Abstract
An unknown vitamin D compound was observed in the HPLC-UV chromatogram of edible mushrooms in the course of analyzing vitamin D2 as part of a food composition study and confirmed by liquid chromatography-mass spectrometry to be vitamin D4 (22-dihydroergocalciferol). Vitamin D4 was quantified by HPLC with UV detection, with vitamin [3H] itamin D3 as an internal standard. White button, crimini, portabella, enoki, shiitake, maitake, oyster, morel, chanterelle, and UV-treated portabella mushrooms were analyzed, as four composites each of a total of 71 samples from U.S. retail suppliers and producers. Vitamin D4 was present (>0.1 µg/100 g) in a total of 18 composites and in at least one composite of each mushroom type except white button. The level was highest in samples with known UV exposure: vitamin D enhanced portabella, and maitake mushrooms from one supplier (0.2–7.0 and 22.5–35.4 µg/100 g, respectively). Other mushrooms had detectable vitamin D4 in some but not all samples. In one composite of oyster mushrooms the vitamin D4 content was more than twice that of D2 (6.29 vs. 2.59 µg/100 g). Vitamin D4 exceeded 2 µg/100 g in the morel and chanterelle mushroom samples that contained D4, but was undetectable in two morel samples. The vitamin D4 precursor 22,23-dihydroergosterol was found in all composites (4.49–16.5 mg/100 g). Vitamin D4 should be expected to occur in mushrooms exposed to UV light, such as commercially produced vitamin D enhanced products, wild grown mushrooms or other mushrooms receiving incidental exposure. Because vitamin D4 coeluted with D3 in the routine HPLC analysis of vitamin D2 and an alternate mobile phase was necessary for resolution, researchers analyzing vitamin D2 in mushrooms and using D3 as an internal standard should verify that the system will resolve vitamins D3 and D4.
Introduction
Vitamin D is a 9,10-secosteroid and 6 forms have been identified [1] . Vitamin D2 (9,10-seco(5Z,7E)-5,7,10(19),22-ergostatetraene-3β-ol; ergocalciferol) and vitamin D3 (9,10-seco(5Z,7E)-5,7,10(19)cholestatriene-3β-ol; cholecalciferol) are the predominant forms of vitamin D relevant to human nutrition. Vitamin D3 originates from animal sources, and vitamin D2 is derived predominantly from fungi, such as yeast [2], [3]. The importance of vitamin D in bone (calcium homeostasis) is well established, and vitamin D has been the subject of increased attention in recent years for its role in muscle function, immunology, heart and cardiovascular disease, cancer, and insulin secretion [4], [5], [6], [7], [8]. A primary source of vitamin D3 in humans and many animals occurs from the conversion of 7-dehydrocholesterol in the epidermis to vitamin D3 during exposure to ultraviolet (UV) radiation present in sunlight [2]. Oily fish and fish liver oils are naturally rich dietary sources of vitamin D3. Other foods in the U.S. marketplace are fortified (typically with vitamin D3), including milk, cheeses, yogurts, cereals, margarines, and orange juice.
Mushrooms are a natural source of vitamin D2. The vitamin D2 content of mushrooms can be increased dramatically by UV irradiation, whereby ergocalciferol is formed from ergosterol [9], [10], [11], [12], [13]. Recent analyses conducted on ten types of mushrooms sampled from the U.S. marketplace showed vitamin D2 concentrations between 0.03–63.2 μg/100 g (1.2–2528 IU/100 g) fresh weight, with the highest levels in mushrooms exposed to UV during production [14]. Ergosterol is also found in yeast and other fungi [15], and vitamin D2 is produced industrially by UV irradiation of yeast [3]. Vitamin D2 is included in some dietary supplements and fortified foods, particularly vegetarian products.
The occurrence of vitamers other than D3 and D2 in the food supply has not been widely reported in the literature, nor have their nutritional value and biological effects. In the available studies evaluating the vitamin D content in different mushroom species (including Mattila et al. [16], [17], [18], Rangel-Castro et al. [19], Teichmann et al. [13]), no vitamers other than D2 have been reported. In our recent analysis of the vitamin D2 and sterol content of ten types of mushrooms [14] a second peak having a UV spectrum consistent with vitamin D was present in the HPLC chromatogram of many samples and occurred at a relatively high level in mushrooms that had been exposed to UV light. The vitamin D4 precursor ergosta-5,7-dienol (22,23-dihydroergosterol) was present in all samples. The purpose of this communication is to report on findings that support the identification of vitamin D4 in mushrooms, and the vitamin D4 content of ten types of mushrooms.
Materials and Methods
General experimental procedures
Reagents and standards for extraction and analysis of vitamin D and sterols were as described previously [14]. Authentic vitamin D4 (manufacturer's specified purity, 98.9% by TLC9) was procured from Lanospharma Laboratories Co., Ltd. (Chongqing, China). Ergosterol and N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) were purchased from Sigma-Aldrich Corp. (St. Louis, MO).
Samples
Samples of white button, crimini, portabella, enoki, shiitake, maitake, oyster, morel, UV-treated portabella, and chanterelle mushrooms were the same as described in detail in the previous report on vitamin D2 and sterols [14] and comprised a total 71 original samples analyzed as four composites of each type of mushroom (two for chanterelle).
