Abstract
Cortisol concentrations in hair collected from young male and female adults were assayed and compared for differences along shaft length and between body sites. No significant differences were found between hair shaft sites, supporting a model of the hair shaft as “alive” and responsive to environmental demand in terms of cortisol production. Hair taken from forearms had significantly higher concentrations of cortisol than hair from lower legs, suggesting a localized hair cortisol response and verifying previous findings. Issues of the link between central and peripheral HPA axes are raised for discussion and further investigation.
Keywords: cortisol, hair, stress, peripheral HPA axis
Introduction
Cortisol is the product of a trophic cascade in the hypothalamic-pituitary-adrenal axis (HPA). That process begins with the synthesis of CRH in the hypothalamus which stimulates ACTH secretion from the pituitary into the bloodstream and thence to the adrenal cortex, which produces cortisol.1 In its role as the principal glucocorticoid in humans, cortisol has vital anti-inflammatory and immunosuppressive influences throughout the body,2,3 as well as a host of other effects that assist metabolism and homeostasis.4 Consequently, a basal concentration of cortisol is required at all times,4 and this may be elevated in response to physical or mental stressors to ensure the adequate physiological responsivity of the organism to environmental demands.5,6
Although the traditional sources for assaying cortisol from the HPA axis have been blood, urine or saliva,7 cortisol has recently also been found to be synthesised in skin8 and hair.9–16 These findings indicate that the CRH-ACTH-cortisol sequence is replicated in melanocytes17 and hair follicles,18 and that the concentration of cortisol in these varies according to physical and psychological stressors in the same fashion as cortisol from the “central” HPA axis, although not in ways that would reflect a single connected HPA axis.14–16 These findings have established the existence of an independent “peripheral” HPA axis in skin and hair,8,17,19 that may respond to threat and demand in similar ways as the central HPA axis but not necessarily be instigated by it. Ito and colleagues18 concluded that hair follicles and their production of cortisol represented a “functional equivalent” but independent HPA axis. That is, as well as being targets for “central” HPA axis activity, skin and hair are also producers of cortisol via their own neuroendocrine systems which include CRH, ACTH and cortisol itself,1 all of which have been shown to be secreted by melanocytes and hair follicles.10,18,20 Although these data strongly support the HPA-like functional equivalent of a CRH-ACTH-cortisol serial synthesis as in the central HPA axis, there remains the possibility that some of the cortisol found in hair may come from the reversible activation of cortisone via 11 betahydroxysteroid dehydrogenase (11betaHSD).
With particular reference to hair, it has previously been accepted that the hair shaft is dead once it leaves the skin,21 but the production of cortisol in the hair follicle plus its presence in the hair shaft challenge this opinion, particularly as these concentrations are not affected by washing of the hair (which has been shown to remove less than 7% of the total hormone extracted),12,22 thereby excluding effects due to secretions from skin or hair follicles near the hair shaft. However, although concentrations of sex steroids have been shown to not vary significantly along the length of the hair shaft,23 few data have previously been reported regarding the relative concentration of cortisol along the hair shaft. Such data would assist in determining if cortisol is transmitted along the supposedly “dead” hair shaft relatively quickly (i.e. if the concentration was continuous along the shaft) or if it is a gradual process (shown by variations in concentration along the shaft), and thus clarify the role of the hair shaft in this process. One recent study24 which collected hair samples from mothers of new-borns, mothers of toddlers and women who had never been pregnant, reported elevated concentrations among both groups of mothers compared to women who had never been pregnant, reflecting the previouslyreported increase in cortisol during pregnancy.25,26 Those authors also reported that hair samples taken closest to the scalp had significantly higher cortisol concentrations than samples further from the scalp, and argued that this may have been due to “washout” effects due to unknown causes.24 These data were in contrast to those reported from monkeys,9 in which there were no significant differences in cortisol concentrations along the hair shaft. These apparent contradictions leave this issue open to further investigation.
Another aspect of potential variability in hair cortisol concentration is that concerning body site. A previous investigation reported no significant differences in cortisol concentrations in hair taken from five different regions of the scalp while subjects were at rest,13 but another study which compared hair from sites which had undergone pain stress versus those which did not, showed significant (but transient) differences in cortisol concentrations across these sites, thus suggesting the presence of some separation in cortisol-production responses across body sites.16 However, no data have yet been reported regarding the variability in cortisol concentrations from different body sites while the subject is at rest. This is a particularly relevant issue in terms of the relationship between the central and peripheral HPA axes. It might be hypothesized that, were the two systems directly linked, then all parts of the external body surface should show very similar (if not identical) hair cortisol concentrations because of blood flow (and ACTH) to these regions. Conversely, if there was a lack of consistency in cortisol concentrations across hair taken from body sites, then it could be concluded that the central and peripheral HPA axes were independent. Moreover, such a lack of significant similarity between the cortisol concentrations in hair from various body sites would argue for a largely independent HPA axis in each hair follicle, as suggested by Ito and colleagues18 or at least independent regions of cortisol-producing hair across the body surface.
