Abstract
How do effector CD4 T cells escape cell death during the contraction of the immune response (IR) remain largely unknown. CD47, through interactions with thrombospondin-1 (TSP-1) and SIRP-α, is implicated in cell death and phagocytosis of malignant cells. Here, we reported a reduction in SIRP-α-Fc binding to effector memory T cells (TEM) and in vitro TCR-activated human CD4 T cells that was linked to TSP-1/CD47-induced cell death. The reduced SIRP-α-Fc binding (CD47low status) was not detected when CD4 T cells were stained with two anti-CD47 mAbs, which recognize distinct epitopes. In contrast, increased SIRP-α-Fc binding (CD47high status) marked central memory T cells (TCM) as well as activated CD4 T cells exposed to IL-2, and correlated with resistance to TSP-1/CD47-mediated killing. Auto-aggressive CD4 effectors, which accumulated in lymph nodes and at mucosal sites of patients with Crohn’s disease, displayed a CD47high status despite a high level of TSP-1 release in colonic tissues. In mice, CD47 (CD47low status) was required on antigen (Ag)-specific CD4 effectors for the contraction of the IR in vivo, as significantly lower numbers of Ag-specific CD47+/+CD4 T cells were recovered when compared to Ag-specific CD47−/− CD4 T cells. In conclusion, we demonstrate that a transient change in the status of CD47, i.e. from CD47high to CD47low, on CD4 effectors regulates the decision-making process that leads to CD47-mediated cell death and contraction of the IR while maintenance of a CD47high status on tissue-destructive CD4 effectors prevents the resolution of the inflammatory response.
Introduction
During an adaptive immune response (IR), naive T cells responding to Ag proliferate vigorously. While the majority of activated T cells will be killed and eliminated (the contraction phase), effector T cells that have passed this checkpoint will survive and execute their memory T cell differentiation program to generate long-lived memory T cells. Central questions are to determine which cells among proliferating effector T cells will live or die, which cells will be cleared or not, and which factors will dictate these crucial decisions [1]. Although re-expression of IL-7R is a determinant for the survival of effectors that will generate memory T cells [2], [3], no surface molecule has been implicated in the control of cell death and elimination during the contraction phase of the IR. Neither differential Fas expression, nor Fas-induced cell death susceptibility can explain why some effectors die during an acute immune response [4], [5].
CD47, known as integrin-associated protein (IAP), contains a single IgV-like extracellular domain, a multiple membrane-spanning domain (MMS) and a short intracytoplasmic tail, which is devoid of signaling motifs [6]. CD47, considered as a marker of self, is expressed on hematopoietic and non- hematopoietic cells and regulates two key functions implicated in the IR: cell death and cell elimination [7]. CD47 interacts in cis with integrins and in trans with two ligands, thrombospondin-1 (TSP-1) and signal regulatory protein alpha (SIRP-α). TSP-1 binds two distinct regions on the CD47 IgV loop while it competes with SIRP-α (D1 distal domain) for one of the two CD47 binding sites [8], [9]. SIRP-α/CD47 interaction controls immune cell elimination. CD47 delivers a negative signal through SIRP-α expressed on resident macrophages or dendritic cells (DCs) to inhibit the clearance of intact hematopoietic cells [10]. In this regard, CD47 expression must be transiently up-regulated on circulating wild type hematopoietic stem cells to spare them from clearance during bone marrow exit [11]. TSP-1/CD47 interaction induces the caspase-independent cell death of malignant B and T lymphocytes [7], [12], [13]. TSP-1 is mainly secreted by antigen presenting cells (APCs) and facilitates the clearance of damaged apoptotic cells by APCs [14]. In addition, increased TSP-1 binding facilitates the elimination of aged erythrocytes by SIRP-α+ macrophages [15]. We recently reported that CD47 status (SIRP-α Fc binding) is transiently regulated on murine CD4 T cells following in vivo immunization. More precisely, CD47high status marked central memory T (TCM) CD4 precursors at an early time point of the IR, while CD47low status identified activated CD4 T cells [16].
In the present study, we demonstrated that CD47 expression and more particularly CD47low status on murine activated CD4 T cells, is key for the contraction phase of the IR in vivo. In addition, we showed that TCR activation induced a transient change in the CD47 status on human CD4 T cells, i.e. from CD47high to CD47low to CD47high, which was linked to TSP-1/CD47-mediated cell death in vitro. Importantly, CD47low status was maintained on CD4 effectors cells in inflamed lymphoid and mucosal tissues of patients with Crohn’s disease (CD). We thus propose that CD47/SIRP-α/TSP-1 axis is involved in the resolution of the inflammatory response.
Results
1. CD47 Status is Differentially Regulated on TCR- Activated Human CD4 T Cell Subsets
We first investigated the modulation of CD47 expression on in vitro activated human CD4 T cell subsets. To this end, we thought to use a SIRP-α-Fc fusion protein and two anti-CD47 monoclonal antibodies (mAbs) that identify different CD47 conformations [15], [17], [18], [19], [20] and/or distinct CD47 epitopes [21]. Hence, B6H12 mAb and SIRP-α-Fc compete for a similar CD47 binding site since B6H12 but not 2D3 inhibits SIRP-α-Fc binding to CD47 [22]. We showed that CD47 expression, as detected by SIRP-α-Fc binding, decreased on a majority of divided naïve CD4 T cells (TN; CD45RA+CCR7+) following stimulation with anti-CD3 and anti-CD28 mAbs (Fig. 1A). The reduced CD47 expression was not observed when activated CD4 T cells were stained with B6H12 anti-CD47 mAb. Thus, decreased SIRP-α-Fc binding to CD47 on activated TN cells was hereafter referred to as CD47low status when compared to SIRP-α-Fc binding to CD47 on undivided TN cells as well as on 50% of activated central memory (TCM; CD45RA-CCR7+CD27+) T cells hereafter referred to as CD47high status (Fig. 1A). Divided CD47low CD4 T cells displayed an effector phenotype (CCR7low) when compared to undivided CD47high CD4 T cells (Fig. 1B).