Extraction and analysis of sterols and vitamin D
Sterols and vitamin D2 were quantified as described previously [14]. Sterols were determined as the trimethylsilyl ether (TMS) derivatives, by gas chromatography with flame ionization detection after alkaline saponification of total lipid extracts, with gas chromatography-mass spectrometry (GC-MS) to confirm component identities. Vitamin D4 was quantified using high-performance liquid chromatography (HPLC) with UV detection and [3H]vitamin D3 as the internal standard as described previously for vitamin D2 [14], except using the HPLC conditions described below.
Identification of the unknown
Mass spectrometry was performed at the High Resolution Mass Spectrometry Facility at the University of Iowa (Iowa City, IA) using a Waters GCT Premier (Waters Corp. Milford, MA). For solid probe high resolution mass spectrometry the ramp temperature used was 100°C/min. For GC-MS the column was a 30m DB-5ms and the ramp started at 170°C, then increased by 10°C per minute with a final temperature of 300°C that was held for 15 minutes. BSTFA derivatization for GC-MS was performed by re-suspending dry samples or standards in a 1∶1 mixture of BSTFA:methylene chloride, warming at 40°C for 60 minutes followed by direct injection of an aliquot of a given mixture onto the GC-MS column.
Quality control
A sample of a mushroom control composite previously described [14], that comprised approximately 50% portabella mushrooms and 50% vitamin D enhanced (UV-treated) portabella mushrooms, was analyzed with each batch of samples and used to monitor run-to-run precision. Validation of recovery of vitamin D2 as described in a previous communication [14] was assumed to apply to the extraction of vitamin D4. The GC-MS analyses described above verified the identity of the analyte peaks.
Data analysis
Means and standard deviations were calculated using Microsoft® Office Excel (Professional Plus edition, 2010; Microsoft Corporation, Redmond, WA), and analysis of variance (α = 0.05) and pairwise comparison of means using the Student-Newman-Keuls test with a 95% confidence interval were performed with XLSTAT (version 2011.2.06; Addinsoft, New York, NY).
Results
Identification of vitamin D unknown in mushrooms
Initially the unknown vitamin D form observed in a variety of mushrooms in previous work [14] was thought to be vitamin D3, because it eluted at the same retention time as a vitamin D3 standard chromatographed under the conditions that were being used for analysis of vitamin D2 and displayed the characteristic UV chromophore. Some literature reports were found on the presence of vitamin D3 in alfalfa, tomato, eggplant and zucchini leaves and some other plants have been reported [20], [21], [22], [23], but none on nutritional quantities of vitamin D3 or other forms besides D2 in mushrooms.
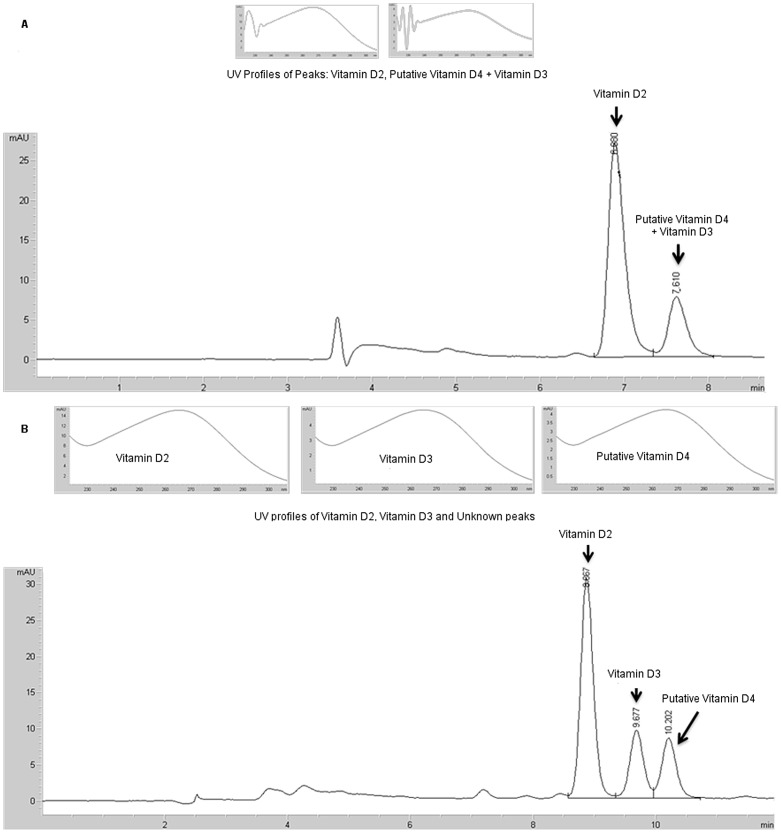
Figure 1 shows the high-performance liquid chromatography (HPLC) chromatogram of a mushroom extract containing the putative vitamin D4 and spiked with vitamin D3. Figure 1A shows the chromatogram from the solvent system routinely used for vitamin D analysis (acetonitrile/methylene chloride (70/30) as described by Phillips et al. [14]; Figure 1B shows the separation of the vitamin D3 and putative vitamin D4 into two components using an alternate solvent system (acetonitrile:methanol, 1∶1), confirming the component was not D3. The unknown was hypothesized to be vitamin D4 (22-dihydroergocalciferol) because it co-eluted with an authentic vitamin D4 using the alternative solvent system and because its precursor is present in mushrooms. Although there have been no previous literature reports of vitamin D4 in mushrooms, vitamin D4 (22,23-dihydroergocalciferol;9,10-seco(5Z,7E)-5,7,10(19)-ergostatriene-3β-ol) is the product of UV irradiation of 22,23-dihydroergosterol, analogous to the formation of vitamin D2 from ergosterol. 22,23-dihydroergosterol (ergosta-5,7-dienol) was present in ten types of mushrooms, as previously reported [14]. Therefore it seemed reasonable to presume conversion of some portion of the 22,23-dihydroergosterol to vitamin D4, and mass spectral studies were conducted to confirm the identity.
