Abstract
Bupropion’s (Zyban® SR) effectiveness to treat symptoms experienced in marihuana withdrawal was tested in a double-blind, placebo-controlled study with chronic, heavy marihuana users. Participants maintained their usual marihuana intake until Quit Day after which they were required to cease intake of THC products for 14 days. A Withdrawal Discomfort Score revealed that for 7 days immediately following cessation, placebo-treated subjects reported more symptoms than bupropion-treated subjects. Self-reported craving for marihuana increased for the placebo-treated group but not for those treated with bupropion. Measures of sleep and cognitive performance were not different between the two groups. Participants in the bupropion treatment arm were more likely to complete the study than those randomized to the placebo arm (50% completion for bupropion vs. 33% completion for placebo). These results suggest that bupropion may be useful for alleviating marihuana withdrawal symptoms and be useful in subject retention during long-term cessation programs.
Keywords: marihuana, withdrawal, pharmacotherapy, bupropion, cognitive performance, sleep
Introduction
This research studied the effectiveness of the anti-depressant bupropion (in the sustained release formula—Zyban® SR) to alleviate withdrawal symptoms in chronic marihuana users. Although evidence of a marihuana withdrawal syndrome has existed for many years1,2 the clinical significance of the condition and the role of the symptoms in relapse has been debated. Recently, the time course of the withdrawal syndrome has been documented3,4 and it has now been demonstrated that the marihuana withdrawal syndrome may very well be an important factor in maintaining use and relapse.5–7 Several components of the withdrawal syndrome are believed to affect the ability of users to quit and to thwart the success of cessation attempts. For example, many users could not quit or quickly relapsed because they experienced significant physical tension, anxiety, irritability and insomnia. Resuming marihuana smoking alleviates these symptoms, which are very similar to those observed after tobacco withdrawal—a condition for which bupropion has been shown to be effective.
Bupropion’s effects on withdrawal symptoms observed during marihuana cessation were reported by Haney et al.8 In an elegant in-patient crossover design with non-treatment seeking individuals, marihuana users smoked marihuana cigarettes repeatedly over 5 days then underwent abrupt cessation and observed for 12 days while being treated with either bupropion or placebo. They concluded that bupropion worsened the marihuana withdrawal syndrome. Participants’ self-ratings of irritability, feeling miserable, restless, unmotivated, and depressed were all greater in the bupropion phase than in the placebo phase. These authors conclude that bupropion may not be an effective pharmacotherapy for treating marihuana dependence.
Preliminary data from our laboratory (unpublished data) indicated that bupropion may be effective in reducing increases in dysphoric mood following marihuana withdrawal in a different paradigm. In an out-patient setting of 21 days of verified abstinence, ratings of anxiety, irritability, and physical tension were collected. These mood effects were attenuated relative to the historical cohort of subjects who were withdrawn without bupropion.4 Given the success of bupropion in tobacco/nicotine cessation programs and our preliminary findings, we embarked to test whether bupropion would alleviate withdrawal symptoms in treatment-seeking cannabis-dependent individuals in a cessation paradigm similar to that used for tobacco cessation programs.
