Abstract
Incretin-based therapies have been gaining much attention recently as a new class of therapeutics for type 2 diabetes worldwide. Among them, glucagon-like peptide-1 receptor agonist liraglutide has been rapidly increasing its global usage. Once daily injection of liraglutide significantly ameliorates glycemic control in patients with type 2 diabetes by enhancing insulin secretion and suppressing glucagon secretion glucose-dependently. Liraglutide delays gastric emptying and suppresses food intakes, both of which contribute to glucose lowering and weight reduction. Efficacy and safety of liraglutide in management of type 2 diabetes have been well documented in several key clinical trials such as series of phase 3 Liraglutide Effect and Action in Diabetes (LEAD) trials, and the liraglutide-versus-sitagliptin trial. Recent two trials dealing with monotherapy and sulfonylurea combination therapy on Japanese patients with type 2 diabetes furthermore indicate liraglutide’s effectiveness in non-obese diabetes. In this review, we summarize results from such clinical trials, and discuss efficacy and safety of liraglutide in management of type 2 diabetes in various countries, along with a pitfall of liraglutide usage in real clinical setting.
Keywords: liraglutide, type 2 diabetes, incretin
Introduction
Management of type 2 diabetes (T2DM) includes improvement and maintenance of glycemic control; however, conventional oral anti-diabetic drugs (OADs) achieve only limited glycemic control and are associated with weight gain or hypoglycemia except for biguanides and alpha-glycosidase inhibitors.1,2 Glycemic control is also compromised by progressive impairment in beta cell function.3 New therapies targeting the incretin system have potentials to improve glycemic control, preserve beta cell function, and minimize hypoglycemia and weight gain.4,5 The incretins, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are a pair of gut hormones that are secreted from the intestine in response to meal ingestions and stimulate insulin secretion glucose-dependently.6–8 GIP and GLP-1 exert their effects by binding to the GIP receptor (GIPR) and GLP-1 receptor (GLP-1R), respectively, both of which are expressed on surfaces of pancreatic beta cells. Upon binding to their ligands, these receptors activate downstream signaling cascades, thereby resulting in enhancement of glucose-induced insulin secretion.6,8 Such glucose-dependent insulinotropic effects of the incretins make them highly attractive candidates for therapeutics against T2DM. Endogenous GIP and GLP-1, however, are rapidly inactivated by the endogenous enzyme dipeptidyl peptidase 4 (DPP-4) with half-lives of only 5 and 2 minutes, respectively.7,8 And, insulinotropic effects of GIP are blunted in hyperglycemic conditions,9 although strong insulinotropic effects of GIP have been shown recovered in patients with their glycemic conditions near-normalized. 10 Thus, two approaches have been successfully taken; 1) GLP-1R agonists (eg, liraglutide and exenatide) that binds to GLP-1R and activates GLP-1R signaling, and 2) DPP-4 inhibitors (eg, sitagliptin, vildagliptin and alogliptin) that inhibit DPP-4-mediated GLP-1 degradation and enhance insulinotropic effects of endogenous GLP–1. Both approaches have been shown effective in management of T2DM.11 In this article, we discuss efficacy and safety of GLP-1R agonist liraglutide in not only obese diabetes but also non-obese diabetes often seen in Asian countries. We also discuss a pitfall of liraglutide treatment in real clinical settings.
Mechanisms of Action
Liraglutide is a GLP-1 agonist with 97% amino acid sequence identity with endogenous human GLP-1 (Fig. 1).12,13 Liraglutide has one amino acid substitution (arginine substituted for lysine) and a C16 palmitic acid side chain attached via a glutamyl spacer.12,13 Because of these modifications, liraglutide has prolonged pharmacologic activity compared to endogenous GLP-1.12,13 Liraglutide is absorbed more slowly from subcutaneous tissues, has reversible albumin binding, and resistance to GLP-1 inactivation by DPP-4.12,13 However, similarly to endogenous GLP-1 (Fig. 1), liraglutide stimulate insulin secretion and decreases glucagon secretion in a glucose-dependent manner, thereby improving 24-hour glycemic control;14,15 this is also accompanied by a delay in gastric emptying.16
Figure 1.
Biological function of GLP-1 related to glycemic control in type 2 diabetes (A) and structure of liraglutide (B). Liraglutide has one amino acid substitution (arginine substituted for lysine) and a C16 palmitic acid side chain attached via a glutamyl spacer. Liraglutide is absorbed more slowly from subcutaneous tissues, has reversible albumin binding, and resistance to GLP-1 inactivation by DPP-4. Like endogenous GLP-1, liraglutide stimulates insulin secretion in a glucose-dependent manner as well as decreases glucagon secretion and delays gastric emptying, all of which contribute to improvement in glycemic control.
Metabolism and Pharmacokinetic Profile
Liraglutide binds reversibly to albumin and is partially resistant to degradation by DPP-4, causing it to be metabolized at a slower rate than endogenous GLP-1.17 In pharmacokinetic studies in healthy men, liraglutide absorption was slow; maximum plasma concentrations were reached approximately 10 to 14 hours after subcutaneous administration.12 The mean elimination half-life ranged from 11 to 13 hours.12 The mean accumulation ratio (Rac) was in the range of 1.4 to 1.5. Pharmacokinetic parameters calculated after one week of treatment were similar to those calculated on day 1.12 Following single and repeated daily doses (5–12.5 μg/kg), Cmax and AUC increased proportionately to the liraglutide dose. Comparison of pharmacokinetic profiles in individuals with varying renal function revealed that renal dysfunction has little effects on pharmacokinetics of liraglutide.18 Consistent with this pharmacokinetic observation, it has been reported that mild renal impairment in patients with type 2 diabetes has little difference on efficacy and safety of liraglutide in a recent meta-analysis of liraglutide’s clinical trials.19
Clinical Trials
The efficacy and safety of liraglutide were studied in six randomized phase 3 Liraglutide Effect and Action in Diabetes (LEAD) trials. The LEAD trials included 4456 patients recruited from more than 600 sites in 40 countries. In these trials, efficacy and safety of liraglutide was investigated as mono-therapy or combination-therapies with different oral anti-diabetic drugs (OADs) in comparison with existing diabetes therapies (Fig. 2).20 Three other recently completed trials compared liraglutide with sitagliptin21 and studied its efficacy and safety in Japanese non-obese patients with T2DM.22,23
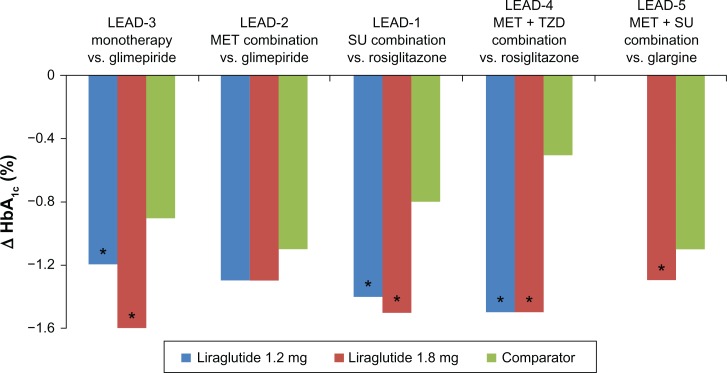
Figure 2.