Results of investigations into these two issues have the potential to advance knowledge and understanding of the nature and process of the peripheral HPA in general, and hair cortisol in particular, and may contribute to a more complete understanding of the overall contribution which the peripheral HPA axis makes to homeostasis and anti-inflammatory processes. Therefore, the present study was designed to investigate these two aspects of hair cortisol as a step in “mapping” hair cortisol responses in greater detail. First, in order to determine the relative concentration (in resting subjects) of cortisol along the hair shaft, long hair was measured at its base, end and the central section between these two extremes in the same way as has previously been done when investigating sex steroid concentrations along the hair shaft.23 Second, to determine if hair cortisol concentrations varied across body sites when subjects were at rest (vs. in the immersed hand which underwent a pain stressor compared to a non-immersed opposite lower leg),16 hair was collected from body extremities (lower legs, forearms and scalp) and tested for cortisol concentrations.
Methods
Participants
Hair shaft concentrations
Twelve healthy young female volunteers (ages ranged from 19 to 26 yr, M = 21.7 yr, SD = 2.3 yr) each had one hair sample of about 100 strands cut from the posterior vertex. Shaft samples varied in length across individuals from 180 mm to 550 mm (M = 326 mm, SD = 113.9). After cutting, each hair sample was taped with sticky paper tape at the base end for identification.
Body site concentrations
Ten healthy young males (age range = 18 to 40 years, M = 24.3, SD = 7.3 yr) volunteered to have hair cut from their posterior scalp vertex and shaved from their lower forearms and lower legs. Samples consisted of about 100 strands each.
Sample collection and Assay
Hair was collected by cutting with scissors (scalp) or shaving with disposable razors (arms and legs) and then placed in a labeled paper envelope. Scissors were washed in methanol between samples and razors were stored in the paper envelopes with the sample of hair they were used to collect. After being emptied from the envelopes and placed in separate glass vials (20 ml), hair was weighed and then chopped with scissors (washed with methanol between chopping samples) before being extracted with 3 ml of methanol for 24 hours. The methanol was then decanted into polypropylene tubes (3.5 mL) and evaporated under vacuum. Gel buffer (100 μL) (phosphate buffered saline, pH 7.5 containing 0.1% gelatin) were added and allowed to stand at room temperature for 60 minutes prior to assay. Cortisol concentrations were determined by radioimmunoassay as previously described.27 Sensitivity of that assay process was 1 ng/mL, well above the observed concentration in hair found here. From previous data,9,16 washing of hair has not been found necessary as concentrations are not significantly different in washed vs. unwashed hair. In addition, although sebum and sweat may contain cortisol, the previous data reporting a lack of significant differences in washed/unwashed hair cortisol concentrations argue that these extra potential sources of cortisol may be effectively discounted in these data.
Procedure
For the hair shaft and body site concentration aspects of this study, participants were all treated individually. After greeting and signing of appropriate consent forms, each participant sat quietly while hair samples were collected. Participants were then thanked for taking part in the experiment. All samples were collected within a 20-min period on one afternoon between the hours of 2.00 pm and 2.20 pm. All procedures were approved by the University of New England Human Experimentation Ethics Committee.