Materials and Methods
Participants
Treatment seeking, chronic, heavy users of marihuana between the ages of 18 to 50 years old were recruited via newspaper advertisements, internet ads, and advertisements on a local radio station. After giving informed consent, potential participants received a physical examination and a Structured Clinical Interview for DSM-IV (SCID-IV).9 Participants had to be physically healthy and meet SCID-IV criteria for marihuana abuse and/or dependence. Additionally, they had to have had at least 3 years of heavy use (smoke 5 out of 7 days per week or greater than 25 times per month) and have experienced 2 or more negative symptoms in previous quit attempts. They could not meet abuse or dependence criteria for any other drug (including nicotine). Forty-five (45) men and women signed informed consent and were screened. Fifteen were found ineligible or declined to participate further; another 8 withdrew consent after the baseline week leaving 22 to be randomized to either active medication or placebo treatment. Of the 22 randomized, 9 completed the study through the 14 day withdrawal assessment period and were used for data analysis. These 9 completers consisted of 5 men, 4 women, aged 31.2±9.6 years old (range 20 to 44 years). Two met criteria for marihuana abuse while the remaining met criteria for abuse and dependence. They were at least daily smokers of marihuana with 1 smoking 2 to 3 times/day and 3 others reporting smoking 3–4 times per day, and had smoked regularly for 3 to 20 years. All had attempted to quit at least once previously. All were regular, light to moderate drinkers of alcohol (1 or 2 drinks per week to 1–2 drinks per day), moderate caffeine users (less than 300 mg per day), and 2 were light tobacco users (less than 5 cigarettes per day). The study was approved by the McLean Hospital Institutional Review Board. Participants provided written informed consent before beginning and they were paid for their participation.
Materials
Daily diary
The diary contained a section for recording any and all drug use in the past 24-hour period (including the study medication), a sleep log, and the Marijuana Withdrawal Checklist.5 The sleep log contained 5 questions and 2 visual analog scales. The questions were: “What time did you go to bed last night?”, “How quickly did you fall asleep (in minutes)?”, “How many times did you wake up during the night for more than just a minute or two (list number and total time awake)?”, “What time did you wake up this morning?”, and “What time did you get out of bed this morning?”. The two visual analog scales assessed the characteristics of the previous sleep period. In response to the question of “When you got up this morning, you felt:”, participants placed a mark along a 100 mm line anchored with “exhausted” on the left end and “refreshed” on the right end. In response to the question of “Overall, your sleep last night was:”, participants placed a mark along a 100 mm line anchored with “very restless” on the left and “very sound” on the right end. This diary incorporates aspects of sleep commonly assessed in clinical and research studies (cf10) and are components of most sleep diaries in current use. The Marijuana Withdrawal Checklist assessed mood and withdrawal symptoms.5 It is comprised of 29 items rated on a 4-point scale (0-none to 3-severe). A withdrawal discomfort score is computed from 10 of the items found to be most sensitive to subjective states following withdrawal from chronic marihuana use (maximum score of 30). The 10 items for the discomfort score are: anger, craving for marihuana, depressed mood, decreased appetite, headaches, irritability, nervousness, restlessness, sleep difficulty, and strange dreams.
Assessment tests
A computerized performance assessment battery was used to assess a variety of cognitive performance abilities including memory, reaction time, and spatial recognition. The specific tasks included simple reaction time (requiring the performer to press the space bar as soon as a snowflake appeared on the screen), logical reasoning (indicate whether a statement about the relationship between two symbols was true or false,11 matching-to-sample (view a 6 × 6 block of red and green squares for 3 seconds and then indicate which of two choices presented after a 5-second delay matched), memory task (the performer studies a 6-letter string and then answers if the appearing letter was in the original string or not), and a running memory continuous performance (‘1-back’) number task (the performer indicates if the currently presented number is the same or different from the one just seen). The battery took approximately 20 minutes to complete.
Other assessments
Changes in self-reported depression and anxiety were assessed using the Beck Depression Inventory12 and Beck Anxiety Inventory.13 These inventories were completed once during the baseline period, on the first day of medication and then weekly (days 7, 14, and 21). The University of Rhode Island Change Assessment (URICA) Scale14 (24-item version) was administered on the first and last day of the study.