Reduction in HbA1c in Liraglutide Effect and Action in Diabetes (LEAD) Studies. Changes in HbA1c from baseline for patients studied in each trials are shown: LEAD-3, monotherapy versus (vs). glimepiride;24 LEAD-2, metformin-combination therapy vs. glimepiride;26 LEAD-1, sulfonylurea-combination therapy vs. rosiglitazone;25 LEAD-4, metformin/thiazolidinedione-combination therapy vs. placebo;27 LEAD-5, metformin/thiazolidinedione-combination therapy vs. glargine.28
Note: *Significant difference vs. comparators.
LEAD-3: Liraglutide as Monotherapy
The LEAD-3 trial investigated the safety and efficacy of two doses of liraglutide monotherapy versus (vs.) glimepiride in patients with T2DM.24 The study patients were either drug-naïve, treated with lifestyle modifications, or had not achieved control with a single oral drug at less than 50% of the maximum approved dose. A total of 745 patients received either once daily subcutaneous liraglutide 1.2 mg (n = 251) or 1.8 mg liraglutide (n = 246) or once daily glimepiride 8 mg (n = 248) for 52 weeks. The primary endpoint was change in HbA1c value from baseline to 52 weeks. Mean HbA1c values decreased from baseline by 0.51% with glimepiride, 0.84% with liraglutide 1.2 mg (P < 0.0014 vs. glimepiride), and by 1.14% with liraglutide 1.8 mg (P < 0.0001 vs. glimepiride). After 52 weeks, 28% of patients treated with liraglutide 1.2 mg and 38% treated with liraglutide 1.8 mg achieved the International Diabetes Federation (IDF) HbA1c target of ≤6.5% vs. 16% of glimepiride patients (P = 0.0025 liraglutide 1.2 mg; P < 0.0001 liraglutide 1.8 mg). Patients treated with liraglutide also had decreased fasting and postprandial plasma glucose, significant weight loss, and decreased systolic blood pressure.
Liraglutide was generally well tolerated. Patients treated with liraglutide had higher rates of gastrointestinal adverse events (liraglutide 1.8 mg 51%, 1.2 mg 49%) than those receiving glimepiride (26%), but most of the events were transient. The most common gastrointestinal adverse event was nausea (liraglutide 1.8 mg 29%, 1.2 mg 27%). Two patients treated with liraglutide had pancreatitis. Both recovered and one continued in the study at the 1.2 mg dose.
LEAD-1, -2, -4, and -5: Liraglutide in Combination with OADs
The LEAD-1 and LEAD-2 trials studied the efficacy and safety of liraglutide in combination with a single OAD. LEAD-1 compared the efficacy of liraglutide (0.6, 1.2 or 1.8 mg/day), rosiglitazone, and placebo when combined with glimepiride in patients with T2DM (n = 1041).25 After 26 weeks, HbA1c had decreased significantly with all doses of liraglutide compared with placebo (P < 0.0001). The 1.2 mg and 1.8 mg doses of liraglutide produced significantly greater decreases in HbA1c compared with rosiglitazone (P < 0.0001). Liraglutide 0.6 mg was non-inferior to rosiglitazone. The American Diabetes Association (ADA) HbA1c target of <7.0% was achieved by significantly more patients in the liraglutide 1.8 mg group (P < 0.0001 vs. rosiglitazone; P < 0.0001 vs. placebo) and in the liraglutide 1.2 mg group (P = 0.0005 vs. rosiglitazone; P < 0.0001 vs. placebo). At week 26, all liraglutide doses were associated with significantly greater decreases in fasting plasma glucose (FPG) compared with placebo (P < 0.0001). Liraglutide 1.2 mg and liraglutide 1.8 mg both produced a 0.7 mmol/L greater reduction in FPG than rosiglitazone (P < 0.01). Treatment differences for postprandial glucose (PPG) were greater with all doses of liraglutide vs. placebo (P < 0.0001) and greater with liraglutide 1.2 mg (P = 0.043) and 1.8 mg (P = 0.0022) vs. rosiglitazone.
Mean body weight decreased from baseline with liraglutide 1.8 mg (−0.2 kg) compared with an increase in weight with rosiglitazone (+2.1 kg); the differences between the drugs were statistically significant (P < 0.0001).
The most common adverse events considered to be possibly or probably related to liraglutide were gastrointestinal and nervous system disorders. The rate of nausea was highest in patients receiving liraglutide 1.2 mg (10.5%). The occurrence of nausea decreased after 4 weeks. One patient receiving liraglutide 0.6 mg developed pancreatitis but completed the trial. Five patients previously diagnosed with pancreatitis completed the trial (4 with liraglutide, 1 with glimepiride) without reporting pancreatitis as an adverse event. There were no significant differences in calcitonin between the liraglutide groups and the placebo or rosiglitazone groups. One major hypoglycemia event was reported in a patient 9 days after starting liraglutide 1.8 mg in combination with glimepiride, which was determined by the investigator to be related to glimepiride. Minor hypoglycemia occurred in <10% of patients in all treatment groups.
LEAD-2 evaluated the efficacy and safety of liraglutide in combination with metformin compared with metformin monotherapy and with metformin plus glimepiride.26 A total of 1091 patients were randomized to liraglutide (0.6, 1.2, or 1.8 mg/day), glimepiride, or placebo, all in combination with metformin. After 26 weeks, glycemic control, as measured by reduction in mean HbA1c, was superior to placebo in the liraglutide 0.6 mg group (−0.8%; 95% CI −1.0 to −0.6), liraglutide 1.2 mg group (−1.1%; 95% CI −1.3 to −0.9), and liraglutide 1.8 mg group [−1.1% (95% CI −1.3 to −0.9)]. The 1.2 mg and 1.8 mg doses of liraglutide were noninferior to glimepiride with respect to HbA1c. At study end, FPG decreases from baseline in all of the liraglutide groups were significantly greater than the increase in the placebo group (P < 0.0001) but similar to the decrease in the glimepiride group. PPG decreased from baseline in all treatment groups, including placebo (P < 0.001 all liraglutide groups vs. placebo). Weight loss was dose-dependent in the liraglutide groups and significantly different from the weight gain observed in the glimepiride group (P < 0.0001). The weight loss in the liraglutide 1.2 mg and 1.8 mg groups was significantly greater than that in the placebo group (P < 0.01). The liraglutide 1.2 mg and 1.8 mg groups had significant reductions in systolic blood pressure of 2 to 3 mm Hg compared with an increase of 0.4 mmHG in the glimepiride group (P = 0.0128 liraglutide 1.2 mg; P = 0.0467 liraglutide 1.8 mg).
Nausea, vomiting, and diarrhea were the most frequently reported adverse events in the liraglutide groups, leading to the withdrawal of 36 (5%) patients receiving liraglutide. Nausea was reported in 11%, 16%, and 19% of patients receiving liraglutide 0.6 mg, 1.2 mg, and 1.8 mg, respectively. Fewer than 10% of these patients experienced nausea on a weekly basis by week 4. One patient in the liraglutide 1.2 mg group and one in the glimepiride group had acute pancreatit is leading to withdrawal from the study. There were no significant differences in calcitonin levels between the groups. Minor hypoglycemia occurred in about 3% of patients in the liraglutide and placebo groups and in 17% of patients in the glimepiride group (P < 0.001 liraglutide vs. glimepiride). No major hypoglycemic events were reported.