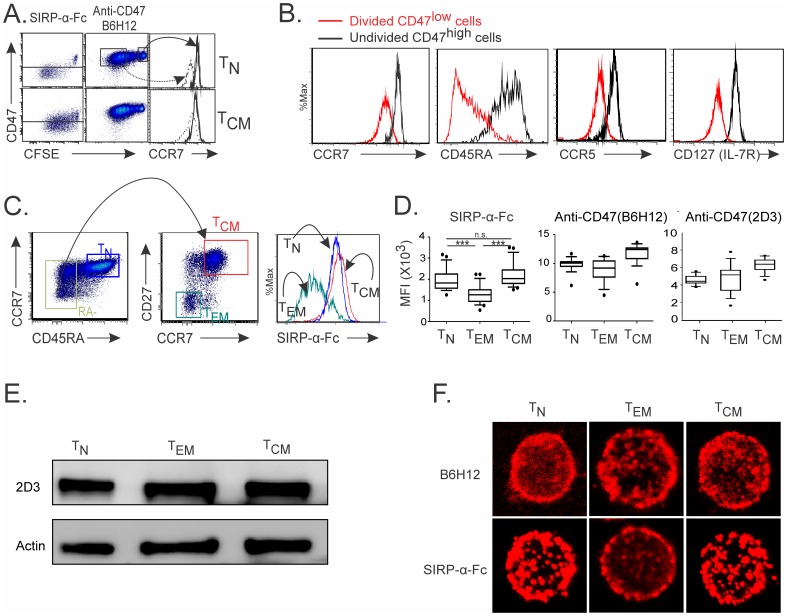
Figure 1. CD47 status is differentially regulated on TCR- activated human CD4 T cell subsets.
(A–B) CFSE-labeled TN and TCM cells were stimulated with immobilized anti-CD3 and soluble anti-CD28 mAbs for 6 days. (A) CD47 (using human SIRP-α-Fc protein or anti-CD47 mAb, clone B6H12) and CCR7 expression was analyzed by flow cytometry. (B) Phenotype of divided CD47low and undivided CD47high cells at day 6 of TN cultures. (C) Strategies to examine CD47 expression on ex vivo isolated human T cells gated on CD4+ T cells. (D) CD47 expression on CD4 T cell subsets using SIRP-α-Fc and anti-CD47 antibodies (B6H12 and 2D3). The mean ± standard deviation (SD) for 16 donors is shown (Anova test: ***p<0.0001). (E) Western blot analysis for CD47 protein on whole-cell lysates using 2D3 mAb. (F) Confocal immunofluorescence of CD47 using SIRP-α-Fc or anti-CD47 (B6H12) antibodies. (A–C; E and F) Data are representative of 3 to 6 independent experiments.
Further studies demonstrated that CD47 status was differentially modulated in ex vivo isolated circulating human CD4 T cell subsets (Fig. 1C). Effector memory (TEM; CD45RA−CCR7−CD27−) T cells, which represent chronically activated T cells by repeated exposure to Ag in the peripheral blood of healthy individuals, displayed a CD47low status when compared to CD47high TN and TCM T cells (Fig. 1D). Transitional memory (TTM, CD45RA−CCR7−CD27+) and terminally differentiated (TTD, CD45RA+CCR7−CD27−) cells were detected as CD47low cells. Alike in vitro TCR-activated CD4 T cells, TN, TEM and TCM expressed similar levels of CD47 expression when they were stained with B6H12 and 2D3 anti-CD47 mAbs, suggesting a change in the conformation rather than in the amount of CD47 protein. Indeed, western blot analysis showed that the three circulating CD4 T cells subsets possessed similar CD47 protein content (Fig. 1E). We next investigated whether differences seen in SIRP-α- Fc binding to CD47 may reflect a differential distribution of CD47 on the cell surface of TN, TEM and TCM. As depicted by confocal microscopy, SIRP-α- Fc staining revealed a homogenous distribution of CD47 molecules on the cell surface of TN and TCM while a distinct and patchy redistribution was observed on TEM (Fig. 1F). In contrast, no characteristic CD47 distribution was found between TN, TEM and TCM using B6H12 mAb. Additionally, SIRP-α-Fc failed to bind CD47 with a truncated transmembrane domain in a modified human T cell line (Fig. S1). We propose that TCR activation elicits a post transcriptional/translational modification of the CD47 molecule that dictates its ability to bind SIRP-α-Fc but not B6H12 or 2D3 mAbs. These data strongly suggested that CD47 status on TEM and TCM subsets reflect different CD47 protein conformations, as these T cells possessed similar protein content.
2. IL-2 Induces a CD47high Status on Human TCR-activated CD47low CD4 T Cells
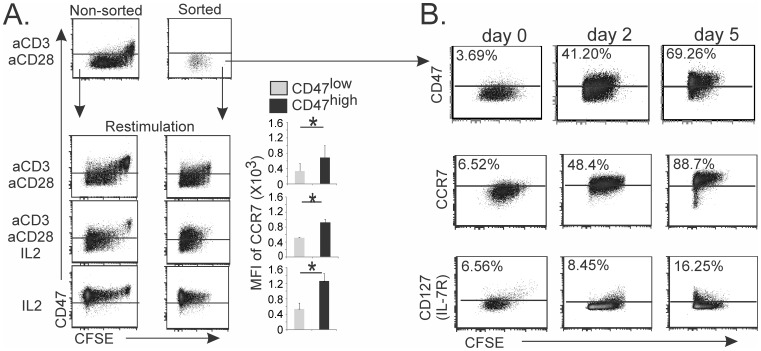
Several cytokines that signal through receptors sharing the common γ chain (γc) are critical for peripheral homeostasis and the generation of memory T cells [23], [24]. We found that a large proportion of TCR-activated naive CD4 T cells regained a CD47high status when these cells were cultured in the presence of IL-2, with or without CD3 restimulation and co-stimulation (Fig. 2A). This suggested that CFSElowCD47high activated TCM cells arose from CD47low T cells. To rule out the possibility that CD47high T cells originated from a few CD47high T cells, which had proliferated and never modified their CD47 status, CD47low CD4 T cells were purified at the end of T cell primary cultures (day 6) and then were re-stimulated. We demonstrated that, indeed, IL-2 induced the re-establishment of a CD47high and central memory (CCR7high) phenotype in FACS sorted purified TCR-activated CD47low CD4 T cells (Fig. 2B). The appearance of CD47high effectors preceded that of CD127 positive cells (Fig. 2C). Thus, human CD4 T cells transiently modulate their CD47 status in response to polyclonal activation, and TCM phenotype is associated with the reacquisition of a CD47high status.