Actiwatch
A wrist-worn activity monitor (Actiwatch® Score, Respironics, Bend, OR) was used for measurements of activity and verification of sleep periods. Sleep periods were scored according to proprietary software which has been shown to be reliably correlated with polysomnoghraphic recordings in young to middle-aged adults.15
Medication
Bupropion sustained release (Zyban® SR) tablets were taken by those randomized to the active medication group in the following manner: For days 1–3, 150 mg once a day and then 150 mg twice a day (mid morning and late afternoon) for the remainder of the study (days 4–21). This regimen is consistent with the manufacturer’s and FDA guidelines for treating tobacco/nicotine addiction. Bupropion tablets were repackaged into gelatin capsules for blind administration. Placebo consisted of identically appearing gelatin capsules that contained gelatin filler (Nature’s Way, 10 gr/650 mg). Additionally, with each medication administration, participants consumed an identically appearing capsule that contained 25 mg of vitamin B2 (riboflavin). Riboflavin can be detected in the urine through the use of ultraviolet light fluorescence and was used as a measure of medication compliance.16
Procedure
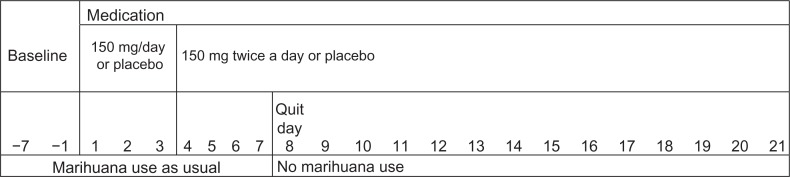
The study was a between-subjects design where participants were randomly assigned to receive either bupropion or placebo following a 7 day baseline period (days −7 to −1; see Fig. 1). A double-blind regimen of either placebo or bupropion began on Day 1 and continued for 21 days. Participants were allowed to continue their usual marihuana use until Day 8—Quit Day, after which no further intake was allowed. Allowing for a one week of treatment with medication before cessation of marihuana use is consistent with established guidelines for the use of bupropion in treating tobacco/nicotine addiction. Female participants were not using oral birth control medication but were required to use other forms of contraception. Urine pregnancy tests were performed at each laboratory visit. Women completed a menstrual cycle diary and began the study on days 5–14 of their cycle. Participants who used tobacco and caffeine continued their use at prestudy levels throughout the study. Participants were trained on the computerized performance tasks during the baseline week in order to establish stable performance, and instructed on how to complete the daily diary.
Figure 1.
Study schematic.
Notes: Participants filled out daily diaries from baseline through day 21. Medication started 7 days before ‘Quit Day’ in a procedure mimicking that recommended for treatment of tobacco cessation.
Participants came to the laboratory daily Monday through Friday. They gave a urine sample that was analyzed quantitatively for THC metabolites (see below) and for the presence/absence of other drugs of abuse (Instant-View® Multi-Drug Screen Urine Test, Alfa Scientific Designs, Inc). The urine was viewed under ultraviolet light for riboflavin fluorescence by two observers. Participants completed a computerized performance assessment battery. Diaries were collected and medication was dispensed.
Analysis of urine samples and verification of abstinence
Urine samples were sent to a commercial laboratory (Quest Diagnostics, Cambridge, MA) where they were screened by immunoassay for the THC metabolite 11-nor-carboy-delta 9-tetrahydrocannabinol (THC-COOH). Urine creatinine concentrations were also measured to assess urine concentration. A threshold for detection of THC-COOH was 20 ng/mL. Samples positive for this metabolite underwent further analysis by gas chromatography-mass spectroscopy (GC/MS) to obtain quantitative THC-COOH concentration. Samples after Day 8 (Quit Day) were further assessed for evidence of new marihuana use. The operational definition for this criterion was that the cannabinoid/creatinine ratio on a given day could not be greater than 50% higher from the ratio obtained in the previous day’s urine sample. This definition of new marihuana use is based on data from controlled clinical studies of urinary excretion profiles of creatinine and marihuana metabolites following marihuana administration in humans17–19 and was used in a previous study in this laboratory.4
Psychosocial support
Participants underwent weekly sessions of a motivational enhancement therapy (MET) that was adapted and used for marihuana dependence.20 This treatment was adapted from manuals used in Project Match21 and the CSAT Multi-Site Marihuana Treatment Project. 22 Three individual MET sessions (once during the week before Quit Day and then once during each week of abstinence) were conducted by a psychologist trained in MET. MET has empirical support for both marihuana dependence23 and alcohol addiction.24
Data analysis
Data were analyzed using a general linear model repeated measures analysis of variance (SPSS 13.0 for Mac OS X, Chicago, IL) using drug group and time as the main factors. Post hoc analyses were performed using Fischer’s least significant difference test when significant main effects were found. Significance level was set at P < 0.05 for all tests. Effect size is reported using the variance-accounted-for statistic Partial Eta-Squared obtained from the SPSS output.