The LEAD-4 and LEAD-5 trials evaluated liraglutide in combinations with two OADs. LEAD-4 investigated liraglutide in combination with metformin and rosiglitazone for 26 weeks in patients with T2DM.27 The patients (n = 533) were randomized to once daily liraglutide 1.2 mg or 1.8 mg or placebo, both in combination with metformin and rosiglitazone. After 26 weeks, the mean HbA1c decreased by 1.5% ± 0.1% in both liraglutide groups and 0.5% ± 0.1% in the placebo group (liraglutide 1.2 mg vs. placebo −0.9% difference, 95% CI − 1.1 to −0.8; liraglutide 1.8 mg vs. placebo −1.1% difference, 95% CI −1.1 to −0.8). HbA1c <7% was achieved by 57.5% of patients in the liraglutide 1.2 mg group and 53.7% in the liraglutide 1.8 mg group vs. 28.1% in the placebo group (P < 0.0001 for both liraglutide groups). FPG decreases were significantly greater in the liraglutide 1.2 mg group (−40 mg/dL) and liraglutide 1.8 mg group (−44 mg/dL) compared with the placebo group (−8 mg/dL) (P < 0.0001). Likewise, PPG values were significantly decreased in the liraglutide 1.2 mg group (−47 mg/dL) and liraglutide 1.8 mg group (−49 mg/dL) vs. the placebo group (−14 mg/dL) (P < 0.001). Mean weight loss was significantly greater in the liraglutide 1.2 mg group (−1.0 kg) and liraglutide 1.8 mg group (−2.0 kg) compared to the placebo group, which had a mean weight gain of 0.6 kg (P < 0.0001).
Gastrointestinal disorders were increased in the liraglutide 1.2 mg group (45%) and the liraglutide 1.8 mg group (56%) vs. the placebo group (19%). Nausea was reported by 29% and 40% of patients in the 1.2 mg and 1.8 mg liraglutide groups, respectively. Nausea generally was transient, occurring in the first 4 weeks of liraglutide treatment. There were no episodes of pancreatitis. More patients in the liraglutide groups withdrew due to adverse events than in the placebo group. Minor hypoglycemia occurred more frequently in the liraglutide groups vs. the placebo group. No major hypoglycemic events were reported. There were no significant differences in calcitonin between the groups.
The objective of the LEAD-5 study was to compare the efficacy and safety of liraglutide vs. placebo and insulin glargine in patients with T2DM.28 A total of 581 patients were randomized to receive once daily liraglutide (1.8 mg), placebo, or insulin glargine, all in combination with metformin and glimepiride. After 26 weeks, the HbA1c reduction from baseline was 1.33% with liraglutide, 0.24% with placebo, and 1.09% with insulin glargine. The reduction in HbA1c was significantly greater with liraglutide than with placebo (P < 0.0001) or insulin glargine (P = 0.0015). Significantly more patients treated with liraglutide achieved HbA1c ≤ 6.5% and <7% vs. placebo and insulin glargine. The mean reduction in FPG was significantly greater with liraglutide vs. placebo (P < 0.0001) but not vs. insulin glargine. Likewise, the reduction in PPG from baseline was significantly greater with liraglutide than with placebo (P < 0.0001) but not vs. insulin glargine.
The weight loss achieved with liraglutide was significantly greater vs. that achieved with placebo (P = 0.0001) and vs. the weight gain observed with insulin glargine (P < 0.0001). The liraglutide group had a significant improvement in beta cell function, as assessed by the proinsulin to C-peptide ratio, compared with the insulin glargine group (P = 0.0019) and the placebo group (P < 0.0001). The liraglutide group had a significant reduction in systolic blood pressure vs. insulin glargine (P = 0.0001) but not vs. placebo.
Minor hypoglycemic episodes occurred in 27.4% of patients treated with liraglutide, 28.9% of patients treated with insulin glargine, and 16.7% of those treated with placebo. Major hypoglycemic events were reported in 5 patients in the liraglutide group (2.2%). Only one of these required medical assistance, none resulted in comas or seizures, and none were nocturnal. The most common adverse events in the liraglutide group were mild to moderate gastrointestinal events, mostly nausea. Nausea was reported in 14% of patients in the liraglutide group but decreased after 1 to 3 weeks of treatment and stabilized to 1.5% after 14 weeks. Total adverse events were more frequent in the liraglutide group but serious adverse events occurred less frequently in the liraglutide group (4%) than in the placebo (7%) and insulin glargine groups (7%). There were no reports of pancreatitis. There was a significant increase in calcitonin levels in both the liraglutide and insulin glargine groups vs. placebo. The estimated mean calcitonin level at 26 weeks was within the normal range for patients in both groups.
LEAD-6: Liraglutide vs. Exenatide
The LEAD-6 trial compared the efficacy and safety of liraglutide with exenatide in patients with T2DM (Fig. 3).29 Exenatide is an exendin-4-based GLP-1 receptor agonist with 53% amino acid identity, with human GLP-1 is eliminated by glomerular filtration, and has a half-life of 2.4 hours. The LEAD-6 comparison between liraglutide and exenatide was conducted because of the structural, metabolic, and pharmacologic differences between the two GLP-1 agonists. Patients from 15 countries with inadequately controlled T2DM on maximally tolerated doses of metformin, Sulfonylurea, or both, were randomized to receive liraglutide 1.8 mg/day (n = 233) or exenatide 10 μg twice a day (n = 231) for 26 weeks. After randomization, patients underwent a 2-week liraglutide dose-escalation period or a 4-week exenatide dose-escalation period to reach the study doses, followed by a 22- to 24-week maintenance period during which dose reductions were not permitted. Background OADs were maintained at prestudy doses, with dose-reductions allowed if unacceptable hypoglycemia occurred. Patients completing the study had the option to enroll in a 52-week liraglutide 1.8 mg extension phase. The primary efficacy endpoint was change in HbA1c from baseline to study end (26 weeks). Secondary efficacy endpoints were proportion of patients reaching HbA1c ADA and IDF targets (<7.0% and <6.5%), changes in FPG, PPG, body weight, beta cell function, glucag on, blood pressure, and lipid profiles. Safety endpoints included adverse events, vital signs, electrocardiogram, biochemical and hematological measures, and patient-reported hypoglycemia episodes.
Figure 3.
Comparison of liraglutide and exenatide in Liraglutide Effect and Action in Diabetes (LEAD) Study-6. Changes in HbA1c (A) and body weight (B) and proportion of patients with nausea are plotted.29 Liraglutide significantly reduced HbA1c more than exenatide did, with significantly lower frequency of nausea. Body weight changes were comparable between two groups. Reproduced from Buse et al., Lancet 2009; 374:39–47, with permission from Elsevier, © 2009.
Baseline characteristics were well balanced between the two treatment groups. There was a greater decrease in HbA1c values in liraglutide-treated patients compared with exenatide-treated patients. The mean change in HbA1c from baseline to week 26 was significantly greater in the liraglutide group (−1.12%) than in the exenatide group (−0.79%), with an estimated treatment difference of −0.33 (95% CI −0.47 to −0.18) (P < 0.0001). Significantly more patients in the liraglutide group than in the exenatide group achieved HbA1c targets <7% (54% vs. 43%; odds ratio [OR] 2.02; 95% CI 1.31 to 3.11; P < 0.0015) and <6.5% (35% vs. 21%; OR 2.73; 95% CI 1.68 to 4.43; P < 0.0001). Patients in the liraglutide group had significantly greater reductions than those in the exenatide group in FPG (−1.61 mmol/L vs. −0.60 mmol/L; P < 0.0001) and PPG after breakfast (P < 0.0001) and dinner (P = 0.0005) but not after lunch. The amount of weight loss and proportion of patients losing weight were similar in both treatment groups. Patients treated with liraglutide had significantly greater increases than those treated with exenatide in fasting insulin (P = 0.0355) and beta-cell function (P < 0.0001). Overall treatment satisfaction was significantly better in the liraglutide group than in the exenatide group (P = 0.0004).