Figure 2. IL-2 induces CD47high status on TCR-activated human CD47low CD4 T cells.
(A) CFSE-labeled TN cells (left panels) were activated with immobilized anti-CD3 and soluble anti-CD28 mAbs for 6 days. Unfractionated activated T cells or FACS sorted CD47low T cells (middle panels) were restimulated for 5 days as indicated. CD47 expression in relation to cell division (CFSE cell dilution) and CCR7 expression in relation to CD47 status. (B) TN were activated as in A and FACS sorted CD47low (day 6) T cells were restimulated in presence of IL-2. Kinetics of CD47 (SIRP-α-Fc protein), CCR7 and CD127 expression is shown. (A–B) Data are representative of at least 4 to 6 independent experiments. Right panel A shows the mean ± standard deviation (SD) for 5 independent experiments. Student t test: *p<0.05.
3. CD47low Status is Linked to TSP-1-induced Cell Death Susceptibility
We next explored whether the transient modulation of CD47 status seen on CD4 T cells might be linked to functional consequences such as T cell death, which occurs during the resolution of the IR. Ligation of CD47 by 4N1K, a peptide that corresponds to the CD47-binding C-terminal domain of TSP-1, kills malignant B cells and T cell lines through a caspase and Fas-independent pathway [13], [25], [26], [27]. We therefore assessed the TSP-1/CD47-mediated cell death of human CD4 T cells in relation to their CD47low or CD47high status. TCR-activated CD47low T cells were susceptible to 4N1K-induced cell death (Fig. 3A). However, they became resistant when they were cultured in the presence of IL-2 and reacquired a CD47high status, linking a change in the CD47 status to susceptibility to TSP-induced cell death. Specific CD47-mediated killing was demonstrated in TEM, while TN and a large fraction of TCM were largely protected from 4N1K-induced cell death (Fig. 3B), corroborating our in vitro findings with TCR-activated T cells. We next asked whether differential CD47 status on CD4 T cell subsets correlated with the switching on of one common “eat me” signal, i.e. calreticulin, which favors cell elimination when CD47/SIRP-α interactions are interrupted [28]. Expression of calreticulin was not detected on viable TN, TEM, or TCM, although it was significantly induced on TEM killed by 4N1K (Fig. 3C). We propose that killing of CD47low T cells occurs upstream of cell clearance, with the latter being mediated by up-regulation of “eat me” signals combined with the interruption of SIRP-α/CD47 interactions.
Figure 3. A CD47low status is linked to TSP-1-induced cell death susceptibility.
(A–B) Specific CD47-mediated killing was performed using 4N1K (TSP-1) or 4NGG (control) peptide on in vitro restimulated FACS sorted activated CD47low T cells as in Figure 2 (A) and ex vivo isolated CD4 T cell subsets (B). (A–B) The mean ± standard deviation (SD) for 5 independent experiments. Student t test: *p<0.05, ***p<0.0001. (C) Calreticulin expression is shown after specific CD47-mediated killing as in B. Data are representative of 4 independent experiments.
4. Chronically Activated T Cells Display a CD47high Status in Lymphoid and Intestinal Tissues of Patients with Crohn’s Disease
Survival of auto-aggressive T cells in tissues prevents the resolution of the inflammatory response and perpetuates disease in patients with inflammatory bowel disease (IBD) [29]. More specifically, lamina propria T cells appear resistant to cell death in Crohn’s disease (CD). We therefore asked whether escape to cell death of mucosal CD4 T cells correlated with their CD47 status in CD patients. To this end, we examined binding of SIRP-α-Fc to CD47 on CD4 T cells in blood, mesenteric lymph nodes (mLNs) and intestinal lamina propria mononuclear cells (LPMC) of CD patients. As expected, the frequency of CD4 effectors (CD45RA−CD27+/−CCR7−) was increased in mLNs and LPMC when compared to PBMC, whereas that of TCM (CD45RA−CD27+CCR7+) was reduced accordingly (Fig. 4A). Despite abundant TSP-1 release in inflamed colonic CD tissues (Fig. 4B), both CCR7+ and CCR7− CD4 T cell subsets that infiltrated mLNs and inflamed gut tissues expressed a CD47high status (Fig. 4C) that could explain the maintenance of auto-aggressive T cells. However, as in healthy donors (Fig. 1), circulating TEM and TCM displayed a CD47low and CD47high status, respectively in patients with CD or an unrelated intestinal disorder (non IBD)(Fig. 4). To verify that absence of differential CD47 status on CCR7+ and CCR7− memory T cells was not a property of T cells that were recruited to peripheral tissues, we also examined mLNs and LPMC of patients with non IBD. As depicted in the same figure, CCR7− effectors displayed a CD47low status in mLNs and colons of non IBD donors as reflected by the ratio of CD47 mean fluorescence intensity (MFI) between CCR7− and CCR7+ T cells. These data suggest that CD4 effectors maintain a CD47high status in inflamed colons, which confers them a resistance to TSP-1-mediated cell death in tissues and favors their accumulation.
Figure 4. CD4 effectors display a CD47high status in lymph nodes and lamina propria of patients with Crohn’s disease.
(A) CD4 T cell subsets were examined in PBMC, mLNs and LPMC of patients with Crohn’s disease. (B) TSP-1 concentration in human colon biopsies. (C) CD47 expression (SIRP-α-Fc protein) after gating on memory (CCR7+) and effector (CCR7−) CD45RA−CD4+ T cells in PBMC, mLNs and LPMC. Data are representative of 4 to 6 independent experiments. The CD47 Mean Fluorescence Intensity (MFI) CCR7−T/CCR7+ T cell ratio was calculated for patients with CD and unrelated IBD patients. (A and C) The mean ± standard deviation (SD) for 5 to 6 independent experiments. Student t test was performed: *p<0.05.