Results
Abstinence and medication compliance
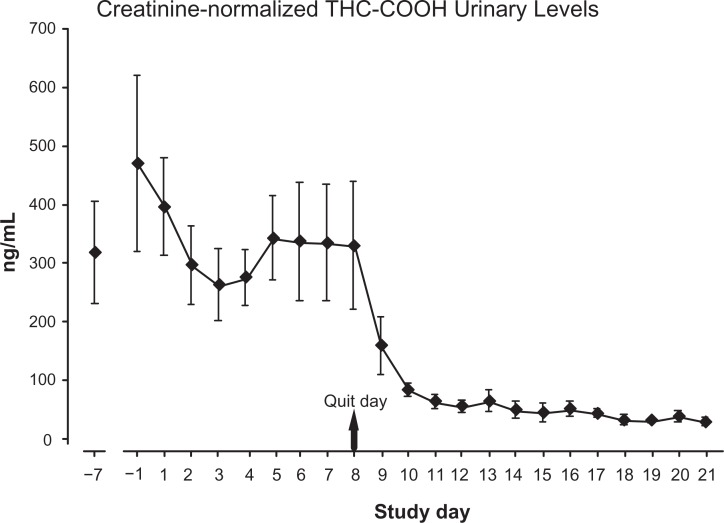
Creatinine-normalized urinary levels for the primary THC metabolite are reported in Figure 2. Average metabolite levels ranged between 260 and 468 ng/mL during study days before Day 8-Quit Day. A sharp decline was observed in the first 2 days of abstinence but detectable levels were still evident (although continuing to decline) at the end of the second week of abstinence. Averaged levels during the last 5 days of the study were 42, 31, 29, 37, and 27 ng/mL. Urine samples collected at each laboratory visit were viewed by two observers for fluorescence. All samples were positive for riboflavin indicating recent ingestion of the medication. As expected, all urine samples were positive for THC metabolites; all samples were negative for other drugs of abuse.
Figure 2.
Urinary THC metabolite levels for 9 participants completing the study.
Note: Values dropped sharply after ‘Quit Day’ and continued to decrease slowly but were still detectable at day 21.
Withdrawal discomfort score
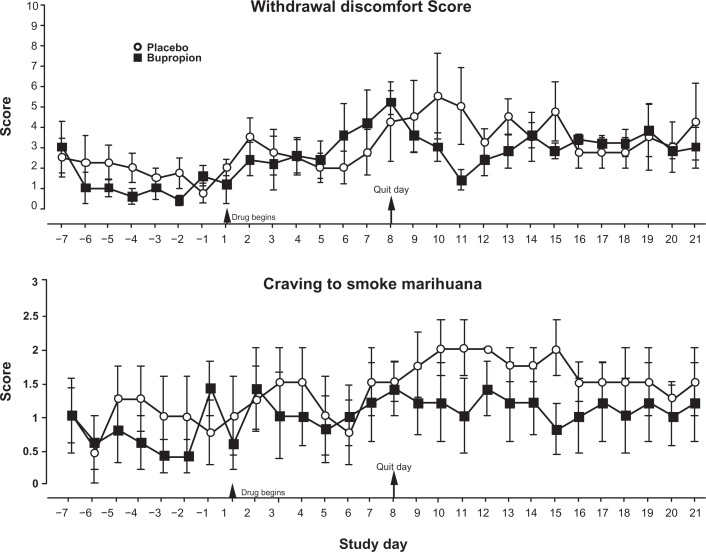
The day by day averages of the 10 item withdrawal discomfort score calculated from the Marijuana Withdrawal Checklist are shown on Figure 3. Generally speaking, scores increased from baseline week through the end of the study (main effect for time: F(21,147) = 1.726, P = 0.033, η2Partial = 0.198), although overall, there was no significant difference between the two groups. Analysis of percent change from baseline values revealed that following cessation of marihuana intake (days 8 through 21), discomfort scores increased significantly for the placebo-treated subjects but not for the bupropion-treated subjects.