Fewer overall adverse events were reported in the liraglutide group (74.9%) than in the exenatide group (78.9%). The most common adverse event in the liraglutide group was dyspepsia (N = 3) and in the exenatide group was nausea (n = 4). Nausea was less persistent with liraglutide, significantly decreasing vs. exenatide after week 4 (P < 0.0001). Patients in the liraglutide group vs. the exenatide group reported more serious adverse events (5.1% vs. 2.6%) and severe adverse events (7.2% vs. 4.7%). One patient developed chronic pancreatitis after 88 days of liraglutide therapy, which the investigator considered unlikely to be related to study drug. There were no major hypoglycemia events in patients treated with liraglutide but two episodes occurred in patients treated with exenatide and a sulfonylurea. Minor hypoglycemia occurred in 26% of liraglutide patients vs. 34% of exenatide patients. The event rates for minor hypoglycemia with liraglutide vs. exenatide were 1.932 vs. 2.600 events per participant per year (p = 0.0131). There were small decreases in calcitonin levels in both treatment groups.
Liraglutide vs. Sitagliptin
Pratley et al evaluated the efficacy and safety of liraglutide vs. the DPP-4 inhibitor sitagliptin, as adjunct treatments to metformin in patients with T2DM who had inadequate control with metformin alone (Fig. 4).21 Participants (n = 665) were randomized to subcutaneous liraglutide 1.2 mg (n = 225) or 1.8 mg (n = 221) once daily or oral sitagliptin 100 mg/day (n = 219) for 26 weeks. Liraglutide was started at 0.6 mg/day and escalated to the allocated dose. Sitagliptin was started and maintained at 100 mg/day. The primary efficacy endpoint was change in HbA1c from baseline to end of study. Secondary endpoints were the proportion of patients reaching the HbA1c targets of <7.0% or <6.5%, FPG, PPG, body weight, beta cell function, fasting lipid profile, cardiovascular risk markers, blood pressure, heart rate, physical measures, treatment satisfaction, and a composite endpoint of the proportion of patients with HbA1c < 7.0%, no hypoglycemia, and weight change of 0 kg or less. Safety assessments included adverse events, self-reported hypoglycemia, and selected hematological and biochemical measures.
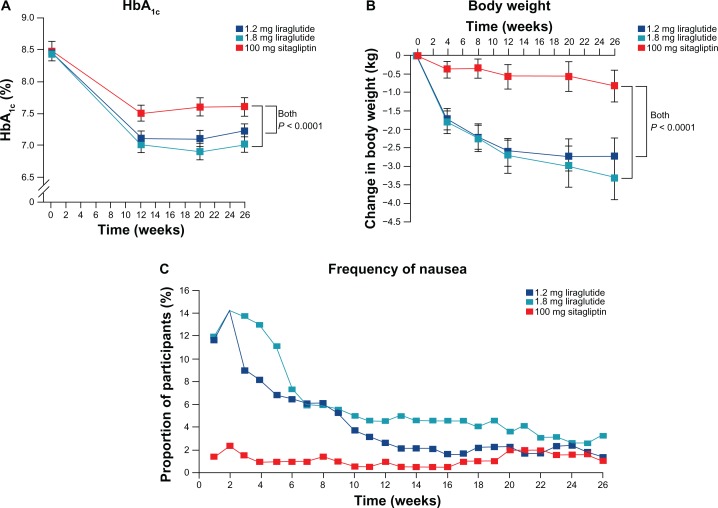
Figure 4.
Comparison of liraglutide and sitagliptin. Changes in HbA1c (A) and body weight (B) and proportion of patients with nausea are plotted.21 Liraglutide significantly reduced HbA1c more than sitagliptin did, with greater reduction in body weight. Although nausea is more frequent in liraglutide groups, proportions of patients suffering from nausea become similar at the end of the trial. Reproduced from Partley et al., Lancet 2010; 375:1447–56, with permission from Elsevier, © 2009.
Of the 665 randomized patients, 658 (99%) received at least one dose of study drug and 554 (83%) completed the trial. Patients in both liraglutide dose groups had greater HbA1c reductions than patients in the sitagliptin group (P < 0.0001). Mean decreases in HbA1c from baseline were −1.50% (95% CI −1.63 to −1.37) with liraglutide 1.8 mg, −1.24% (95% CI −1.37 to −1.11) with liraglutide 1.2 mg, and −0.90% (95% CI −1.03 to −0.77) for sitagliptin. The HbA1c targets were achieved by significantly more patients treated with liraglutide than with sitagliptin. Liraglutide produced significantly greater decreases in FPG compared with sitagliptin (P < 0.0001 for both doses vs. sitagliptin). The mean FPG decreases were −2.14 mmol/L (95% CI −2.43 to −1.84) with liraglutide 1.8 mg, −1.87 mmol/L (95% CI −2.16 to −1.57) with liraglutide 1.2 mg, and −0.83 mmol/L (95% CI −1.13 to −0.54) with sitagliptin. Weight loss was significantly greater with liraglutide than with sitagliptin (P < 0.0001 for both doses vs. sitagliptin). Mean weight loss was −3.38 kg (95% CI −3.91 to −2.84) with liraglutide 1.8 mg, −2.86 kg (95% CI −3.39 to −2.32) with liraglutide 1.2 mg, and −0.96 kg (−1.50 to −0.42) with sitagliptin.
Significantly greater reductions in waist circumference were observed with both doses of liraglutide vs. sitagliptin (1.2 mg P = 0.0010; 1.8 mg P = 0.0017). Both liraglutide doses vs. sitagliptin produced significant improvements in beta-cell function (homeostatic model assessment-beta) (P < 0.0001), C-peptide concentration (P = 0.0008), and proinsulin-to-insulin ratio (P = 0.0004). Diastolic blood pressure was significantly reduced with liraglutide 1.8 mg vs. sitagliptin (P = 0.0210). Heart rate increased with both liraglutide doses and decreased with sitagliptin; although the differences were small, they were significant. Liraglutide 1.8 mg was associated with a significantly greater decrease in total cholesterol vs. sitagliptin (P = 0.0332). The composite endpoint was achieved by 46% of patients in the liraglutide 1.8 mg group (OR 5.46; 95% CI 3.37 to 8.85; P < 0.0001 vs. sitagliptin), 37% in the liraglutide 1.2 mg group (OR 3.45; 95% CI 2.12 to 5.61; P < 0.0001 vs. sitagliptin), and 14% in the sitagliptin group. Patients in the liraglutide 1.8 mg group had significantly higher treatment satisfaction than those treated with sitagliptin (difference 1.39; 95% CI 0.13 to 2.64; P = 0.0300).