6. Expression of CD47 is Required on Murine Ag-specific CD4 T Cells for the Contraction Phase in vivo
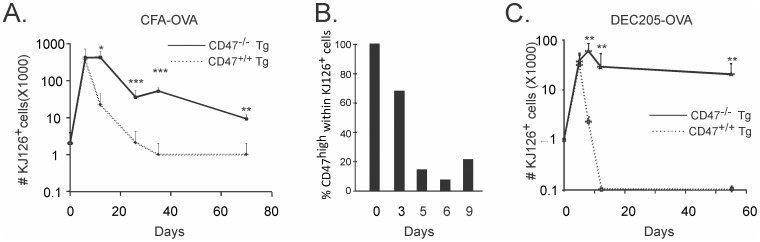
We recently reported that CD47low status is observed on activated murine CD4 T cells in vivo, independently of the route and the methods of immunization [16]. We hypothesized, here, that CD47low status and CD47-mediated cell death are involved in the crash of the IR, while the reestablishment of a CD47high status might offer an advantage to pre-committed TCM cells to escape cell death and elimination. Indeed, CD47high status marked TCM precursors at an early time point of IR [16]. We therefore determined whether CD47 expression and/or CD47 status on murine CD4 T cells had an impact on the contraction phase of the IR in vivo. CD47 is implicated in cell elimination [10]. Hence, viable CD47−/− Tg T cells are readily cleared from wild type hosts, whereas they can be adoptively transferred into CD47-deficient hosts without being cleared [30], [31]. Here, CD47−/− hosts were passively transferred with Tg CD47−/− or CD47+/+ CD4 T cells and immunized subcutaneously with CFA/OVA. Kinetics revealed that proliferation of Tg CD47+/+ T cells occurred at an early time point followed by cell contraction (Fig. 5A). The latter correlated with a change to a CD47low status (Fig. 5B). Tg CD47+/+ T cells also proliferated, albeit at a lower rate, in DEC205-OVA when compared to CFA/OVA immunized mice before their elimination from hosts (Fig. 5C). Although the recovery of Tg CD47+/+ and CD47−/− CD4 T cells was similar until day 9, the absence of CD47 on CD4 T cells significantly increased the yield of Ag-specific T cells at later time points in both immunogenic (CFA/OVA) and tolerogenic (DEC205-OVA) responses (Fig. 5). Tg CD47−/− T cells were still retraced 70 days after immunization. Therefore, we demonstrate that CD47 expression, and specifically a CD47low status, is required on CD4 T cells for the contraction phase during an acute immune response.
Figure 5. CD47 on CD4 T cells regulates the contraction of the immune response in vivo.
One day after adoptive transfer of CD47+/+ or CD47−/− Tg T cells isolated from DO11.10 mice into CD47−/− BALB/c hosts, mice were immunized s.c. with CFA-OVA or DEC205-OVA. (A and C) Kinetic of the recovery of viable Tg T cells is shown. (B) CD47 status (SIRP-α-Fc protein) gated on CD4 KJ126+(Tg) T cells post immunization. Data are representative of 4 to 6 independent experiments, student t test was performed on 8 to 12 mice. *p<0.05, ***p<0.001.
Discussion
The pathway to memory T cell generation can be divided into 3 sequential and critical steps during an acute immune response: 1) resistance to massive cell death, 2) prevention of viable cell elimination, and 3) cell survival. CD47 is implicated in the two first steps [7]. We propose here that a change to a CD47low status is key to determine the cell’s decision to die while the commitment to cell clearance occurs as a downstream event. The CD47low status was detected by staining CD4 T cells with a SIRP-α-Fc fusion protein, although it was not observed using two anti-CD47 mAbs that recognize distinct epitopes. Combined with flow cytometry data, the confocal microscopy and Western blot analysis suggest that CD47 status is linked to a post-translational modification and/or cell surface redistribution rather than to differences in the amounts of CD47 protein expression. A change in the CD47 conformation has been reported in several earlier studies. In fact, binding of B6H12, 2D3 mAbs and SIRP-α-Fc to CD47 is regulated by several factors such as temperature, the cell type on which CD47 is expressed and the presence of cholesterol [17], [32]. For example, affinity of B6H12 and 2D3 mAb for CD47 is higher when monocytes but not RBC, are incubated at 37C instead of 0C. In addition, CD47 displays a different conformation on sickle RBC when compared to normal RBC [18]. 2D3 binds with greater affinity than B6H12 mAb to CD47 on sickle [18] and aged erythrocytes [15], which results in adhesion to TSP-1 under flow and static conditions. A loss of SIRP-α-Fc but not B6H12 and 2D3 binding, thus acquisition of CD47low status, can be artificially induced either by replacing the transmembrane region of CD47 by CD7, by cholesterol removal, or by a double cysteine mutation that disrupts the S-S disulfide bridge between the transmembrane and extracellular CD47 domains [19], [20]. Nonetheless, the precise molecular mechanism behind the physiologic conformational modification of CD47 remains to be elucidated. Reduced or enhanced binding to SIRP-α-Fc might result from either CD47 association with other surface molecules, since CD47 was originally identified as an integrin-associated molecule [33], CD47 redistribution at the membrane [28], and/or a modified glycosylation pattern [22].
In the present study, we investigated the functional consequences provoked by the change in CD47 status on CD4 T cells. Upon activation, human CD4 T cells transiently displayed a CD47low status and become sensitive to CD47-mediated cell death by TSP-1. This may represent one mechanism involved in the contraction of the IR, as well as in the resolution of the inflammatory response. We here showed that the absence of CD47 on murine Ag-specific T cells significantly impaired the contraction of the IR in vivo, demonstrating that the presence of CD47, and more particularly a CD47low status, was necessary for this process to occur. Furthermore, a transient change of CD47low status on CD4 T cells is required to mediate TSP-1-induced cell death in vitro in humans. IL-2 induced a re-expression of CD47high status on human TCR-activated CD4 T cells. T cells themselves represent a source of IL-2 and TSP-1 and CD3 stimulation leads to an increase in the availability of TSP-1 on the cell surface of recently activated T cells [34]. CD47 ligation inhibits early T cell activation, IL-2 production, and CD25 expression [35]. The latter is transiently expressed on activated CD4 T cells in vivo, and CD4+CD25−/− or IL-2−/− effector T cells survive very poorly and generate low numbers of memory T cells in non lymphopenic naive mice [36]. TSP-1 and SIRP-α bind CD47 IgV loop [37] and TSP-1 can inhibit SIRP-α-Fc binding to CD47 expressing-Jurkat cells [38]. We therefore postulate that the reestablishment of a CD47high phenotype on TCM and re-encounter with SIRP-α+ myeloid cells (macrophages or DCs) might offer an advantage to avoid TSP-1-induced cell death whereas CD47low status promotes TSP-1 binding that favors cell death and elimination. A direct interaction between CD47low effectors and SIRP-α+ DCs may also induce IL-2 secretion by T cells. Rebres et al. have demonstrated that SIRP-α-Fc ligation synergizes with CD3 for T cell activation and induces PKC θ translocation, resulting in IL-2 production by T cells [19]. DCs, through autocrine secretion of IL-2, trans-present IL-2 to T cells for optimal clonal expansion and effector function [39]. Thus, in addition to T cell-derived IL-2, DCs also could reverse a CD47low to a CD47high status. We showed here that a CD47high status was maintained on CD4 effectors in inflamed CD tissues. This suggests that auto-aggressive T cells that contribute to tissue destruction, might possess a deregulation in the conformational change process of CD47 which is revealed by an increase in SIRP-α-Fc binding. CD47high status confers resistance to TSP-1-induced killing to CD4 tissue effectors that accumulate in tissues, as we observed abundant TSP-1 release in CD tissues. In that regard, we recently reported that CD47high status on CD4 effectors identifies functional long-lived memory T cell progenitors [16]. Therefore, maintenance of a CD47high status in pathology may be deleterious to the host and perpetuate chronic inflammatory response.
