Abstract
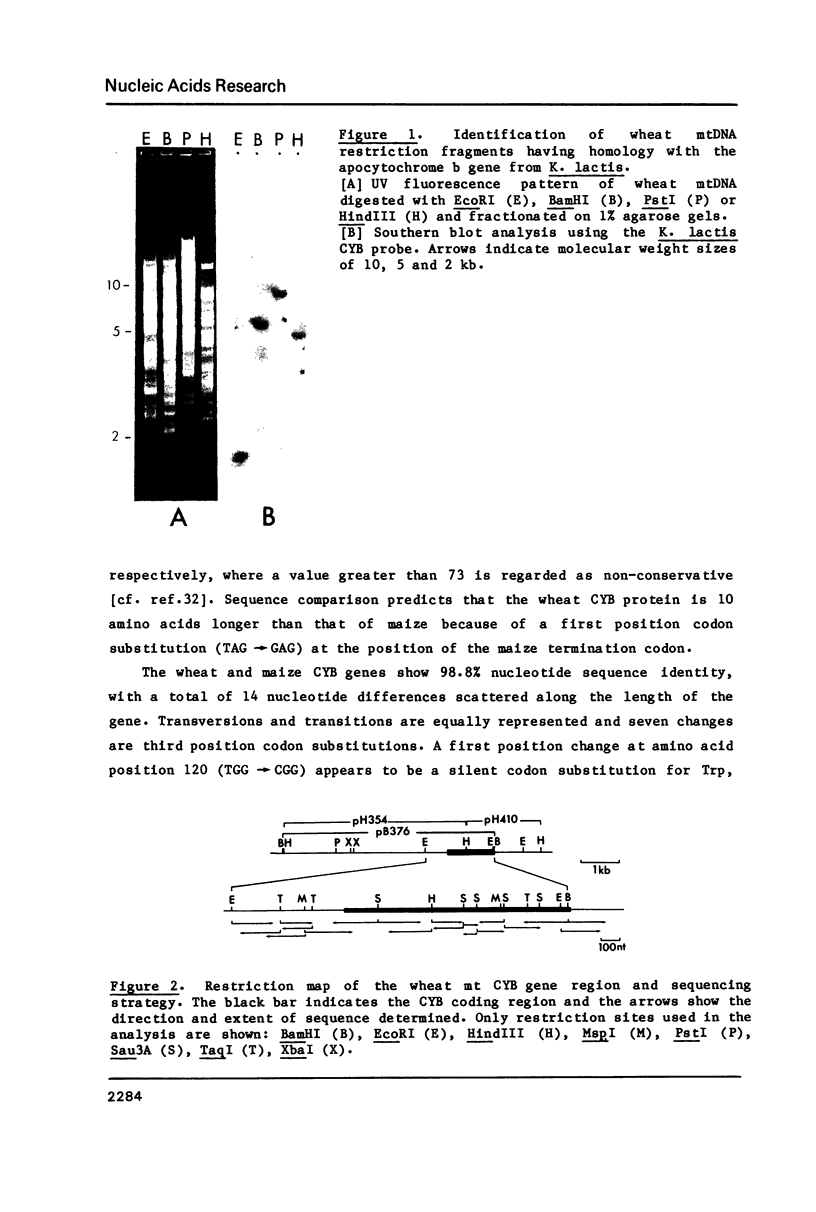
The wheat mitochondrial gene for apocytochrome b (CYB) has been identified by its hybridization to a yeast CYB probe and its nucleotide sequence has been determined. The wheat CYB sequence predicts a cytochrome b apoprotein of 398 amino acids; it is almost identical to that of maize but has ten additional amino acids at the carboxy terminus. No introns are present in the wheat CYB gene, but an internal segment of the gene is repeated at another genomic location. Transcript analysis reveals a single wheat CYB mRNA of approximately 2.4 kb with a long untranslated leader. Sequences upstream of the CYB coding region are very similar in wheat and maize but the stretch proposed to be a ribosome binding site in maize is not conserved in wheat. The corresponding leader regions of the wheat mitochondrial mRNAs for cytochrome oxidase subunits I and II also lack complementarity to the 3'-end of the small subunit rRNA. We conclude that alternative signals are involved in the initiation of translation in plant mitochondria.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bonen L., Boer P. H., Gray M. W. The wheat cytochrome oxidase subunit II gene has an intron insert and three radical amino acid changes relative to maize. EMBO J. 1984 Nov;3(11):2531–2536. doi: 10.1002/j.1460-2075.1984.tb02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Gray M. W. Organization and expression of the mitochondrial genome of plants I. The genes for wheat mitochondrial ribosomal and transfer RNA: evidence for an unusual arrangement. Nucleic Acids Res. 1980 Jan 25;8(2):319–335. doi: 10.1093/nar/8.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. G., Simpson M. V. Novel features of animal mtDNA evolution as shown by sequences of two rat cytochrome oxidase subunit II genes. Proc Natl Acad Sci U S A. 1982 May;79(10):3246–3250. doi: 10.1073/pnas.79.10.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Brown W. M., Wilson A. C. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984 Mar;106(3):479–499. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao S., Sederoff R. R., Levings C. S. Partial Sequence Analysis of the 5S to 18S rRNA Gene Region of the Maize Mitochondrial Genome. Plant Physiol. 1983 Jan;71(1):190–193. doi: 10.1104/pp.71.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao S., Sederoff R., Levings C. S., 3rd Nucleotide sequence and evolution of the 18S ribosomal RNA gene in maize mitochondria. Nucleic Acids Res. 1984 Aug 24;12(16):6629–6644. doi: 10.1093/nar/12.16.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., Mendu N., Ginsburg H., Kridl J. C. Sequence analysis of the maize mitochondrial 26 S rRNA gene and flanking regions. Plasmid. 1984 Mar;11(2):141–150. doi: 10.1016/0147-619x(84)90019-2. [DOI] [PubMed] [Google Scholar]