Figure 3.
Daily withdrawal discomfort and craving scores.
Notes: The withdrawal discomfort is a composite score of 10 items from the behavioral checklist of symptoms with a maximum score or 30. Craving to smoke marihuana was rated on a 0 to 3 point scale from ‘none’ to ‘severe’.
Craving scores
Similarly, craving scores tended to increase from baseline days through the end of the study, although this effect missed statistical significance (main effect for time: F(21,147) = 1.522, P = 0.078, η2Partial = 0.179) (Fig. 3). However, a further analysis using percent change in scores revealed that placebo-treated subjects significantly increased their craving ratings following marihuana cessation and these ratings remained significantly elevated from baseline for the two treatment weeks (main effect for time: F(3,21) = 3.416, P = 0.036, η2Partial = 0.328; time by group interaction: F(3,21) = 4.511, P = 0.014, η2Partial = 0.392). Bupropion-treated subjects did not show an increase in craving ratings, and furthermore, these ratings were significantly lower than the placebo-treated group during the withdrawal weeks.
Beck Depression and Anxiety Inventories; URICA
Scores on the depression inventory were below 10 with one exception for a placebo subject during baseline and remained low throughout the study (data not shown). No significant changes were observed for study phase or medication treatment. Scores on the anxiety inventory similarly were low (below 6 except for the same subject during baseline) but did show a significant decrease overall through the study [F(4,28) = 3.856, P = 0.013, η2Partial = 0.355]. Scores were significantly lower after one week of withdrawal in comparison to baseline for both groups. There were no differences due to medication treatment. Analysis of the Readiness to Change score from the URICA scale did not reveal a significant time effect or group difference.
Sleep measures
Analysis of the actiwatch recordings and daily diary entries indicated that total sleep time decreased slightly for both groups during the first withdrawal week and recovered somewhat during the second withdrawal week (data not shown). From the diary entries, participants slept on the average of 7.3 hours during baseline, 6.8 hours (30 minutes less) during the first week of withdrawal, and 7 hours during the second week of withdrawal. These changes were not statistically significant. Both groups showed similar patterns and there were no significant differences between the groups. Latency to fall asleep did not vary by drug group or study week, but during the first week of withdrawal, once participants fell asleep, those in the bupropion group tended to sleep better: wake after sleep onset (WASO) analysis showed that the bupropion group was awake 10.1% of the sleep period vs. 14.2% for the placebo-treated group. This difference was not statistically significant. Visual analog scale ratings of sleep quality remained steady throughout the study. Average weekly ratings of feeling exhausted to refreshed upon awakening were between 61 and 65 (on a 100 mm line). Average weekly ratings of feeling exhausted to refreshed upon awakening were between 61 and 65 (on a 100 mm line). Average weekly ratings of restless to sound overall sleep were between 66 and 73. Stanford Sleepiness Scale ratings decreased slightly (indicating more alertness) over the course of the study (from an average of 2.6 in baseline to 2.0 in the second week of withdrawal) but were not statistically significant between the groups or over the study weeks.
Cognitive tests
Performance on two of the cognitive assessment tests improved over the study weeks. Throughput measures of performance (ie, correct responses per unit of time) on the continuous performance (‘1-back’) task improved from an average of 102 correct responses per minute during baseline to 124 during the second week of withdrawal (main effect for time: F(3,18) = 25.92, P < 0.001, η2Partial = 0.812; data not shown). Logical Reasoning values increased from an average of 28.4 during baseline to 36.4 (main effect for time: F(3,18) = 5.524, P = 0.007, η2Partial l = 0.479; data not shown). Performance on the other tests also showed generally increasing scores, but these changes were not statistically significant. There were no statistically significant differences between the placebo-and bupropion-treated groups.