How effector T cell death is regulated during the contraction phase is not fully understood. For many years, the Fas death receptor was considered to be the only T cell surface molecule implicated in the contraction phase of the IR. Fas-mediated signaling leads to activation-induced caspase-dependent apoptosis of TCR-expanded T cells [40], [41]. Mice lacking functional genes for Fas or its ligand (FasL), show uncontrolled lympho-proliferation and developed autoimmunity [42], [43]. CD47 has been linked to Fas [44]. Yet, CD47−/− mice do not display lympho-proliferative disorders as seen in Fas-deficient mice [45]. Fas signaling, like CD47, kills TEM cells and spares TCM as well as TN cells. However, unlike differential CD47 status, Fas expression is similar on TEM and TCM cell subsets [46]. CD47 augments Fas-mediated apoptosis, but CD47-initiated signaling is not required to enable Fas killing. This process is unidirectional since Fas is not necessary for CD47-mediated killing [44]. We thus propose that CD47, rather than Fas-mediated cell death, plays a key role in the dampening of an acute response. We showed here an increased yield of Ag-specific CD47−/− T cells in CD47−/− hosts while Ag-specific CD47+/+ T cell were barely detectable 70 days after primary immunization. In fact, the role of Fas in contraction phase has been challenged by Alexander et al, who showed that the elimination of effector T cells is completely independent of caspase activation. Administration of 11 different regimens of a pan-caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp (OMe)-fluoromethylketone (zVAD) in vivo showed no significant impact on effector or memory CD8 or CD4 T cell development [4]. Neither the activation of caspases nor that of pro-apoptotic members of the Bcl-2 family, such as Bax, Bak or Bim, or the release of apoptogenic proteins AIF (apoptosis-inducing factor), cytochrome c, endonuclease G (EndoG), Omi/HtrA2 and Smac/DIABLO from mitochondria tocytosol is observed in CD47-mediated cell death [12], [25]. Instead, the molecular pathway of CD47-caspase–independent cell death involves Drp1 translocation from the cytosol to the mitochondria, a process controlled by chymotrypsin-like serine proteases [25]. Once inside the mitochondria, Drp1 provokes an impairment of the mitochondrial electron transport chain, resulting in dissipation of mitochondrial transmembrane potential, reactive oxygen species generation, and a drop in ATP levels. However, a physical interaction between CD47 and the proapoptotic Bcl-2/adenovirus E1B 19-kDa interacting protein 3 (BNIP3), which is expressed upon T cell activation, inhibits BNIP3 degradation by the proteasome, thereby sensitizing T cells to apoptosis [26], [47].
At the end of the contraction phase, macrophages and neutrophils must eliminate unwanted (apoptotic or damaged) and “unfit” cells via phagocytosis [48]. CD47 serves as a “don’t eat me” signal, which inhibits cell clearance when delivered to SIRP-α+ cells [49]. Viable CD47−/− T cells are quickly eliminated from a CD47+/+, but not CD47−/− host, by SIRP-α+ cells [30], [31]. Notably, since CD47−/− mice are viable, clearance of CD47−/− cells does not occur in CD47−/− mice because these SIRP-α+ macrophages must be educated by CD47 +/+ stromal cells to acquire functional phagocytosis via interruption of the CD47/SIRP-α pathway [50]. Nonetheless, the contraction of CD4+CD44hiCD47low T cells occurred in the immunized CD47−/− host. In fact, a CD47low status does not equate to absence of CD47. Of note, only 10% to 20% of normal CD47 expression on RBC is sufficient to prevent cell clearance [51]. Furthermore, Weissman and others demonstrated that concealing CD47 with antibodies on live cells is necessary but insufficient to trigger phagocytosis in vivo, since phagocytosis required the expression of calreticulin, which is upregulated on malignant cells [52]. In fact, the “turning off” of non-phagocytic signals must be coupled to the “switching on” of phagocytic signals to provoke cell elimination [48]. Among others, calreticulin serves as a pro-phagocytic signal, which, through binding to its macrophage counter-receptor low-density lipoprotein–related protein (LRP), leads to engulfment of the target cell [28]. In the present study, calreticulin expression was not detected on viable human memory CD4 T cell subsets. In contrast, killing by 4N1K peptide induced calreticulin expression on TEM dying cells, indicating that CD47-mediated cell death represents an upstream event to the elimination of unwanted cells. CD47 expression/redistribution on apoptotic cells also appear to augment phagocytosis [28], [53].
Taken all, we present a key role for CD47 on CD4 T cells in the resolution of an inflammatory response and propose that the following sequence of events accounts for the elimination of a large number of effector T cells. Ag encounter induces a CD47low status on TCR-activated CD4 T cells. Unless rescued by IL-2, which reverses their phenotype to CD47high status, the majority of CD47low T cells will become susceptible to killing by TSP-1 and then augment their expression of pro-phagocytic signals to promote their clearance by SIRP-α+ cells. Further studies that permit to modulate CD47’s status and T cell death may provide novel strategies for improved vaccination and/or the elimination of unwanted, auto-aggressive T cells in inflamed tissues such as in CD.