Figure 1. HPLC chromatograms and UV spectra of vitamin D components in a mixed mushroom extract.
Chromatography on a Vydac® ODS column developed using (A) acetonitrile:methylene chloride (70∶30) (the solvent system used previously for quantitation of vitamin D2 [14]), showing co-migration of the putative vitamin D4 with vitamin D3 in this system; (B) developed with acetonitrile:methanol (1∶1) mobile phase, showing separation of the peak containing putative vitamin D4 and vitamin D3 into two components.
Mass spectral confirmation
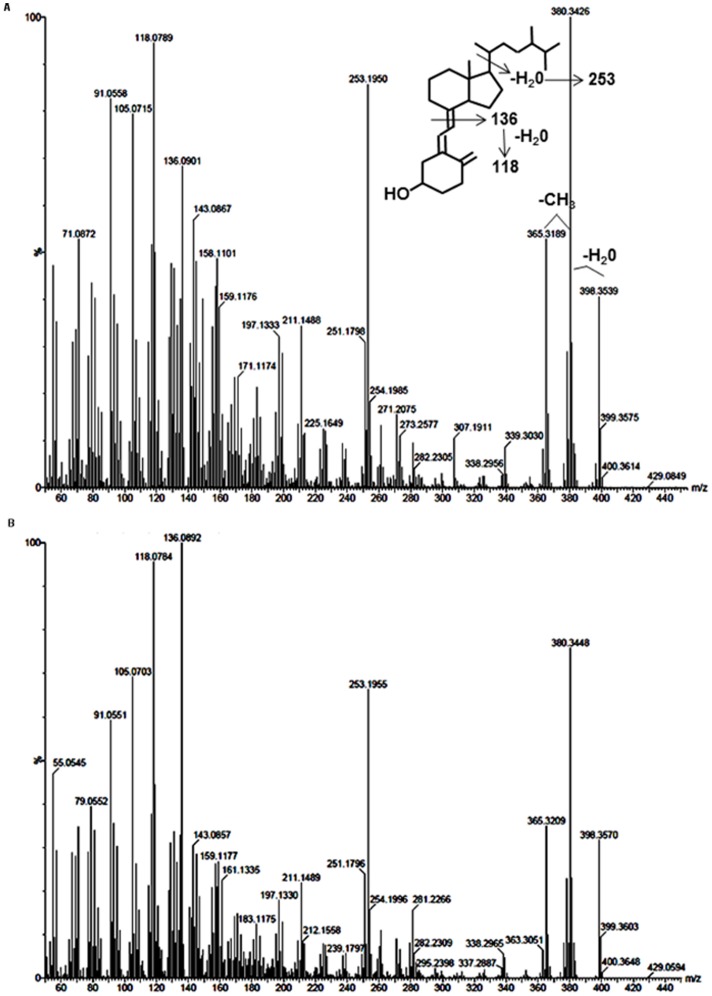
Material was collected from the putative vitamin D4 peak of a mixture of mushroom types and analyzed by high resolution mass spectrometry and compared with an authentic vitamin D4 standard run under identical conditions. As seen in Figure 2A, the mushroom compound produced a parent molecular ion at m/z 398.3539, in good agreement with the calculated mass value of 398.3549 for vitamin D4. Losses of water and a methyl group are readily apparent (m/z 380.3426 and 365.3189). The prominent peak at 253.1950 corresponds to loss of the vitamin D4 side chain in combination with a water molecule, while peaks at 136.0901 and 118.0789 are characteristic for cleavage of the secosteroid structure and subsequent water loss. All of these fragments were also observed with the authentic vitamin D4 compound when subjected to the same high resolution analysis (Figure 2B). In addition, low resolution mass spectrometry of TMS-derivatized samples of both the mushroom isolate and vitamin D4 standard produced analogous mass spectral fragmentations, with parent ions of m/z 470.5 (data not shown), thus verifying the presence of a single hydroxyl moiety and further corroborating the identity of this compound from the mushroom isolate as vitamin D4.
Figure 2. High resolution mass spectral comparison of putative vitamin D4 isolated from mushroom.
(A) Spectrum of HPLC-purified mushroom isolate corresponding to vitamin D4 with structure and breakdown products highlighted. (B) Spectrum of vitamin D4 standard.