Completion analysis
Twenty-two (22) participants completed the baseline week and were randomized to a treatment group. Of the 10 randomized to the bupropion treatment, 50% finished the 21-day study. Of the 12 randomized to the placebo treatment, 4 completed the study (33% completion).
Discussion
Bupropion has been shown to be effective in reducing withdrawal symptoms and reducing cravings when quitting tobacco cigarette smoking.25,26 One rationale for the use of bupropion to treat cannabis dependence and withdrawal symptoms rests on the similarity of withdrawal symptoms between cigarettes and cannabis use.27,28 However, reports on the use of bupropion to treat marihuana withdrawal symptoms have been negative. Carpenter et al29 concluded that bupropion did not lessen withdrawal symptoms (specifically irritability) during a 13-week outpatient treatment study. Haney et al’s8 in-patient study of enforced marihuana smoking followed by placebo smoking showed that bupropion increased rather than decreased some withdrawal symptoms. Both sets of researchers conclude that bupropion would have limited, if any, usefulness in treating marihuana dependence. The present study tested bupropion in a paradigm modeled after smoking cessation programs and assessed effectiveness during the period when symptoms are typically the strongest3 and demonstrated that two important measures of subjective marihuana withdrawal effects—a composite withdrawal discomfort score and self-reported cravings to smoke—increased for participants treated with placebo but remained constant for the bupropion-treated participants during a 14-day withdrawal period.
The selection of participants for this study included individuals who met DSM-IV criteria for abuse and/or dependence, but more importantly, potential participants were queried further to insure that regardless of whether they strictly met abuse and/or dependence criteria, they had had difficulty in reducing or stopping their use in the past. We wanted to maximize our efforts to study individuals who had experienced discernable (and unpleasant) withdrawal efforts in previous attempts to cut down or stop using marihuana. We felt that the best opportunity to assess bupropion’s effects would be in individuals who had problems in the past and not simply including individuals who met DSM-IV criteria.
A limitation of the study is its small sample size. We designed the assessment to include only individuals who completed the 14-day withdrawal period. A more definitive assessment of bupropion’s ability to alter marihuana withdrawal effects will need to include a larger sample size of completers and perhaps the incorporation of more sophisticated statistical methods to handle the data from the non-completers.
Long-term, chronic marihuana intake has been shown to affect cognitive abilities. Solowij et al30 compared performance on 9 standard neuropsychological tests of attention, memory, and executive functioning in three groups of subjects: long-term users with a mean of over 20 years of regular use (at least twice per month), short-term users with a mean of 10 years of use, and nonusers. In comparison to nonusers or short-term users, long-term users performed significantly poorer on verbal learning and time estimation tasks. Despite these demonstrated effects of such use, it appears that they are not permanent. Pope et al,31 for example, studied cognitive performance in long-term, chronic users recruited to stop smoking and refrain from all THC intake for 28 days. Tests included assessments of memory, attention, abstraction ability, and ability to learn and recall visuospatial information. Current users performed poorer than former users or control subjects for the first 7 days of withdrawal, consistent with residual levels of THC remaining in the body. By day 28 however, there were no differences among the groups, indicating that impaired cognitive performance is a function of recent marihuana use. The results of the present study are in agreement with gradual improvements in psychomotor and cognitive performance reported following cessation of chronic drug intake. Performance on the tests included in the battery used in the current study did not show any withdrawal-related decrease: performance was not poorer during withdrawal for either treatment group. These results show that the ability of these tests to be indicative of an acute withdrawal syndrome appears to be limited, but may be more useful for assessing long-term changes following cessation of use.