Materials and Methods
Ethics Statement
All mouse experimental protocols were approved by “Comité institutionnel de protection des animaux (CIPA) du Centre de recherche du Centre hospitalier de l’Université de Montréal (CRCHUM)” that follows the guidelines of the Canadian Council on Animal Care (CCAC). All the experiments were approved by “Comité d’éthique de la recherche du Centre hospitalier de l’Université de Montréal (CHUM)” and written informed consent was obtained from all donors. Human samples were obtained from healthy volunteers, umbilical cord blood and the patients recruited from the Gastroenterology and Surgery Division at CHUM.
Clinical Samples
Peripheral blood samples were collected from all donors, and tissue samples were obtained from endoscopic biopsies or surgically resected specimens. Intestinal tissue samples were taken from unaffected areas of donors with non inflammatory bowel diseases (non IBD) or inflamed regions of Crohn’s disease (CD) patients. Mesenteric lymph nodes (mLNs) were collected after surgery by the pathologists.
Animals
CD47−/−129sv/eg mice were backcrossed onto CD47+/+ BALB/c mice for 16 to 18 generations. Mice expressing the DO11.10 TCR transgene, which is specific for the peptide residues 323–339 of chicken OVA, were purchased from Charles River Laboratories and backcrossed into CD47−/− mice. Female mice 6 to 10 weeks old were used in all experimental protocols and were maintained under specific pathogen-free conditions.
Isolation of Cells
Peripheral blood mononuclear cells (PBMC) or umbilical cord blood mononuclear cells (CBMC) were prepared by density gradient centrifugation of heparinized peripheral blood. Lamina propria mononuclear cells (LPMC) were prepared from intestinal specimens using a modified protocol described by Bull and Bookman (1977). Briefly, the dissected mucosal tissue was cut into small pieces, incubated in HBSS (Sigma) with 1 mM DTT (Sigma) and 1 mM EDTA (Sigma) for 45 min at 37°C, followed by enzymatic digestion with 0.25 mg/ml of collagenase D (Roche) and 0.01 mg/ml of DNase I (Roche) for 45–60 min at 37°C, combined with mechanical dissociation by Dissociator (Miltenyi Biotech). Mesenteric LNs were harvested and squeezed on a 70 mm pore mesh to obtain a cellular suspension.
Antibodies and Reagents
All the antibodies were purchased from Biolegend (USA) unless otherwise indicated. 2D3 cell line was obtained from Dr. E. Brown (Genentech, USA). Monoclonal antibodies against the following human antigens were used for labeling and sorting: CD45 (HI30) CD4 (RPA-T4), CD45RA (HI100), CD62L (DREG-56), CCR7 (TG8/CCR7), CD47 (B6H12 and 2D3), CD27 (MT271, BD) CCR5 (2D7/CCR5), CD127 (HIL-7R-M21) and Calreticulin (FMC 75, Assay designs). Antibodies against mouse antigens: CD4 (RM4–5), TCR (DO11.10) (KJ126). Human and mouse CD47 expression was also revealed using huSIRP-α-Fc and muSIRP-α-Fc fusion proteins that contain SIRP-αD1D2D3 domains fused to mutated human Fc IgG (Novartis, Basel, Switzerland), respectively [54]. For in vitro human T cell stimulation, anti-CD3 (OKT3, Janssen-Ortho) and anti-CD28 (CD28.2) mAbs were used.
Flow Cytometry for Phenotypic Analysis
CD47 expression was examined with huSIRP-α-Fc or with anti-CD47 mAbs after gating on naive (TN: CD4+CD45RA+CCR7+), effector memory (TEM: CD4+CD45RA−CCR7−CD27−), and central memory (TCM: CD4+CD45RA−CCR7+CD27+) T cells. Staining was performed in FACS buffer (PBS supplemented with 2% FCS, 2 mM EDTA, and 0.01% sodium azide at 4°C for 30 min).
Cell Culture
TN and TCM cells were isolated from PBMC or CBMC using a FACS Aria II sorter (BD). Purity was more than 99%. 1×106 CFSE-labeled TN or TCM cells were stimulated in RPMI (Wisent Inc.) supplemented with 10% fetal calf serum (Wisent Inc), 500 U/ml penicillin, and 500 ug/ml streptomycin with immobilized anti-CD3 (10 ug/ml) and soluble anti-CD28 (2 ug/ml) mAbs for 6 days in 24-well plates (Costar). For secondary cultures, 0.5×106 activated TN cells were restimulated with coated anti-CD3 (10 ug/ml) and soluble anti-CD28 (2 ug/ml) with/without IL-2 (100 U, R&D system) or expanded only in IL-2 for 5 days in 48-well plates (Costar). For some experiments, CFSE-labeled activated CD4 T cells were stained with huSIRP-α-Fc protein and FACS-sorted according to CD47 status before restimulation.
Protein Extractions and Immunoblot
Whole-cell extracts were prepared in 20 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 2 mM EDTA, and antiprotease mixture (Roche). Protein content was determined with the Bio-Rad DC kit and 10 dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After blotting, Nitrocellulose filters were probed with 2D3 mAb and anti β-actin. Both were detected according to standard procedures.
TSP-1 Production by Human Colonic Specimens
TSP-1 concentration (ng/ml) was measured with ELISA kit (Chemicon International) in human colon tissue lysates after homogenization and normalized per milligram of tissue.
Confocal Microscopy
TN, TEM and TCM were FACs sorted from PBMC (purity >99.9%), stained with SIRP-α-Fc or anti-CD47 (B6H12) biotinylated and followed by streptavidin Dylight 649 (Biolegend). Samples were mounted in ProLong Gold (Invitrogen) and analyzed with a confocal microscope (Leica).
CD47-mediated Killing Assays
Purified T cells (4×105) isolated from PBMC were labeled with antibodies for CD4 T cell subsets before incubating with 200 uM of TSP-1-specific (4N1K, KRFYVVMWKK) or control (4NGG, KRFYGGMWKK) peptides (McGill University/Sheldon Biotechnology, Montreal) for 30 min at 37°C. Apoptotic cells were revealed with Annexin V binding (BD Bioscience) after gating on TN, TEM, and TCM T cells. For some experiments, CFSE-labeled TN were activated for 6 days and then FACS-sorted according to CFSElow before in vitro secondary stimulation. After 5 days, CD47-mediated killing assays were performed under the same conditions as in ex vivo studies. Specific CD47-mediated killing was calculated as follows: percentage of Annexin+ cells in 4N1K-stimulated T cells, minus the percentage of Annexin+ cells in 4NGG-stimulated T cells.