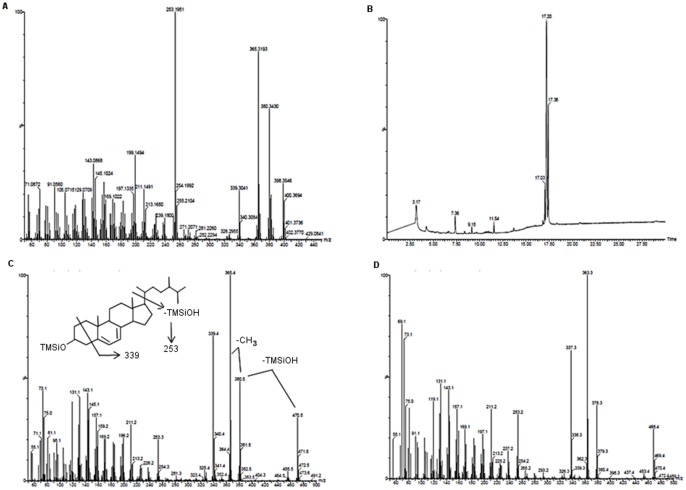
In a similar manner, high resolution mass spectrometry was also performed on the purported 22,23-dihydroergosterol collected from the mixed mushroom sample; however, in contrast the spectra revealed the presence of at least 2 compounds, with molecular ions evident at m/z 398.3546 and 400.3694 (Figure 3A). The lower mass was in agreement with the prediction for 22,23-dihydroergosterol (C28H46O; calculated value 398.3549), while the higher mass suggested an additional saturation of a diene bond, presumably of a 22,23-dihydroergosterol-like molecule (C28H48O; calculated value of 400.3705). Because of the apparent complexity of the sample, the mixture was derivatized with BSTFA and subjected to gas chromatography-mass spectrometry (GC-MS). As seen in Figure 3B, the gas chromatogram of the TMS-derivatized mushroom isolate revealed the presence of 3 peaks. The major peak (17.2 min) produced a parent ion of m/z 470.5, in keeping with the derivatization of a single hydroxyl moiety and consistent with the expected ion mass for the TMS derivative of 22,23-dihydroergosterol (Figure 3C). Ions corresponding to loss of trimethylsilanol (m/z 380.5) followed by a methyl group (m/z 365.4) were readily apparent. The decrease of 131 mass units to produce the ion at m/z 339 is proposed to arise from fragmentation of the A-ring, most likely involving loss of C-2, C-3, C-4 and their substituents [24], [25]. Importantly, the presence of the m/z 253.3 ion, representing the core ring structure resulting from loss of the side chain and trimethylsilanol fragments indicates the additional saturation with hydrogen molecules occurred in the side chain. By way of comparison, an authentic ergosterol standard was similarly derivatized with BSTFA and subjected to GC-MS, which produced a single peak at 17.4 minutes (data not shown). As seen in Figure 3D the fragmentation pattern for derivatized ergosterol standard essentially paralleled that of the mushroom isolate, including the presence of the m/z 253.2 ion; except for the observed decrease in the molecular ion due to unsaturation of the side chain in the standard material. Thus, the data are consistent with the isolation of 22,23-dihydroergosterol from the mushroom extract. Finally, the other 2 peaks observed in the GC trace from the derivatized mushroom isolate (17.03 and 17.38 min) both produced parent ions at m/z 472 and fragments at m/z 255 (data not shown). As noted above, we suspect these may be isomers corresponding to additional saturation of one or the other of the diene bonds in the B-ring of 22,23-dihydroergosterol to produce, for instance, 22,23-dihydrobrassicasterol. Additional experiments will need to be performed to confirm these suspicions; however, the loss of the diene entity would explain the extent to which these compounds could co-migrate with the 22,23-dihydroergosterol and escape detection by HPLC utilizing an ultraviolet light detector to track the purification of the mushroom compounds.
Figure 3. Spectral analysis of putative dihydroergosterol in a mushroom isolate.
(A) High resolution mass spectrum of purified mushroom isolate corresponding to dihydroergosterol. (B) Gas chromatogram of products obtained following derivatization of the purified mushroom isolate with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA). (C) Low resolution GC-MS of derivatized mushroom product at t = 17.20 min corresponding to dihydroergosterol with structure and breakdown products highlighted. (D) Low resolution GC-MS of commercially available ergosterol standard following derivatization with BSTFA.
The quantitative values for 22,23-dihydroergosterol that are reported were obtained in the previously reported GC and GC-MS analysis [14], which provided better resolution and eliminated the interference of the other components that were shown to coelute with 22,23-dihydroergosterol in the HPLC system.