Changes in sleep patterns have long been associated with withdrawal from chronic drug use. Early studies by Feinberg et al,32,33 Jones et al,1 and Karacan et al34 showed sleeplessness associated with an increase in rapid eye movement sleep following the abrupt termination of access to marihuana. More recently, Budney et al6 reported that ‘sleep difficulties’ were recorded by over 40% of their subjects, although the exact nature of these difficulties were not detailed. Haney et al’s7,35 in-patient studies reported that withdrawal from 4 days of oral THC administration, but not 4 days of smoked marihuana, produced significant disruptions in sleep: decreases in self-reported quantity and quality of sleep were observed. The changes observed during withdrawal in the current study were not statistically significant. This is somewhat surprising given the previous reports of sleep alterations during withdrawal. The reasons for the lack of significant changes need further examination.
The higher completion rate by the bupropion group is encouraging. It is possible that those treated with placebo experienced more severe withdrawal symptoms overall and thus were more likely to drop out, but this will require additional experimentation. Success in cessation treatment is often related to the ability to keep patients enrolled and keep receiving pharmacotherapy and psychosocial support.36 If bupropion is alleviating some of the withdrawal symptoms, this may contribute to improved retention. Bupropion is a prescription medication that should not cause problems in marihuana-dependent but otherwise healthy individuals. When prescribed at the recommended doses for nicotine withdrawal, side effects included a low incidence of reported dry mouth, skin rashes, shakiness, and dizziness. Insomnia was reported by 31% of those taking the medication in a dose-response trial.37 More serious side effects such as seizures are possible and therefore the medication is not recommended for individuals with a history of seizures or in bulimia patients, especially at higher than the recommended doses. Our experience (in an admittedly small sample) would indicate that when prescribed at the recommended doses, bupropion is well tolerated in marihuana using individuals.
This small sample study indicates that bupropion may be effective in relieving some withdrawal symptoms that occur following cessation of chronic marihuana use. Changes observed in withdrawal discomfort scores and cravings in the placebo-treated group were not observed in the bupropion-treated group. These are preliminary but encouraging results for the use of bupropion as a potential pharmacotherapeutic agent in the treatment of marihuana withdrawal.
Footnotes
Author Contributions
Conceived and designed the experiment: DMP, SEL. Analyzed the data: DMP. Wrote the first draft of the manuscript: DMP. Contributed to the writing of the manuscript: DMP, ARL, ETR, MAM, SEL. Agree with manuscript results and conclusions: DMP, ARL, ETR, MAM, SEL. Jointly developed the structure and arguments for the paper: DMP, SEL. Made critical revisions and approved final version: DMP, ARL, ETR, MAM, SEL. All authors reviewed and approved of the final manuscript.
Competing Interests
DMP is a NIDA grant recipient and has also received travel support. All other authors disclose NIDA grants.
Funding
This work was supported by the National Institute on Drug Abuse [grant numbers DA017275 (DMP) and DA000343 (SEL)]. This study is registered on ClinicalTrials.gov (# NCT 00142870).