More treatment-emergent adverse events (TEAEs) were reported with liraglutide vs. sitagliptin. The most common adverse events were gastrointestinal disorders and infections. Gastrointestinal disorders occurred more frequently with liraglutide 1.8 mg (40%) and liraglutide 1.2 mg (33%) than with sitagliptin (21%). More patients in the liraglutide 1.2 mg and 1.8 groups experienced nausea compared to the sitagliptin group. Nausea with liraglutide was transient, with a median duration of 13 days with liraglutide 1.2 mg and 8 days with liraglutide 1.8 mg vs. 26 days with sitagliptin. There were similar rates of serious (2%–3%) and severe (3%–4%) adverse events in the three treatment groups. There were no reports of pancreatitis. Changes in serum calcitonin from baseline were similar across all treatment groups.
Liraglutide Monotherapy in Japanese Patients with Type 2 Diabetes
This study evaluated the efficacy and safety of liraglutide 0.9 mg/day monotherapy compared with glibenclamide monotherapy in Japanese patients with T2DM (Fig. 5).30 A total of 411 Japanese patients with T2DM were randomized 2:1 to liraglutide 0.9 mg/day (n = 272) or glibenclamide 2.5 mg administered once or twice per day (n = 139) for 24 weeks. The initial 24-week double-blind period was followed by an ongoing 28-week open-label period to evaluate the long-term safety and efficacy of liraglutide. The primary efficacy endpoint was HbA1c at 24 weeks. Secondary endpoints included FPG, 7-point self-measured blood glucose (SMBG) profiles, PPG, body weight, lipid profile, cardiovascular biomarkers, and percentage of patients achieving HbA1c targets of <7.0% ADA or <6.5% IDF. Safety endpoints included the incidence of adverse events, vital signs, and clinical laboratory assessments, and self-reported hypoglycemic episodes.
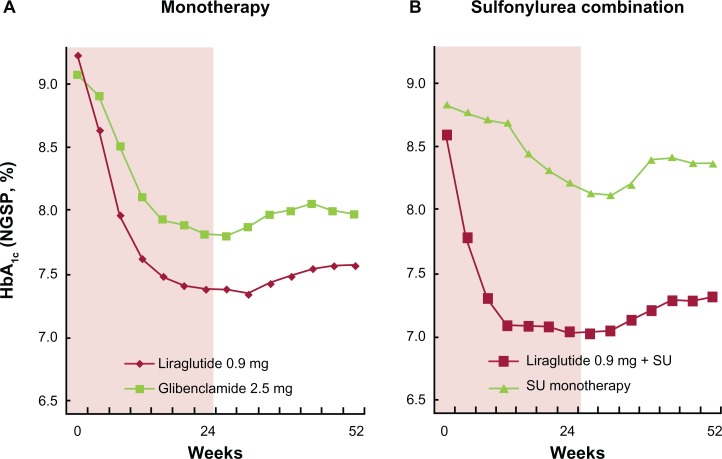
Figure 5.
Liraglutide mono- and sulfonylurea combination therapies in Japanese patients with type 2 diabetes. Changes in HbA1c are shown for liraglutide monotherapy22,30 (A) and sulfonylurea combination therapy22,23 (B). In both trials, treatment over the first 24 weeks was given in a double blind fashion, followed by a 28-week open label treatment period.
A total of 366 patients completed 24 weeks of treatment. Baseline characteristics were well matched between the two groups. HbA1c reductions were significantly greater with liraglutide compared with glibenclamide. At 24 weeks, the estimated mean HbA1c was 6.99% in the liraglutide group (change from baseline −1.88%) and 7.50% in the gibenclamide group (change from baseline −1.38%), with a treatment difference of −0.50% (95% CI −0.70 to −0.30; P < 0.0001). The change in HbA1c from baseline was −1.74% with liraglutide and −1.18% with glibenclamide. The ADA HbA1c target of <7.0% was achieved by 49% of liraglutide patients vs. 30.8% of glibenclamide patients (P < 0.0001). The IDF HbA1c target of <6.5% was achieved by 27.8% of liraglutide patients vs. 10.8% of glibenclamide patients (P < 0.0001). Patients treated with liraglutide had significantly greater improvements vs. glibenclamide in FPG (P < 0.0001), PPG mean area under the curve (P < 0.0001), SMPG (P < 0.0001), body weight (P < 0.0001), brain natriuretic peptide (P < 0.0001), and high-sensitivity C-reactive protein (P = 0.0476).
TEAEs were reported in 73.1% of liraglutide patients and 74.2% of glibenclamide patients. The most common TEAEs in both groups were nasopharyngitis, diarrhea, constipation, and upper respiratory infection. More gastrointestinal adverse events were reported with liraglutide vs. glibenclamide, including diarrhea (6.3% vs. 3.8%), constipation (5.6% vs. 3.8%), and nausea (4.5% vs. 1.5%). Serious adverse events occurred in 4.9% of liraglutide patients and 6.1% of glibenclamide patients. The proportion of patients who withdrew from the trial because of adverse events was 3.7% in the liraglutide group and 3.0% in the glibenclamide group. There were no reports of pancreatitis in either group. No significant change in calcitonin levels was observed in the liraglutide group but there was a significant shift to a lower category in the glibenclamide group. There were no major hypoglycemic events reported in either treatment group. The overall rate (events/patient/year) of minor hypoglycemic events was 0.25 in the liraglutide group compared with 1.58 in the glibenclamide group (P < 0.0001).
Recently, one year extension of the same study has been reported.22 At week 52, the change in HbA1c from baseline was −1.5% with liraglutide and −1.0% with glibenclamide with treatment difference of −0.49% (95% CI −0.71 to −0.29). Fasting plasma glucose fell from 202.8 and 202.1 mg/dL, respectively, to 145.3 and 156.7 mg/dL, respectively. Mean plasma glucose and mean postprandial plasma glucose increment were lower in the liraglutide group. Mean bodyweight was reduced by 0.8 kg in the liraglutide group and increased by 1.0 kg in the glibenclamide group. The proportion of patients reporting at least one TEAE in the liraglutide and glibenclamide groups was 91.4% and 91.7%, respectively. Most TEAEs were mild in severity. No major hypoglycemic episode was observed.
Liraglutide Combination with Sulfonylurea in Japanese Patients with Type 2 Diabetes
This study evaluated the efficacy and safety of two doses of liraglutide (0.6 and 0.9 mg/day) vs. placebo, both added to a sulfonylurea, in Japanese patients with T2DM (Fig. 5).30 A total of 264 Japanese patients with T2DM currently treated with glibenclamide, glicazide, or glimepiride were included in this double-blind, 24 week trial. The patients were randomized to receive liraglutide 0.6 mg (n = 88), liraglutide 0.9 mg (n = 88), or placebo (n = 88). All patients continued on their pretrial sulfonylurea treatment. The primary endpoint was HbA1c level at 24 weeks. Secondary endpoints included 7-point SMPG, body weight, FPG, PPG, lipid profile, cardiovascular biomarkers, and the percentage of patients achieving the HbA1c targets of <7.0% or <6.5%. Safety endpoints include incidence of hypoglycemic episodes and adverse events, vital signs, and clinical laboratory assessments.
Baseline characteristics were well balanced between the treatment groups. HbA1c levels were significantly reduced and sustained with both liraglutide doses (P < 0.0001). Estimated HbA1c values were significantly lower with liraglutide 0.6 mg (7.14%) and 0.9 mg (6.67%) than with placebo (8.06%) (P < 0.0001). The percentages of patients achieving HbA1c target values of <7.0% and <6.5% were significantly greater in the liraglutide group than in the placebo group. Significant reductions were reported with liraglutide vs. placebo in FPG (P < 0.0001 both doses), and mean 7-point self monitored plasma glucose (SMPG) (P < 0.0001 both doses). Mean body weight did not change in the two liraglutide groups and decreased in the placebo group.