Vitamin D4 content of mushrooms
Table 1 summarizes the assayed concentration (fresh weight basis) of vitamin D4 and its precursor, 22,23-dihydroergosterol in ten types of mushrooms (white button, crimini, portabella, enoki, shiitake, maitake, oyster, morel, and UV-treated portabella, and chanterelle) sampled from retail outlets in the U.S. Overall, vitamin D4 was detected (>0.1 µg/100 g) in 18 of the total of 38 composites analyzed and was present at an average concentration of 5.2 µg/100 g. However there was wide variability between and within samples different types of mushrooms. There were 7 samples known to contain mushrooms that had been exposed to UV light during production: the Mushroom CC, the vitamin D enhanced portabella, and the two maitake composites from supplier G (Table 1). All of these samples contained vitamin D4, and in some the concentration was similar to or greater than that of vitamin D2 (previously reported in Phillips et al. [14]). The two maitake mushroom samples that were high in vitamin D2 (63.2 and 48.9 µg/100 g) were also high in vitamin D4 (35.4 and 22.5 µg/100 g, respectively). These mushrooms were presumed to have been exposed to UV light under the growing conditions reportedly used by this producer [26]. Of the mushrooms not known to have received UV exposure, vitamin D4 occurred in at least one composite of each type except white button. In oyster mushrooms the composite highest in vitamin D2 (2.59 µg/100 g) had a vitamin D4 content more than two-fold higher (6.29 µg/100 g). Vitamin D4 exceeded 2 µg/100 g in the morel and chanterelle mushroom samples that contained D4 (all but two morel composites).
Table 1. Vitamin D4 and pre-vitamin D4 (22,23-dihydroergosterol; ergosta-5,7-dienol) content of ten types of mushrooms.
| Vitamin D4 | 22,23-Dihydroergosterol | |||||||||
| Mushroom | Scientific name | NDB no.a | Com- positeb | Moisture (g/100g) | µg/100g fresh weightc | Mean | SD | Std Err | mg/100g fresh weight | Mean |
| White button | Agaricus bisporus | 11260 | 1 | 92.85 | – | –B | – | – | 5.97 | 6.03 B,C |
| 2 | 92.81 | – | 5.79 | |||||||
| 3 | 92.35 | – | 5.86 | |||||||
| 4 | 92.47 | – | 6.49 | |||||||
| Enoki | Flammulina veluptipes | 11950 | A1 | 87.68 | – | 0.10 B | 0.21 | 0.10 | 17.0 | 16.5 A |
| A2 | 88.47 | – | 18.0 | |||||||
| G1 | 88.28 | 0.41 | 17.0 | |||||||
| 1 | 89.30 | – | 13.8 | |||||||
| Shiitake | Lentinus edodes | 11238 | 1 | 86.90 | 0.27 | 0.51 B | 0.48 | 0.24 | 7.31 | 6.51 B,C |
| 2 | 91.41 | 0.67 | 7.25 | |||||||
| 3 | 90.53 | 1.11 | 6.15 | |||||||
| A1 | 90.11 | – | 5.34 | |||||||
| Maitake | Grifola frondosa | 11993 | A1 | 88.37 | – | 14.5 A | 17.5 | 8.76 | 8.90 | 6.34 B,C |
| A2 | 88.59 | – | 9.00 | |||||||
| C1 | 92.30 | 35.4 | 3.53 | |||||||
| C2 | 91.92 | 22.5 | 3.92 | |||||||
| Oyster | Pleurotus ostreatus | 11987 | A1 | 89.70 | 0.81 | 1.77 AB | 3.00 | 1.52 | 8.55 | 8.89 B |
| 1 | 88.77 | – | 11.7 | |||||||
| 2 | 90.38 | 6.29 | 8.16 | |||||||
| 3 | 90.54 | – | 7.13 | |||||||
| Crimini | Agaricus bisporus | 11266 | 1 | 91.92 | – | 0.31 B | 0.61 | 0.31 | 5.25 | 5.92 B,C |
| 2 | 91.22 | 1.22 | 6.11 | |||||||
| A1 | 93.08 | – | 5.42 | |||||||
| B1 | 92.07 | – | 6.92 | |||||||
| Portabella | Agaricus bisporus | 11265 | 1 | 90.96 | – | 0.14B | 0.27 | 0.14 | 6.75 | 6.18 B,C |
| 2 | 92.22 | – | 5.45 | |||||||
| 3 | 91.29 | 0.55 | 6.53 | |||||||
| 4 | 91.25 | – | 5.97 | |||||||
| Portabella, uv treated | Agaricus bisporus | 11998 | A1 | 94.86 | 0.20 | 3.62 AB | 3.22 | 1.61 | 4.57 | 4.70 C |
| A2 | 95.12 | 1.66 | 3.94 | |||||||
| B1 | 94.76 | 7.05 | 5.10 | |||||||
| B2 | 93.68 | 5.56 | 5.20 | |||||||
| Chanterelle | Cantharellus californicus or C. cibarius | 11239 | D1 | 91.09 | 0.82 | 1.62 AB | 1.13 | 0.80 | 5.23 | 4.49 C |
| D2 | 88.61 | 2.42 | 3.75 | |||||||
| Morel | Morchella spp. | 11240 | E1 | 89.46 | 2.36 | 1.13 B | 1.31 | 0.65 | 7.13 | 5.79 B,C |
| E2 | 90.38 | 2.15 | 5.75 | |||||||
| F1 | 89.44 | – | 5.31 | |||||||
| F2 | 89.18 | – | 4.98 | |||||||
Database entry number from United States Department of Agriculture (USDA) National Nutrient Database for Standard Reference [53]; b Composites are combinations of samples from statisitical sampling locations in the U.S., or retail suppliers, as described in Phillips et al. [14]. Composites designated with the same capital letter were from the same supplier. c – indicates less than the limit of detection (0.1 μg/100 g fresh weight).
