et al. Effect of rosiglitazone, metformin, and glyburide on bone biomarkers in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010 Jan;95(1):134–42. doi: 10.1210/jc.2009-0572. [DOI] [PubMed] [Google Scholar]
- 81.McClain CJ, Mokshagundam SP, Barve SS, et al. Mechanisms of nonalcoholic steatohepatitis. Alcohol. 2004;34:67–79. doi: 10.1016/j.alcohol.2004.07.007. [DOI] [PubMed] [Google Scholar]