TEAEs were reported in 76.1% of patients receiving liraglutide 0.6 mg, 78.4% receiving liraglutide 0.9 mg, and 75% receiving placebo. The most common adverse events were nasopharyngitis, diarrhea, and constipation. More gastrointestinal adverse events were reported in the liraglutide groups during the first 4 weeks than in the placebo group but there were no major differences across all groups. No major hypoglycemic episodes were reported. The rate of minor hypoglycemic events (events/patient/year) was higher in the liraglutide 0.6 mg group (2.17) and the liraglutide 0.9 mg group (1.96) than in the placebo group (1.01). Serious TEAEs were reported by 3 patients in the liraglutide 0.6 mg group, 2 in the liraglutide 0.9 mg group, and 3 in the placebo group. Plasma concentrations of calcitonin decreased in all groups. There were no reports of pancreatitis.
Recently, one year extension of the same study has been reported.23 At week 52, HbA1c in the liraglutide 0.6 mg, liraglutide 0.9 mg and placebo groups was reduced from 9.00 to 7.91%, from 8.61 to 7.33%, and from 8.85 to 8.79%, respectively. The mean difference of HbA1c (95% CI) in the liraglutide 0.6 and 0.9 mg groups vs. the placebo group was −0.96 (−1.25 to −0.67) and −1.33 (−1.62 to −1.04), respectively. Mean fasting plasma glucose at week 52 was lower in the liraglutide groups compared with the placebo group, and mean bodyweight remained unchanged in the liraglutide groups. Most subjects in all three treatment groups reported mild adverse events. No major hypoglycemic episode was reported.
In both monotherapy and sulfonylurea combination therapies, changes of HbA1c from baseline were greater in Japanese T2DM patients22,23,30,31 than those observed in LEAD-3 and -1,24,25 despite of lower dosages of liraglutide used in the Japanese. This could partly explained by the fact that pathophysiology of Japanese T2DM is characterized with impaired early phase insulin secretion rather than insulin resistance,32 and liraglutide could ameliorate defects in early phase insulin secretion. In addition, meal-induced enhancement of GLP-1 secretion has been reported negligible in Japanese T2DM.33,34 Meal-induced enhancement of GLP-1 secretion might not be responsible for pathogenesis of Japanese T2DM because it is also observed in healthy volunteers,33 however it could explain why liraglutide exerts glucose lowering effects at such a low dose in Japanese.
Liraglutide in Patients with Type 2 Diabetes from China, Korea and India
This study evaluated the efficacy and safety of liraglutide 0.6, 1.2, and 1.8 mg/day vs. 4 mg of glimepiride, both added to metformin in Asian patients with T2DM.35 A total of 929 patients with T2DM from China, Korea and India were included in this doubleblind, 16 week trial. The patients were randomized to receive liraglutide 0.6 mg (n = 231), 1.2 mg (n = 233), 1.8 mg (n = 233), or 4 mg glimepiride (n = 231). All patients received metformin. The primary endpoint was HbA1c level at 16 weeks. Secondary endpoints included 7-point SMPG, body weight, FPG, systolic blood pressure, and the percentage of patients achieving the HbA1c targets of <7% or <6.5%. Safety endpoints include incidence of hypoglycemic episodes and adverse events, vital signs, and clinical laboratory assessments.
Baseline characteristics were well balanced between the treatment groups. HbA1c levels were reduced and sustained with all liraglutide doses. After 16 weeks of treatment, the observed mean HbA1c reduction from baseline was 1.0% in the liraglutide 0.6 mg group, 1.3% in the liraglutide 1.2 mg group, 1.4% in the liraglutide 1.8 mg group, and 1.3% in the glimepiride group. No significant difference was shown in the percentage of subjects HbA1c target values of <7.0% and <6.5% among the liraglutide 1.2 mg group, the liraglutide 1.8 mg group, and the 4 mg glimepiride group (42.9, 44.6 and 43.7% for <7.0%, respectively; 26.2, 30.0 and 29.4% for 6.5%, respectively).
A rapid decrease in FPG was observed within the first 2 weeks after randomization, and FPG remained stable during the remaining 14 weeks of the trial in all treatment groups. At the end of the trial, the mean PPG values (mean of three main meals) from 7-point SMPG decreased significantly greater in the liraglutide 1.8 mg group than the liraglutide 0.6 mg group, the liraglutide 1.2 mg group, and the glimepiride group. The estimated changes in mean PPG were comparable among the liraglutide 0.6 mg group, the liraglutide 1.2 mg group, and the glimepiride group.
Liraglutide groups showed 1.8–2.4 kg mean weight reduction, while a 0.1 kg mean increase in the glimepiride group (P < 0.0001). A dose-dependency was observed for the reduction seen in all liraglutide treatment groups in favor of the liraglutide 1.8 mg group (−1.8 ± 2.2, −2.3 ± 2.4 and −2.4 ± 2.6 kg for liraglutide 0.6, 1.2 and 1.8 mg, respectively). A decrease of more than 3 mmHg in mean SBP was observed in liraglutide groups, while a decrease of 0.9 mmHg in mean systolic blood pressure was observed in glimepiride group. The reduction in mean systolic blood pressure in the liraglutide 1.2 and 1.8 mg groups was significantly higher than that in the glimepiride.
No major hypoglycaemia was reported for liraglutide, while two subjects treated with glimepiride reported major hypoglycaemia. The liraglutide groups were associated with approximately 10-fold lower incidence of minor hypoglycaemia than the glimepiride group. Serious adverse events that resulted in withdrawal from the study were reported in 1.7%–3.4% of subjects in four groups (3.9% in the liraglutide 0.6 mg group, 9.4% in the liraglutide 1.2 mg group, 12.8% in the liraglutide 1.8 mg group and 1.3% in the glimepiride group). The most frequently reported adverse events in liraglutide groups were those from the gastrointestinal system, such as diarrhea, nausea and vomiting, which were transient and occurred primarily during the first 4 weeks of treatment and decreased markedly over time. No events of pancreatitis were reported in this trial.
The results of this study in patients with type 2 diabetes in China, Korea, and India are consistent with efficacy and safety of liraglutide reported in previous LEAD studies. Interestingly, HbA1c lowering effects of liraglutide on Japanese type 2 diabetes22,23,30,31 are much greater than those in this study. Although the study in China, Korea and India are designed different from that in Japan (eg, the presence or absence of metformin combination), it is interesting, in future, to investigate if differences in HbA1c lowering effects of liraglutide are resulted from yet-to-characterized differences in pathophysiology of type 2 diabetes among Asians.
Safety
The liraglutide clinical trials have shown that liraglutide is generally well tolerated. The most common adverse events are gastrointestinal disorders, especially nausea. Nausea was the most frequently reported adverse event in the LEAD-3 (27%–29%),24 LEAD-1 (7%–11%),25 LEAD-2 (16%–19%),26 LEAD-4 (29%–40%),27 LEAD-5 (14%),28 and the trial comparing liraglutide and sitagliptin (21%–27%).21 However, nausea was transient, decreasing after about 4 weeks. Most episodes of nausea were mild to moderate in nature and were dose-dependent.20,36 Nausea episodes were less frequent in the Japanese studies,22,23 which may be due to the lower liraglutide doses used in those studies.