Results for a total of 26 analyses of a control composite (Mushroom CC) across multiple assays provided an estimate of the analytical uncertainty in the vitamin D4 concentrations assayed in individual composites. The mean vitamin D4 concentration in the Mushroom CC was 0.14 µg/100g with a standard deviation of 0.042 µg/100 g (standard error, 0.008 µg/100 g). Greater precision at higher concentrations would be expected [27].
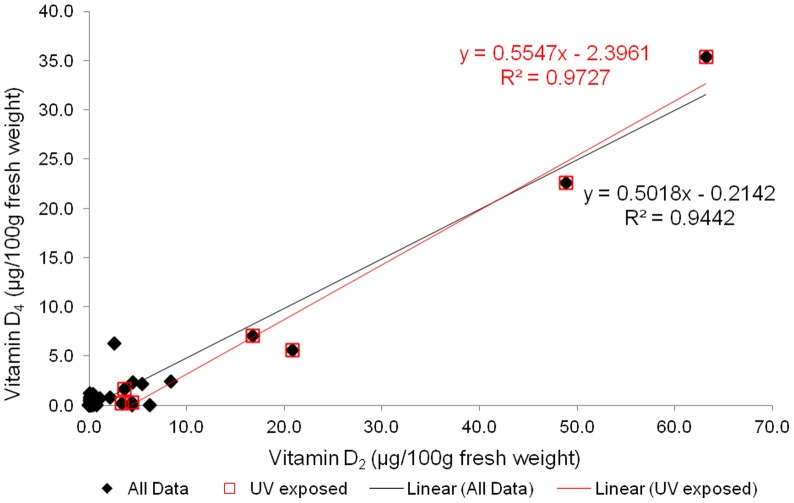
The presence of vitamin D4 in all mushrooms with known UV exposure but with no consistency in other samples suggests that vitamin D4 in mushrooms results from incidental or intentional UV exposure. Interestingly, Wang et al. [28] reported variability in the vitamin D level in lichens (Cladina spp.) as related to UV exposure at different latitudes. Figure 4 illustrates vitamin D4 concentration as a function of vitamin D2 concentration (previously reported [14]) in the 38 composites of ten types of mushrooms that were analyzed. Overall there was a positive correlation between vitamins D4 and D2. In a separate study of white button mushrooms subjected to controlled UV exposure [29], all of the UV-treated samples contained vitamin D4, with an average of 2.43 μg/100 g fresh weight (range 1.95–2.74), whereas the concentration was <0.1 μg/100 g in the unexposed mushrooms.
Figure 4. Relationship between the vitamin D4 and vitamin D2 concentrations in ten types of mushrooms ( Table 1 ).
Data for vitamin D2 were previously reported [14].
Vitamin D4 precursor in mushrooms
The vitamin D4 precursor 22,23-dihydroergosterol was present in all mushroom composites (Table 1). The levels were not correlated with vitamin D4, but differed among species. Enoki mushrooms had a notably higher 22,23-dihydroergosterol content, with an average of 16.5 mg/100g compared to 4.49–8.89 mg/100 g in other types of mushrooms.
There have been other, limited reports on 22,23-dihydroergosterol in mushrooms, although the diversity in common nomenclature for sterols often makes the synonymous identity or close structural similarity among various sterols not readily apparent (see Moss [30] for detailed information on steroid nomenclature). 22-23-Dihydroergosterol [(24R)-24-methylcholesta-5,7-dien-3β-ol] is ergosta-5,7-dienol, and ergosta-5,7-dienol in wild and cultivated mushrooms [Cantharellus cibarius and C. tubaeformis (chanterelle), Boletus edulis (king bolete), Lentinus edodes (shiitake), Pleurotus ostreatus (oyster), and Agaricus bisporus (white button, brown button, crimini), portabella] was reported by Teichmann et al. [13]. Vitamin D2 levels were also analyzed in that study but no chromatograms from the vitamin D analysis were published, so it is not possible to determine if vitamin D4 may have been present. Shao et al. [31] recently reported the ergosterol content of stems and caps of white and brown button mushrooms at different stages of development and identified an “ergosterol analogue” in their HPLC analysis. This component is likely 22,23-dihydroergosterol based on comparison of the concentrations reported to those in the present study, and the fact that this component was identified in all samples of white and brown mushrooms in the present investigation. In the Shao et al. study [31] the sum of the concentration of the “ergosterol analogue” in the saponified extracts of the stems and caps was 0.71–0.95 mg/g dry wt and 0.42–0.65 mg/g dry wt in brown mushrooms (11.2–14.0% and 6.9–10.5% of the ergosterol concentration, respectively). These concentrations were similar to the averages of 0.82 mg/g dry wt and 0.75 mg/g dry wt for 22,23-dihydroergosterol (10.7% and 9.8% of the ergosterol concentration, respectively) in this study (Table 2).