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981;21(Suppl 8–9):143S–52. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 2.Tennant FS. The clinical syndrome of marijuana dependence. Psychiatric Annals. 1986;16:225–34. [Google Scholar]
- 3.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 4.Kouri EM, Pope HG. Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8(4):483–92. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- 5.Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58(10):917–24. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- 6.Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94(9):1311–22. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- 7.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999;141(4):395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 8.Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology (Berl) 2001;155(2):171–9. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- 9.First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders (Research Version) NY: Biometrics Research, NY State Psychiatric Institute; 2002. [Google Scholar]
- 10.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133(12):1382–8. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 11.Baddeley AD. A 3-minute reasoning test based on grammatical transformations. Psychon Sci. 1968;10:341–2. [Google Scholar]
- 12.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 14.DiClemente CC, Hughes SO. Stages of change profiles in outpatient alcoholism treatment. J Subst Abuse. 1990;2(2):217–35. doi: 10.1016/s0899-3289(05)80057-4. [DOI] [PubMed] [Google Scholar]
- 15.Sadeh A, Alster J, Urbach D, Lavie P. Actigraphically based automatic bedtime sleep wake scoring: Validity and clinical applications. J Ambulatory Monitoring. 1989;2:209–216. [Google Scholar]
- 16.Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20:1412–17. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 17.Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol. 1998;22(6):445–54. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- 18.Huestis MA, Mitchell JM, Cone EJ. Detection times of marijuana metabolites in urine by immunoassay and GC-MS. J Anal Toxicol. 1995;19(6):443–9. doi: 10.1093/jat/19.6.443. [DOI] [PubMed] [Google Scholar]
- 19.Huestis MA, Mitchell JM, Cone EJ. Urinary excretion profiles of 11-nor-9-carboxy-delta 9-tetrahydrocannabinol in humans after single smoked doses of marijuana. J Anal Toxicol. 1996;20(6):441–52. doi: 10.1093/jat/20.6.441. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg KL, Roffman RA, Carroll KM, et al. Tailoring cannabis dependence treatment for a diverse population. Addiction. 2002;97(Suppl 1):135–42. doi: 10.1046/j.1360-0443.97.s01.5.x. [DOI] [PubMed] [Google Scholar]
- 21.Project MRG. Matching alcoholism treatments to client heterogeneity: Project Match posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- 22.Stephens RS, Babor TF, Kadden R, Miller M. The Marijuana Treatment Project: rationale, design and participant characteristics. Addiction. 2002;97(Suppl 1):109–24. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]
- 23.Sinha R, Easton C, Renee-Aubin L, Carroll KM. Engaging young probation-referred marijuana-abusing individuals in treatment: a pilot trial. Am J Addict. 2003;12(4):314–23. [PubMed] [Google Scholar]
- 24.Bien TH, Miller WR, Tonigan JS. Brief interventions for alcohol problems: a review. Addiction. 1993;88(3):315–35. doi: 10.1111/j.1360-0443.1993.tb00820.x. [DOI] [PubMed] [Google Scholar]
- 25.Hurt RD, Sachs DP, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337(17):1195–202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- 26.Jorenby D. Clinical efficacy of bupropion in the management of smoking cessation. Drugs. 2002;62(Suppl 2):25–35. doi: 10.2165/00003495-200262002-00003. [DOI] [PubMed] [Google Scholar]
- 27.Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92(1–3):48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandrey RG, Budney AJ, Moore BA, Hughes JR. A cross-study comparison of cannabis and tobacco withdrawal. Am J Addict. 2005;14(1):54–63. doi: 10.1080/10550490590899853. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: Double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18:53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solowij N, Stephens RS, Roffman RA, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287(9):1123–31. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- 31.Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58(10):909–15. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 32.Feinberg I, Jones R, Walker J, Cavness C, Floyd T. Effects of marijuana extract and tetrahydrocannabinol on electroencephalographic sleep patterns. Clin Pharmacol Ther. 1976;19(6):782–94. doi: 10.1002/cpt1976196782. [DOI] [PubMed] [Google Scholar]
- 33.Feinberg I, Jones R, Walker JM, Cavness C, March J. Effects of high dosage delta-9-tetrahydrocannabinol on sleep patterns in man. Clin Pharmacol Ther. 1975;17(4):458–66. doi: 10.1002/cpt1975174458. [DOI] [PubMed] [Google Scholar]
- 34.Karacan I, Fernandez-Salas A, Coggins WJ, et al. Sleep electroencephalographic-eletrooculographic characteristics of chronic marijuana users: Part I. Ann N Y Acad Sci. 1976;282:348–74. doi: 10.1111/j.1749-6632.1976.tb49909.x. [DOI] [PubMed] [Google Scholar]
- 35.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 1999;141(4):385–94. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- 36.Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68(6):1051–61. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- 37.PDR . Physicians’ Desk Reference. 56th Edition. Montvale, NJ: Thompson PDR; 2002. [Google Scholar]