The occurrence of major hypoglycemia with liraglutide was rare. There were no major episodes with liraglutide monotherapy and only 7 when liraglutide was used in combination therapy. Six of the episodes were in patients treated with liraglutide plus sulfonylureas. One episode occurred in a patient treated with liraglutide 1.2 mg and metformin the trial comparing liraglutide and sitagliptin; this episode was associated with a blood glucose level of 3.6 mmol/L and was not associated with seizure or coma.21 The phase 3 trials demonstrate that the glycemic benefits associated with liraglutide therapy are not accompanied by an increased risk of hypoglycemia compared with other therapies.20
The LEAD studies also demonstrated that liraglutide is not associated with an increased risk of pancreatitis. 20 Only five acute and 2 chronic cases of pancreatitis were reported during the phase 3 trials,20 for an incidence rate of 2.2 cases per 1000 patients-years.36 Continuous exposure to GLP-1 agonists has been shown to cause C-cell tumors, benign adenomas and malignant carcinomas in rodents.36 Although increased cases of malignant C-cell carcinomas were observed in rodents treated with liraglutide, liraglutide treatment did not affect their overall survival rates. In human, the incidence of medullary thyroid cancer is limited, which makes it infeasible to conduct a clinical trial to detect an increased risk of C-cell tumors in liraglutide-treated patients. However, serum calcitonin levels were not found to be significantly different among liraglutidetreated patients and controls.20 In addition, no association of exenatide and C-cell tumors has been reported. However, liraglutide, as well as other incretin-based drugs, are relatively new drugs and potential associations with cancers and pancreatitis needs to be evaluated by prospective randomized trials in future rather than retrospective analyses.37,38
Efficacy
Liraglutide was associated with significant reductions in HbA1c in the phase 3 LEAD trials, the trial comparing liraglutide and sitagliptin, and the two Japanese trials. As the primary endpoint in the LEAD trials, HbA1c reductions ranged from 0.84% to 1.50% with liraglutide 1.2 and 1.8 mg.20 In the trial comparing liraglutide and sitagliptin, HbA1c was reduced by 1.24% to 1.50% in the liraglutide groups compared with 0.90% in the sitagliptin group.21 When liraglutide 0.9 mg was compared with glibenclamide in Japanese patients, HbA1c was reduced from baseline to 24 weeks by 1.88% with liraglutide vs. 1.38% with glibenclamide.30 When combined with sulfonylureas in Japanese patients with type 2 diabetes, liraglutide was associated with a 1.46% to 1.56% decrease in HbA1c.31 Liraglutide also reduced FPG and PPG levels across the phase 3 trials.20 The improvement in glycemic control was accompanied by weight loss of up to a mean −3.2 kg in the LEAD-1 through -5 trials.20 In the LEAD-6 trial, where patients received liraglutide in combination with a sulfonylurea, a mean weight gain of 0.3 kg was reported.29 Weight loss was significantly greater with liraglutide than with sitagliptin in the trial comparing liraglutide and sitagliptin.21 Significantly greater reductions in waist circumference were also observed with both doses of liraglutide vs. sitagliptin.21 Systolic blood pressure was reduced in patients treated with liraglutide in the LEAD trials, potentially leading to a reduction in risk of cardiovascular disease.
The phase 3 trials also demonstrated an improvement in beta cell function when combined with OADs, as measured by the homeostasis model assessment-beta. Increases in beta cell function ranged from 23% to 32% after 26 weeks of treatment in these trials.21,27,29,30
Patient Preference
The LEAD-3, LEAD-6, and the trial comparing liraglutide and sitagliptin assessed patient satisfaction or quality of life (QOL) of the study participants. In the LEAD-3 trial of liraglutide monotherapy, Patient Reported Outcomes (PRO) were assessed to determine the effect of liraglutide on QOL.39 Patients completed the 77-item questionnaire on general perceived health, mental and emotional health, cognitive functioning, overall QOL, weight image, weight concern, body size and body distress, at baseline and weeks 28 and 52. Patients in the liraglutide 1.8 mg group had improved QOL scoring for physical and emotional domains compared with patients in the glimepiride group (P = 0.02).39 The improvements appeared to result from improvements in weight image and weight concern in the liraglutide group vs. the glimepiride group (P < 0.01).39 In a pooled analysis of all groups, decreases in body mass index (BMI) were associated with improvements in weight image and decreases in weight concern (P < 0.0001).39 Decreases in weight concern were associated with increases in overall QOL (P < 0.0001), general perceived health (P < 0.0001), and mental and emotional health (P = 0.002). Decreases in HbA1c were associated with increases in general perceived health (P = 0.0002).39
Patients in the LEAD-6 trial receiving liraglutide (n = 161) reported significantly better overall treatment satisfaction than those in the exenatide group (n = 143) (P = 0.0004).29 In the trial comparing liraglutide and sitagliptin, treatment satisfaction was assessed with the Diabetes Treatment Satisfaction Questionnaire in a subgroup of 505 patients. The results showed that patients in the liraglutide 1.8 mg group had significantly higher treatment satisfaction than those treated with sitagliptin (difference 1.39; 95% CI 0.13 to 2.64; P = 0.0300).29
A study by Polster et al compared patient preferences for liraglutide and exenatide for the treatment of T2DM.40 The EQ-5D index, EQ-5D visual analogue scale, a time trade-off (TTO) exercise in which respondents compared the profiles of liraglutide and exenatide, a conjoint exercise in which respondents compared a series of TTO exercises for hypothetical product profiles, and demographic measures were used. The surveys were conducted on the internet. The TTO exercise included four attributes of the products: change in HbA1c, incidence of nausea, incidence of hypoglycemia, and dosing frequency. A total of 382 patients with T2DM participated in the survey. The profile representing liraglutide was preferred by 96% of the patients over the profile representing exenatide. The mean TTO scores were 0.978 for liraglutide vs. 0.94 for exenatide, for a mean difference of 0.038 (P < 0.05). Estimated preference scores from the conjoint analysis showed that the most important attribute to patients was efficacy measured by HbA1c, followed by nausea, hypoglycemia, and dosing schedule.
Safety in Real Life
Clinical trials described so far indicate that liraglutide is indeed a safe and effective to manage T2DM worldwide. Yet, incidents of severe hyperglycemia or ketoacidosis have been reported recently in Japan when insulin therapy is terminated and switched to liraglutide in insulin-dependent T2DM patients. Although it is an injection similar to insulin, liraglutide cannot be a replacement of insulin therapy. Therefore, when insulin is switched to liraglutide, beta cell function needs to be carefully evaluated beforehand by measuring urinary C-peptide and serum C-peptide before and after glucagon administration or meal ingestions, and patients should be instructed to perform frequent SMPG after insulinto-liraglutide switch to avoid serious hyperglycemia and diabetic ketoacidosis. Recent data indicate that liraglutide is more effective when introduced in patients with short durations and less OADs used, whose beta cell function could be highly maintained. 41 While clinical characteristics of patients who respond well to liraglutide are largely unknown, it is certain that use of liraglutide early in the disease progression provides greater clinical benefits and potential improvement in beta cell function.