Table 2. Comparison of assayed concentrations of ergosterol (vitamin D2 precursor) and 22,23-dihydroergosterol (vitamin D4 precursor) in white and brown button mushrooms.
| Range (μg/100g dry weight) | |||
| Component | This study | Shao et al. [31] a | |
| Ergosterol | White button | 740–795 | 563–681 |
| Brown buttonc | 725–821 | 475–938 | |
| 22,23-Dihydroergosterol | White button | 77–86 | 71–95 b |
| Brown buttonc | 65–87 | 42–65 b | |
| 22,23-Dihydroergosterol | White button | 10.0–11.2 | 11.2–14.0 |
| (as percent of ergosterol) | Brown buttonc | 7.9–12.0 | 6.9–10.5 |
In this study for four samples of each type, and as reported by Shao et al. [31] for one sample at each of three stages of maturity for each mushroom type.
values show the sum of the concentrations in the separately assayed stems and caps.
reported as “ergosterol analogue”.
crimini.
Discussion
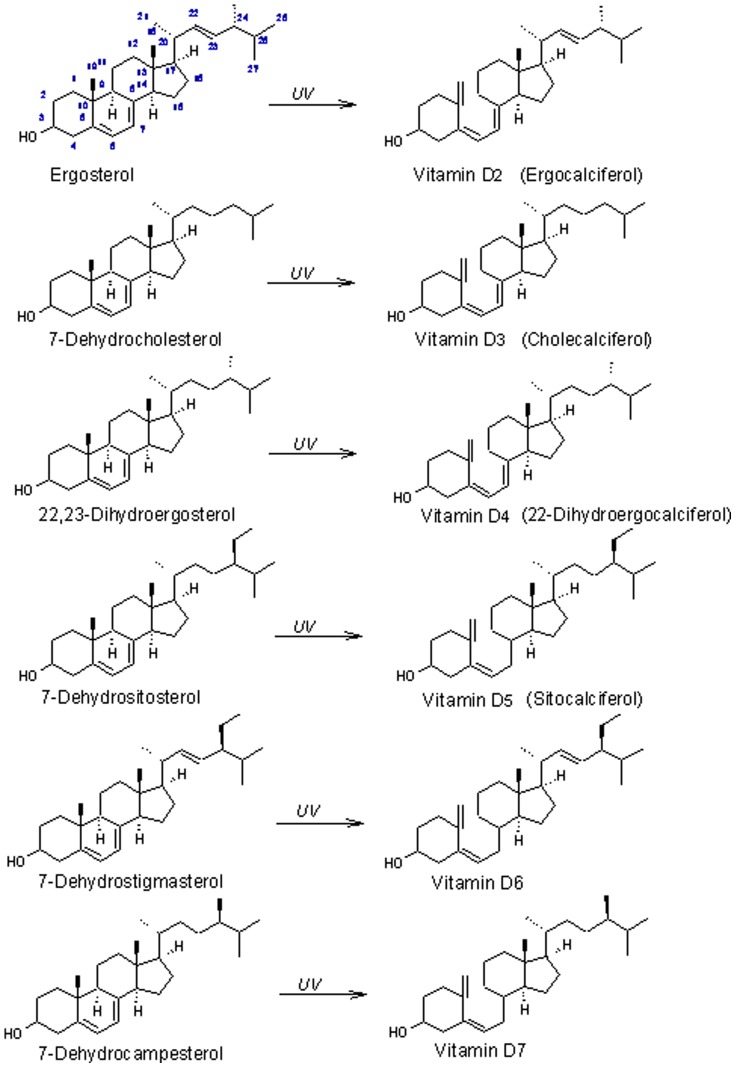
The conjugated unsaturation at C-5 and C-7 in the B-ring is the key structural feature of sterols that are converted to vitamin D by UV irradiation. Figure 5 shows the sterol precursors of vitamin D compounds, which differ in the side chain at C-24 and the C22–23 bond. Excellent reviews are available on the metabolism and physiology of vitamin D [5], [32], [33]. Overall there is very little published on the physiological significance of vitamers other than D3 or their occurrence in foods and other natural products aside from vitamin D2 in mushrooms. Vitamin D3 and D2 are metabolized in vivo to the biologically active forms, 1α,25-dihydroxyvitamin D3 and D2 [22], [34]. The bioavailability of vitamin D3 is well established, and the bioavailability of vitamin D2 from mushrooms in humans has been shown to be comparable to that of a vitamin D2 supplement [35], [36]. Forms other than D3 have shown lower biological activity in vitamin D dependent cellular functions in some studies. DeLuca et al. [37] synthesized 22,23-[3H]vitamin D4 and compared its metabolism to 22,23-[3H]vitamin D3 in the rat. Vitamin D4 metabolites had a tissue distribution similar to vitamin D3 but were excreted more quickly but also appear to have lower toxicity in high doses compared to D3 [38].
Figure 5. Structure of six forms of vitamin D their sterol precursors.
The lower potential toxicity of vitamin D compounds other than D3 has spurred interest in their development as vitamin D analogs for use as potential pharmaceutical agents. The synthetic derivative of vitamin D5, 1α-hydroxyvitamin D5, has shown anti-tumor activity and been studied as an anti-cancer treatment [39], [40], [41]. Tachibana and Tsuji [42] found the metabolism of 1α,25-dihydroxyvitamin D4 to be similar to that of 1α,25-dihydroxyvitamin D2 in a study involving rats. Jones [43] has written an excellent review on vitamin D analogs, their pharmaceutical applications, and potential mechanisms of action.