Results
Hair shaft concentrations
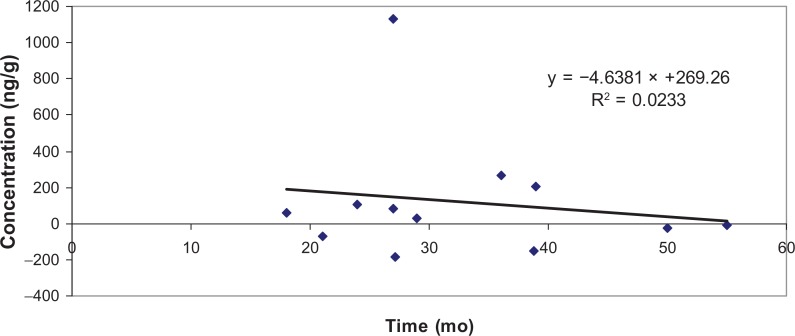
Table 1 shows the means and SE for sample weights and cortisol concentrations (ng/g) across the three hair shaft sites. From Table 1, it is apparent that there was some variability in cortisol among the hair shaft sites, with average concentrations becoming greater from the base to the end of the shaft. However, while ANOVA on the ng/g cortisol concentration data showed a significant difference between subjects for the raw ng/g data (F(11, 35) = 5.17, p < 0.001), there was no significant difference in cortisol concentrations across base, middle and end of shaft (F(2, 35) = 1.33, ns). Examination of the difference between individual Ss’ cortisol concentrations from the base to the end of hair shaft (M = 117.5 ng/g) showed that this difference was not significant (F(1, 23) = 0.025, ns). Moreover, when the hypothesis that cortisol concentrations at the end of the shaft would be “washed out” by time24 was tested by comparing change in cortisol concentration from base (i.e. the most recently grown hair) to end of shaft (the oldest hair) with the length of hair shaft from individual Ss, there was no significant correlation between length of shaft (and thus time the hair strand had been growing) and change in cortisol concentration (r = 0.153, ns), thus rejecting that hypothesis, and representing a finding that was congruent with data previously reported from monkeys9 but not female humans.24 By applying the rule of thumb that hair grows about 10 mm/month, the effect of time of growth on difference in cortisol concentrations between the end and the base of the shaft may be seen in Figure 1.
Table 1.
Mean (SE) weight and ng/g cortisol concentrations across the three hair shaft sites.
| Site | Weight (g) | ng/g |
|---|---|---|
| Base | 0.0719 (0.0173) | 312.6667 (83.0793) |
| Middle | 0.0516 (0.0137) | 332.8333 (54.4473) |
| End | 0.0437 (0.0098) | 430.4167 (106.9753) |
Figure 1.
Base-end cortisol concentration differences by time of growth.
In a final test of the suggestion that cortisol would show a “continuously decline” after three months,24 examination of individual Ss’ hair shaft cortisol concentrations indicated that, of the 12 females sampled, five had their highest concentration at the end of the shaft, with hair shaft lengths of between 270 mm and 390 mm (i.e. 27 to 39 mo).
Body site concentrations
Table 2 presents the means and SE for sample weights and ng/g cortisol concentrations across the five body sites. There was a significant difference between subjects’ hair cortisol concentrations (F(9, 35) = 2.35, p < 0.05) but only a non-significant trend among body sites (F(4, 35) = 2.31, p = 0.07). Because of the exploratory nature of this study, further examination of the hair cortisol concentration differences across body sites was undertaken. Table 2 indicates that the highest cortisol concentrations were observed in the arms, and the lowest in the legs, with the scalp values falling between these two, and this was verified by statistical testing: arms (M = 1057.323 ng/g, SE = 87.73), legs (M = 644.900 ng/g, SE = 85.51) (F(1, 38) = 11.324, p < 0.005). There was no significant difference between left side limbs (M = 789.47 ng/g, SE = 99.42) and right side limbs (M = 899.20 ng/g, SE = 96.91).
Table 2.
Mean (SE) weight and ng/g cortisol concentrations across the five body sites.