Conclusions
Liraglutide is a relatively newer treatment option with the potential to address unmet needs of patients with T2DM. The phase 3 studies demonstrate that liraglutide provides significant improvements in glycemic control with a low risk of hypoglycemia. Additionally, liraglutide induces weight loss and has protective effects on beta cell function. Liraglutide is generally well tolerated. The most common adverse event is mild to moderate nausea, which usually is transient. There is, to date, no direct evidence that liraglutide is associated with an increased risk of pancreatitis or a significant change in calcitonin levels.
Acknowledgments
The authors have no conflict of interest. The authors deeply apologize for citing only part of the relevant work in this field (due to limited space) and are indebted to many authors for their contributions. The authors thank Ms. Aya Sanagi for secretarial assistance. HbA1c values were shown in National Glycohemoglobin Standardization Program values as recommended by the Japan Diabetes Society.42,43
Abbreviations
- ADA,
American Diabetes Association;
- BMI,
body mass index;
- GI,
gastrointestinal;
- T2DM,
type 2 diabetes;
- OAD,
oral anti-diabetic drugs;
- FPG,
fasting plasma glucose;
- GIP,
glucose-dependent insulinotropic polypeptide;
- GLP-1,
glucagon-like peptide-1;
- GIPR,
GIP receptor;
- GLP-1R,
GLP-1 receptor;
- PPG,
postprandial glucose;
- LEAD,
Liraglutide Effect and Action in Diabetes;
- QOL,
quality of life;
- vs.,
versus;
- SMPG,
self-measured plasma glucose;
- TEAE,
treatment-emergent adverse event;
- TTO,
time trade-off.
Footnotes
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2009;52:17–30. doi: 10.1007/s00125-008-1157-y. [DOI] [PubMed] [Google Scholar]
- 3.UK prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes. 1999;44:1249–58. [PubMed] [Google Scholar]
- 4.Drucker DJ, Sherman SI, Gorelick FS, et al. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010;33:428–33. doi: 10.2337/dc09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med. 2011;124:S3–18. doi: 10.1016/j.amjmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 8.Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the Two Incretin Hormones: Similarities and Differences. J Diabet Invest. 2010;1:9–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilsboll T, Krarup T, Madsbad S, et al. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia. 2002;45:1111–9. doi: 10.1007/s00125-002-0878-6. [DOI] [PubMed] [Google Scholar]
- 10.Hojberg PV, Vilsboll T, Rabol R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 11.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 12.Agerso H, Jensen LB, Elbrond B, et al. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 13.Agerso H, Vicini P. Pharmacodynamics of NN2211, a novel long acting GLP-1 derivative. Eur J Pharm Sci. 2003;19:141–50. doi: 10.1016/s0928-0987(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 14.Chang AM, Jakobsen G, Sturis J, et al. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52:1786–91. doi: 10.2337/diabetes.52.7.1786. [DOI] [PubMed] [Google Scholar]
- 15.Degn KB, Juhl CB, Sturis J, et al. One week’s treatment with the longacting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–94. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 16.Juhl CB, Hollingdal M, Sturis J, et al. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes. 2002;51:424–9. doi: 10.2337/diabetes.51.2.424. [DOI] [PubMed] [Google Scholar]
- 17.Elbrond B, Jakobsen G, Larsen S, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care. 2002;25:1398–404. doi: 10.2337/diacare.25.8.1398. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen LV, Hindsberger C, Robson R, et al. Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br J Clin Pharmacol. 2009;68:898–905. doi: 10.1111/j.1365-2125.2009.03536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson JA, Brett J, Falahati A, et al. Mild Renal impairment has no effect on the efficacy and safety of liraglutide. Endocr Pract. 2010:1–31. doi: 10.4158/EP10215.RA. [DOI] [PubMed] [Google Scholar]
- 20.Raskin P, Mora PF. Glycaemic control with liraglutide: the phase 3 trial programme. Int J Clin Pract. 2010;64(Suppl):21–7. doi: 10.1111/j.1742-1241.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 21.Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 22.Kaku K, Rasmussen MF, Nishida T, et al. Fifty-two-week, randomized, multicenter trial to compare the safety and efficacy of the novel glucagonlike peptide-1 analog liraglutide vs. glibenclamide in patients with type 2 diabetes. Journal of Diabetes Investigation. 2011. in press. [DOI] [PMC free article] [PubMed]
- 23.Seino Y, Rasmussen MF, Nishida T, et al. Glucagon-like peptide-1 analog liraglutide in combination with sulfonylurea safely improves blood glucose measures vs. sulfonylurea monotherapy in Japanese patients with type 2 diabetes: Results of a 52-week, randomized, multicenter trial. Journal of Diabetes Investigation. 2011;2:280–6. doi: 10.1111/j.2040-1124.2011.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 25.Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–78. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–30. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs. insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046–55. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 30.Seino Y, Rasmussen MF, Nishida T, et al. Efficacy and Safety of the once-daily human GLP-1 analogue, liraglutide, vs. glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin. 2010;26:1013–22. doi: 10.1185/03007991003672551. [DOI] [PubMed] [Google Scholar]
- 31.Kaku K, Rasmussen MF, Clauson P, et al. Improved glycaemic control with minimal hypoglycaemia and no weight change with the once-daily human GLP-1 analogue liraglutide as add-on to sulfonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:341–7. doi: 10.1111/j.1463-1326.2009.01194.x. [DOI] [PubMed] [Google Scholar]
- 32.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Research and Clinical Practice. 2004;66S:S37–44. doi: 10.1016/j.diabres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 33.Yabe D, Kuroe A, Lee S, et al. Little Enhancement of Meal-Induced GLP-1 Secretion in Japanese: Comparison of Type 2 Diabetes and Healthy Controls. J Diabet Invest. 2010;1:56–9. doi: 10.1111/j.2040-1124.2010.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yabe D, Watanabe K, Sugawara K, et al. Comparison of Incretin Immunoassays with or without Plasma Extraction: Incretin Secretion in Japanese Patients with Type 2 Diabetes. J Diabet Invest. 2011. in press. [DOI] [PMC free article] [PubMed]
- 35.Yang W, Chen L, Ji Q, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial(*) Diabetes Obes Metab. 2011;13:81–8. doi: 10.1111/j.1463-1326.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 36.Peterson GE, Pollom RD. Liraglutide in clinical practice: dosing, safety and efficacy. Int J Clin Pract. 64(Suppl):35–43. doi: 10.1111/j.1742-1241.2010.02498.x. [DOI] [PubMed] [Google Scholar]
- 37.Elashoff M, Matveyenko AV, Gier E, et al. Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon-Like Peptide-1–Based Therapies. Gastroenterology. 2011;141:150–6. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes Care. 33:2349–54. doi: 10.2337/dc10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bode BW, Testa MA, Magwire M, et al. Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:604–12. doi: 10.1111/j.1463-1326.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polster M, Zanutto E, McDonald S, et al. A comparison of preferences for two GLP-1 products—liraglutide and exenatide—for the treatment of type 2 diabetes. J Med Econ. 2010;13:655–61. doi: 10.3111/13696998.2010.529377. [DOI] [PubMed] [Google Scholar]
- 41.Garber A, Matthews D, Zinman B, et al. The Effect of disease stage, indicated by number of previous oral antidiabetic agents, on the response to liraglutide in type 2 diabetes. Diabetes. 2011 [Google Scholar]
- 42.Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Journal of Diabetes Investigation. 2010;1:212–28. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seino Y, Nanjo K, Tajima N, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Diabetology International. 2010;1:2–20. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]