Adoptive Transfer Experiments
BALB/c CD47+/+ or CD47−/− mice were passively transferred (intravenously, i.v.) with 2×106 CFSE-labeled CD4 T cells isolated from CD47+/+ or CD47−/− DO11.10 (Tg) mice. After 1 day, mice were immunized either subcutaneously (s.c.) with OVA protein (100ug/ml, Sigma) emulsified in complete Freund’sFreud adjuvant (MP Biomedicals, LLC) or anti-DEC205 coupled with OVA peptide (gift from R. Steinman, Rockefeller University). The phenotype of Tg T cells was analyzed by flow cytometry at different time points in draining LNs.
Statistical Analysis
Statistical analyses were performed using the unpaired Student t test. One-way Anova test was used to compare the variation of CD47 expression between human CD4 T cell subpopulations. Data represent mean ± standard deviation (SD). (***P<.001; **P<.01; *P<.05).
Supporting Information
JinB8, a CD47-negative Jurkat cell line, was transfected with various cDNA constructs of CD47 as previously described [12]. Cell lines were stained with anti-CD47 (B6H12 or 2D3) mAbs or huSIRP-α-Fc protein. Data are representative of 2 independent experiments.
(TIF)
Acknowledgments
We would like to thank J-P Wu and G.Delespesse for critical readings and comments, H. Mehta for editing of the manuscript and K. Yurchenko And Janet Crosby Laganière for technical contributions.
Funding Statement
This work was supported by Canadian Institutes of Health Research – CIHR (no MOP-102562) and Crohn’s and Colitis Foundation of Canada. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gerlach C, van Heijst JW, Schumacher TN (2011) The descent of memory T cells. Ann N Y Acad Sci 1217: 139–153. [DOI] [PubMed] [Google Scholar]
- 2. Li J, Huston G, Swain SL (2003) IL-7 promotes the transition of CD4 effectors to persistent memory cells. The Journal of experimental medicine 198: 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sprent J, Surh CD (2011) Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nature immunology 131: 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nussbaum AK, Whitton JL (2004) The contraction phase of virus-specific CD8+ T cells is unaffected by a pan-caspase inhibitor. Journal of immunology 173: 6611–6618. [DOI] [PubMed] [Google Scholar]
- 5. Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, et al. (2008) Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity 28: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown EJ, Frazier WA (2001) Integrin-associated protein (CD47) and its ligands. Trends Cell Biol 11: 130–135. [DOI] [PubMed] [Google Scholar]
- 7. Sarfati M, Fortin G, Raymond M, Susin S (2008) CD47 in the immune response: role of thrombospondin and SIRP-alpha reverse signaling. Curr Drug Targets 9: 842–850. [DOI] [PubMed] [Google Scholar]
- 8. Floquet N, Dedieu S, Martiny L, Dauchez M, Perahia D (2008) Human thrombospondin’s (TSP-1) C-terminal domain opens to interact with the CD-47 receptor: a molecular modeling study. Archives of biochemistry and biophysics 478: 103–109. [DOI] [PubMed] [Google Scholar]
- 9. Hatherley D, Graham SC, Turner J, Harlos K, Stuart DI, et al. (2008) Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Molecular cell 31: 266–277. [DOI] [PubMed] [Google Scholar]
- 10. Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, et al. (2003) By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115: 13–23. [DOI] [PubMed] [Google Scholar]
- 11. Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, et al. (2009) CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138: 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mateo V, Brown EJ, Biron G, Rubio M, Fischer A, et al. (2002) Mechanisms of CD47-induced caspase-independent cell death in normal and leukemic cells: link between phosphatidylserine exposure and cytoskeleton organization. Blood 100: 2882–2890. [DOI] [PubMed] [Google Scholar]
- 13. Pettersen RD, Hestdal K, Olafsen MK, Lie SO, Lindberg FP (1999) CD47 signals T cell death. J Immunol 162: 7031–7040. [PubMed] [Google Scholar]
- 14. Ren Y, Savill J (1995) Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol 154: 2366–2374. [PubMed] [Google Scholar]
- 15. Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R (2012) CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood. [DOI] [PubMed] [Google Scholar]
- 16. Van VQ, Raymond M, Baba N, Rubio M, Wakahara K, et al. (2012) CD47high Expression on CD4 Effectors Identifies Functional Long-Lived Memory T Cell Progenitors. Journal of immunology. [DOI] [PubMed] [Google Scholar]
- 17. Green JM, Zhelesnyak A, Chung J, Lindberg FP, Sarfati M, et al. (1999) Role of cholesterol in formation and function of a signaling complex involving alphavbeta3, integrin-associated protein (CD47), and heterotrimeric G proteins. J Cell Biol 146: 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brittain JE, Mlinar KJ, Anderson CS, Orringer EP, Parise LV (2001) Integrin-associated protein is an adhesion receptor on sickle red blood cells for immobilized thrombospondin. Blood 97: 2159–2164. [DOI] [PubMed] [Google Scholar]
- 19. Rebres RA, Green JM, Reinhold MI, Ticchioni M, Brown EJ (2001) Membrane raft association of CD47 is necessary for actin polymerization and protein kinase C theta translocation in its synergistic activation of T cells. J Biol Chem 276: 7672–7680. [DOI] [PubMed] [Google Scholar]
- 20. Rebres RA, Vaz LE, Green JM, Brown EJ (2001) Normal ligand binding and signaling by CD47 (integrin-associated protein) requires a long range disulfide bond between the extracellular and membrane-spanning domains. J Biol Chem 276: 34607–34616. [DOI] [PubMed] [Google Scholar]
- 21. Brown E, Hooper L, Ho T, Gresham H (1990) Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. The Journal of cell biology 111: 2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Subramanian S, Boder ET, Discher DE (2007) Phylogenetic divergence of CD47 interactions with human signal regulatory protein alpha reveals locus of species specificity. Implications for the binding site. J Biol Chem 282: 1805–1818. [DOI] [PubMed] [Google Scholar]
- 23. Osborne LC, Abraham N (2010) Regulation of memory T cells by gammac cytokines. Cytokine 50: 105–113. [DOI] [PubMed] [Google Scholar]
- 24. Kameyama K, Nemoto Y, Kanai T, Shinohara T, Okamoto R, et al. (2010) IL-2 is positively involved in the development of colitogenic CD4(+) IL-7Ralpha(high) memory T cells in chronic colitis. Eur J Immunol. [DOI] [PubMed] [Google Scholar]