- Dawson A. J., Jones V. P., Leaver C. J. The apocytochrome b gene in maize mitochondria does not contain introns and is preceded by a potential ribosome binding site. EMBO J. 1984 Sep;3(9):2107–2113. doi: 10.1002/j.1460-2075.1984.tb02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Leaver C. J. The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell. 1981 Nov;26(3 Pt 1):315–323. doi: 10.1016/0092-8674(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Grantham R. Amino acid difference formula to help explain protein evolution. Science. 1974 Sep 6;185(4154):862–864. doi: 10.1126/science.185.4154.862. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Sankoff D., Cedergren R. J. On the evolutionary descent of organisms and organelles: a global phylogeny based on a highly conserved structural core in small subunit ribosomal RNA. Nucleic Acids Res. 1984 Jul 25;12(14):5837–5852. doi: 10.1093/nar/12.14.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesel R., Brennicke A. Cytochrome oxidase subunit II gene in mitochondria of Oenothera has no intron. EMBO J. 1983;2(12):2173–2178. doi: 10.1002/j.1460-2075.1983.tb01719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kao T., Moon E., Wu R. Cytochrome oxidase subunit II gene of rice has an insertion sequence within the intron. Nucleic Acids Res. 1984 Oct 11;12(19):7305–7315. doi: 10.1093/nar/12.19.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K., Kobayashi M., Yaginuma K., Taira M., Yoshida E., Imai M. Nucleotide sequence and evolution of the rat mitochondrial cytochrome b gene containing the ochre termination codon. Gene. 1982 Dec;20(2):177–185. doi: 10.1016/0378-1119(82)90036-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981 Apr 9;290(5806):470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- Rambach A., Hogness D. S. Translation of Drosophila melanogaster sequences in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5041–5045. doi: 10.1073/pnas.74.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M. Location of haem-binding sites in the mitochondrial cytochrome b. FEBS Lett. 1984 Jan 30;166(2):367–372. doi: 10.1016/0014-5793(84)80114-3. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Gray M. W. 3'-Terminal sequence of wheat mitochondrial 18S ribosomal RNA: further evidence of a eubacterial evolutionary origin. Nucleic Acids Res. 1982 Jul 10;10(13):3921–3932. doi: 10.1093/nar/10.13.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederoff R. R., Levings C. S., Timothy D. H., Hu W. W. Evolution of DNA sequence organization in mitochondrial genomes of Zea. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5953–5957. doi: 10.1073/pnas.78.10.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. F., Bonen L., Gray M. W. Primary sequence of wheat mitochondrial 5S ribosomal ribonucleic acid: functional and evolutionary implications. Biochemistry. 1981 Jul 7;20(14):4022–4029. doi: 10.1021/bi00517a011. [DOI] [PubMed] [Google Scholar]
- Spencer D. F., Schnare M. N., Gray M. W. Pronounced structural similarities between the small subunit ribosomal RNA genes of wheat mitochondria and Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jan;81(2):493–497. doi: 10.1073/pnas.81.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Ward B. L., Anderson R. S., Bendich A. J. The mitochondrial genome is large and variable in a family of plants (cucurbitaceae). Cell. 1981 Sep;25(3):793–803. doi: 10.1016/0092-8674(81)90187-2. [DOI] [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]