| Site | Weight (g) | ng/g |
|---|---|---|
| Left arm | 0.0045 (0.0087) | 1038.5600 (146.6205) |
| Right arm | 0.0048 (0.0009) | 1073.9000 (144.7293) |
| Left leg | 0.0065 (0.0009) | 565.3000 (72.7997) |
| Right leg | 0.0066 (0.0010) | 724.5000 (123.2836) |
| Scalp | 0.0109 (0.0032) | 838.0000 (249.0307) |
Discussion
These data on hair shaft cortisol concentrations agree with one previous study on monkeys9 but not with another on young women24 in failing to find any significant differences in hair cortisol concentrations along the length of hair shafts that were also collected from young women. However, in the study which did report differences in concentration along the hair shaft, those authors commented that cortisol significantly declined in hair samples after three months for “unknown” reasons. By contrast, hair collected in the present study showed no “wash out” in cortisol concentrations, even after periods of more than three years. The lack of statistically significant variability in hair shaft site concentrations of cortisol found here argues for a “live” hair shaft interpretation, at least in terms of cortisol being moved along the shaft so that sites up to 500 mm apart (i.e. 50 months of growth) showed similar concentrations of cortisol. These data are congruent with those previously reported which showed immediate and transient variability in hair cortisol concentrations in body sites which had received a pain stressor16 because they confirm the active response nature of the peripheral hair HPA axis previously identified via in vitro studies.18
The second part of this study presents the first data on cortisol concentrations in hair collected from extremes of the body while the S was at rest. The lack of a significant difference across all body sites appears to argue for the presence of a “centrally-mediated” overall hair cortisol-production system while the S was not under major stressor demand. However, that difference was trending towards statistical significance at traditional levels and thus leaves this issue open to further investigation, particularly in view of our previous finding that arm and leg hair cortisol concentrations were clearly independent when the arm underwent major pain stress.16 The significant difference found in this study between leg and arm hair cortisol concentrations provides further support for the “disentanglement” hypothesis that all hair follicles are independent producers of the CRH-ACTH-cortisol cascade, as demonstrated by Ito and colleagues.18
Together, these two sets of data suggest that the production of cortisol in hair is a dynamic rather than a static process, and that various body sites (i.e. arms vs. legs) may respond more powerfully to environmental demand, particularly when they are subject to localized stress.16 As mentioned in the introductory section of this paper, cortisol has anti-inflammatory effects,4 and the function of localized hair cortisol responsivity may be related to a wide range of anti-inflammatory actions for specific skin puncture and localized pathogenic infection.28 The selective advantage of an independent peripheral HPA axis may be argued, both because of the immediacy of cortisol response to stress at a particular limb site16 rather than the usual delay of about eight minutes for cortisol to appear in the bloodstream,4 and also because of the economic use of this powerful hormonal response for localized infection. That is, as well as being a valuable anti-inflammatory agent, cortisol can also have significant deleterious effects upon the organism if serum levels are consistently elevated, including atherosclerosis,29 bowel disease,4 mood disorders, neurodegenerative diseases, diabetes and cancer.30 Thus, frequent central HPA responses to minor peripheral injuries might produce harmful overall circulating levels of cortisol, whereas more locally-focused hair follicle HPA axis release of cortisol into the specific area of injury, with no clear avenue for this cortisol to enter the bloodstream and contribute to elevated serum (and whole body) levels, could provide an adaptive advantage to the organism. One potential hypothesis that is consistent with the findings presented herein is that hair cortisol is a product of both the central HPA axis (to provide a “background” or resting level of cortisol) and also of the local follicle production when the immediate site if under stress. Further investigation of the relationship between central and peripheral HPA axes is required to clarify this issue.
Limitations of this study include those associated with generalization of findings. Because of hair fashion, males’ hair was not of sufficient length to provide comparisons of cortisol concentration along their hair shafts. Similarly, females did not have sufficient leg and arm hair to allow reliable assay procedures to be applied to hair from these areas. Thus, the issue of gender-specific effects remains unaddressed. Similarly, although there was a reasonable distribution in ages of participants, no old or very young participants were recruited. All participants were volunteers and, while this should not present any obvious limitations upon generalisability of hair cortisol data, it does reflect a limitation in sampling. Finally, cortisol may be reverse activated from cortisone by 11betaHSD. Although the in vitro data reported by Ito and colleagues argue strongly against this being a potential confounding source of cortisol in this study, future research could address this issue more conclusively.
As mentioned above, future research should address the presence of any central HPA-to-peripheral HPA axis linkage. While this has been investigated in a pilot study for Ss under pain stress to a specific body region,16 the collection of data from central and peripheral cortisol production while participants were undertaking normal daily activities would assist in clarifying this relationship. A diurnal variation in central HPA axis activity has previously been demonstrated4 but no data have yet been reported from repeated collections of hair over a 24-hour period to test the extent of the central HPA-peripheral HPA axis link and disentanglement hypothesis suggested above.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors report no conflicts of interest.