- 25. Bras M, Yuste VJ, Roue G, Barbier S, Sancho P, et al. (2007) Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol 27: 7073–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamy L, Ticchioni M, Rouquette-Jazdanian AK, Samson M, Deckert M, et al. (2003) CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J Biol Chem 278: 23915–23921. [DOI] [PubMed] [Google Scholar]
- 27. Mateo V, Lagneaux L, Bron D, Biron G, Armant M, et al. (1999) CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med 5: 1277–1284. [DOI] [PubMed] [Google Scholar]
- 28. Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, et al. (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123: 321–334. [DOI] [PubMed] [Google Scholar]
- 29. Kaser A, Zeissig S, Blumberg RS (2010) Inflammatory bowel disease. Annual Review of Immunology 28: 573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouguermouh S, Van VQ, Martel J, Gautier P, Rubio M, et al. (2008) CD47 expression on T cell is a self-control negative regulator of type 1 immune response. J Immunol 180: 8073–8082.18523271 [Google Scholar]
- 31. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, et al. (2000) Role of CD47 as a marker of self on red blood cells. Science 288: 2051–2054. [DOI] [PubMed] [Google Scholar]
- 32. Rosales C, Gresham HD, Brown EJ (1992) Expression of the 50-kDa integrin-associated protein on myeloid cells and erythrocytes. Journal of immunology 149: 2759–2764. [PubMed] [Google Scholar]
- 33. Lindberg FP, Gresham HD, Schwarz E, Brown EJ (1993) Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol 123: 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li SS, Liu Z, Uzunel M, Sundqvist KG (2006) Endogenous thrombospondin-1 is a cell-surface ligand for regulation of integrin-dependent T-lymphocyte adhesion. Blood 108: 3112–3120. [DOI] [PubMed] [Google Scholar]
- 35. Avice MN, Rubio M, Sergerie M, Delespesse G, Sarfati M (2001) Role of CD47 in the induction of human naive T cell anergy. J Immunol 167: 2459–2468. [DOI] [PubMed] [Google Scholar]
- 36. Dooms H, Wolslegel K, Lin P, Abbas AK (2007) Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. The Journal of experimental medicine 204: 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Floquet N, Dedieu S, Martiny L, Dauchez M, Perahia D (2008) Human thrombospondin’s (TSP-1) C-terminal domain opens to interact with the CD-47 receptor: a molecular modeling study. Arch Biochem Biophys 478: 103–109. [DOI] [PubMed] [Google Scholar]
- 38. Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, et al. (2009) Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. The Journal of biological chemistry 284: 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wuest SC, Edwan JH, Martin JF, Han S, Perry JS, et al. (2011) A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nature medicine 17: 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krammer PH (2000) CD95’s deadly mission in the immune system. Nature 407: 789–795. [DOI] [PubMed] [Google Scholar]
- 41. Ashkenazi A, Dixit VM (1998) Death receptors: signaling and modulation. Science 281: 1305–1308. [DOI] [PubMed] [Google Scholar]
- 42. Nagata S (1998) Human autoimmune lymphoproliferative syndrome, a defect in the apoptosis-inducing Fas receptor: a lesson from the mouse model. Journal of human genetics 43: 2–8. [DOI] [PubMed] [Google Scholar]
- 43. Choi Y, Ramnath VR, Eaton AS, Chen A, Simon-Stoos KL, et al. (1999) Expression in transgenic mice of dominant interfering Fas mutations: a model for human autoimmune lymphoproliferative syndrome. Clinical immunology 93: 34–45. [DOI] [PubMed] [Google Scholar]
- 44. Manna PP, Dimitry J, Oldenborg PA, Frazier WA (2005) CD47 augments Fas/CD95-mediated apoptosis. J Biol Chem 280: 29637–29644. [DOI] [PubMed] [Google Scholar]
- 45. Van VQ, Darwiche J, Raymond M, Lesage S, Bouguermouh S, et al. (2008) Cutting edge: CD47 controls the in vivo proliferation and homeostasis of peripheral CD4+ CD25+ Foxp3+ regulatory T cells that express CD103. J Immunol 181: 5204–5208. [DOI] [PubMed] [Google Scholar]
- 46. Ramaswamy M, Cruz AC, Cleland SY, Deng M, Price S, et al. (2010) Specific elimination of effector memory CD4(+) T cells due to enhanced Fas signaling complex formation and association with lipid raft microdomains. Cell Death Differ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, et al. (2007) Interactions between CD47 and thrombospondin reduce inflammation. J Immunol 178: 5930–5939. [DOI] [PubMed] [Google Scholar]
- 48. Krysko DV, D’Herde K, Vandenabeele P (2006) Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis : an international journal on programmed cell death 11: 1709–1726. [DOI] [PubMed] [Google Scholar]
- 49. Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, et al. (2001) CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med 194: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang H, Madariaga ML, Wang S, Van Rooijen N, Oldenborg PA, et al. (2007) Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci U S A 104: 13744–13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsai RK, Discher DE (2008) Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. The Journal of cell biology 180: 989–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, et al. (2010) Calreticulin Is the Dominant Pro-Phagocytic Signal on Multiple Human Cancers and Is Counterbalanced by CD47. Sci Transl Med 2: 63ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tada K, Tanaka M, Hanayama R, Miwa K, Shinohara A, et al. (2003) Tethering of apoptotic cells to phagocytes through binding of CD47 to Src homology 2 domain-bearing protein tyrosine phosphatase substrate-1. Journal of immunology 171: 5718–5726. [DOI] [PubMed] [Google Scholar]
- 54. Raymond M, Rubio M, Fortin G, Shalaby KH, Hammad H, et al. (2009) Selective control of SIRP-alpha-positive airway dendritic cell trafficking through CD47 is critical for the development of T(H)2-mediated allergic inflammation. The Journal of allergy and clinical immunology 124: 1333–1342 e1331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
JinB8, a CD47-negative Jurkat cell line, was transfected with various cDNA constructs of CD47 as previously described [12]. Cell lines were stained with anti-CD47 (B6H12 or 2D3) mAbs or huSIRP-α-Fc protein. Data are representative of 2 independent experiments.
(TIF)