References
- 1.Aron DC, Findling JW, Tyrrell JB. Glucocorticoids and adrenal androgens. In: Greenspan FS, Gardner D, editors. Basic and Clinical Endocrinology. 8th Ed. G Lange Medical Books/McGraw-Hill; New York: 2007. pp. 346–395. [Google Scholar]
- 2.Klaitman V, Almog Y. Corticosteroids in sepsis: A new concept for an old drug. Israeli Med Ass Jour. 2003;5:51–5. [PubMed] [Google Scholar]
- 3.Weissmann G, Thomas L. Studies on lysosomes: II. The effect of cortisone on the release of acid hydrolases from a large granular fraction of rabbit liver induced by an excess of vitamin A. J Clin Inv. 1963;42:661–9. doi: 10.1172/JCI104757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyton AC, Hall JE. Textbook of Medical Physiology. 11th ed. Elsevier; Philadelphia, Penn: 2006. [Google Scholar]
- 5.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 6.Sharpley CF, McLean S. Use of salivary cortisol as an indicator of biobehavioural reactivity to a brief psychological task. Scan J Behav Ther. 1992;21:35–45. [Google Scholar]
- 7.Umeda T, Hiramatsu R, Iwaoka T, Shimada T, Miura F, Sato T. Use of saliva for monitoring unbound free cortisol levels in serum. Clin Chem Acta. 1981;110:245–53. doi: 10.1016/0009-8981(81)90353-3. [DOI] [PubMed] [Google Scholar]
- 8.Arck PC, Slominski A, Theoharides TC, Peters EMJ, Paus R. Neuroimmunology of Stress: Skin takes center stage. J Invest Dermatol. 2006;126:1697–704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 2006;147:255–61. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Klein J, Karaskov T, Stevens B, Yamada J, Koren G. Hair cortisol-a potential biological marker for chronic stress. Clin Pharm Therap. 2004;75:44. [Google Scholar]
- 11.Koren L, Mokady O, Karaskov T, Klein J, Koren G, Geffen E. A novel method using hair for determining hormonal levels in wildlife. Animal Behav. 2002;63:403–6. [Google Scholar]
- 12.Raul JS, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 2004;37:1105–11. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Sauve B, Koren G, Walsh G, Tokmakejian S, Van Uum AHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 30:E183–91. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 14.Van Uum SSH, Sauve B, Fraser LA, Morley-Forster P, Paul TL, Kpren G. Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress. Stress. 18 doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- 15.Yamada J, Stevens B, De Silva N, et al. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatol. 2007;92:42–49. doi: 10.1159/000100085. [DOI] [PubMed] [Google Scholar]
- 16.Sharpley CF, Kauter KG, McFarlane JR. An initial exploration of in vivo hair cortisol responses to a brief pain stressor: Latency, localisation and independence effects. Physiol Res. doi: 10.33549/physiolres.931544. In press. [DOI] [PubMed] [Google Scholar]
- 17.Slominski A, Zbytek B, Szczesniewski A, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–6. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 18.Ito N, Ito T, Kromminga A, Betterman A, Takigawa M, Keesd F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB Jour. 2005;19:1332–4. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 19.Paus R, Theoharides TC, Arck P. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immuno. 2006;27:32–9. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Kalra S, Klein J, Karaskov T, Woodland C, Einarson A, Koren G. Use of hair cortisol as a biomarker of chronic stress in pregnancy. Clin Pharm Therap. 2005;77:69. [Google Scholar]
- 21.Harkey MR. Anatomy and physiology of hair. For Sci Inter. 1993;63:9–18. doi: 10.1016/0379-0738(93)90255-9. [DOI] [PubMed] [Google Scholar]
- 22.Cirimele V, Kinyz P, Dumestre V, Goulle JP, Ludes B. Identification of ten corticosteroids in human hair by liquid chromatography-ionspray mass spectrometry. For Sci Int. 2000;107:381–8. doi: 10.1016/s0379-0738(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang HZ, Lan J, Meng YJ, Wan XJ, Han DW. A preliminary study of steroid reproductive hormones in human hair. J Steroid Biochem Molec Biol. 1998;67:447–50. doi: 10.1016/s0960-0760(98)00120-4. [DOI] [PubMed] [Google Scholar]
- 24.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production—Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroend. 2009;34:32–7. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Erickson K, Thorsen P, Chrouson G, Grigoriadis DE, Khongsaly O, McGregor J, et al. Preterm birth: Associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol. 2001;86:2544–52. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- 26.Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental cortisotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Yuen BSJ, Owens PC, Symonds ME, et al. Effects of Leptin on fetal plasma Adrenocorticotropic Hormone and Cortisol concentrations and the timing of parturition in the sheep. Biol Reprod. 2004;70:1650–1657. doi: 10.1095/biolreprod.103.025254. [DOI] [PubMed] [Google Scholar]
- 28.Klaitman V, Almog Y. Corticosteroids in sepsis: A new concept for an old drug. Israeli Med Assoc J. 2003;5:51–55. [PubMed] [Google Scholar]
- 29.Silverthorn DU. Human Physiology. 4th ed. San Francisco: Pearson; 2007. [Google Scholar]
- 30.Jope RS, Yuskaitis CJ, Beurel E. Gycogen synthase kinase-3 (GSK3): Inflammation, diseases, and therapeutics. Neuroche Res. 2007;32:577–95. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]