Knowledge of the occurrence of lesser known forms of vitamin D and their sterol precursors, particularly in foods, herbal medicines, and materials that may be sources of these compounds is therefore valuable, given the potential value of vitamin D compounds. Some other organisms in which 22,23-dihydroergosterol (ergosta-5,7-dienol; 22-dihydroergosterol) has been reported include Chlorella species [44] and various yeasts and fungi [15], [45]. It has been found in Mucor pusillus [46], a source of a milk curdling protease used in cheese production. Interestingly, anobiid beetles have been shown to synthesize cholesterol from 22-dihydroergosterol supplied by symbiotic yeast, with 7-dehydrocholesterol (the precursor of vitamin D3) as the intermediate [47]. 22,23-dihydroergosterol and also 7-dehydrostigmasterol (another Δ5,7-sterol) and the precursor of vitamin D6 (Fig. 1) have been reported in Trypanosoma cruzi, the organism responsible for Chagas disease [48]. Vitamin D5 is the product of UV irradiation of 7-dehydrositosterol (Fig. 5). 7-dehydrositosterol has been reported in Rauwolfia serpentina (snakeroot), a plant commonly used in Chinese herbal medicine [49] and also in algae [50]. 7-dehydrocampesterol, the C-24 epimer of 22,23-dihydroergosterol [51] and the precursor to vitamin D7, has been found in Crithidia fasciculate [48] and in Helianthus annuus (sunflower) seed oil [52].
Because the vitamin D4 precursor 22,23-dihydroergosterol occurred in all mushrooms analyzed and vitamin D4 was found in approximately half of the samples overall and in all mushrooms with know UV exposure, its presence should be expected in mushrooms exposed to UV light in the commercial production of vitamin D enhanced products, or in wild grown or other mushrooms receiving incidental UV exposure.
Wide variability in the occurrence and vitamin D4 concentration in this relatively large sampling of mushrooms also suggests that the common practice of using vitamin D3 as an internal standard in the HPLC analysis of vitamin D2 in mushrooms will result in errors unless the separation of vitamins D3 and D4 by the chromatographic system is assured.
Further study of the biological activity of vitamin D4 is warranted, given its presence in many commonly consumed mushrooms.
Acknowledgments
The authors would like to thank Mr. Vic Parcell at the University of Iowa High Resolution Mass Spectrometry Facility for his technical expertise.
Funding Statement
This work was supported by the United States Department of Agriculture Agricultural Research Service as part of the National Food and Nutrient Analysis Program, by cooperative agreement 59-1235-7-146 between the USDA Nutrient Data Laboratory and Virginia Tech. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bills CE (1938) The chemistry of vitamin D. J Am Med Assoc. 110: 2150–2155. [Google Scholar]
- 2.Chen TC, Lu Z, Holick MF (2010) Photobiology of vitamin D. In: Holick MF, editor. Vitamin D physiology, molecular biology, and clinical applications, 2nd edition. New York: Springer. 35–60. [Google Scholar]
- 3.Hirsch AL (2011) Industrial aspects of vitamin D. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D, 3rd edition. San Diego: Academic Press. 73–93. [Google Scholar]
- 4. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84: 18–28. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF (2010) editor (2010) Vitamin D physiology, molecular biology, and clinical applications, 2nd edition. New York: Springer. 1048 p. [Google Scholar]
- 6.Ross AC, Taylor CL, Yaktine AL, Del Valle HB (2010) editors (2010) Dietary reference intakes for vitamin D and calcium. Washington DC: Institute of Medicine of the National Academies. Available: http://www.iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/DRI-Values.aspx. Accessed 2011 Jul 25. [Google Scholar]
- 7. Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, et al. (2010) Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 91: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 8. Zhao X-Y, Feldman D (2001) The role of vitamin D in prostate cancer. Steroids 66: 293–300. [DOI] [PubMed] [Google Scholar]
- 9. Jasinghe VJ, Perera CO (2006) Ultraviolet irradiation: the generator of vitamin D2 in edible mushrooms. Food Chem 95: 638–643. [Google Scholar]
- 10. Koyyalamudi SR, Jeong S-C, Song C-H, Cho KY, Pang G (2009) Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation. J Agric Food Chem 57: 3351–3355. [DOI] [PubMed] [Google Scholar]
- 11. Koyyalamudi SR, Jeong SC, Pang G, Teal A, Biggs T (2011) Concentration of vitamin D2 in white button mushrooms (Agaricus bisporus) exposed to pulsed UV light. J Food Comp Anal 24: 976–979. [Google Scholar]
- 12. Roberts JS, Teichert A, McHugh TH (2008) Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J Agric Food Chem 56: 4541–4544. [DOI] [PubMed] [Google Scholar]
- 13. Teichmann A, Dutta PC, Staffas A, Jagerstäd M (2007) Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: effect of uv radiation. LWT – Food Sci Technol 40: 815–822. [Google Scholar]
- 14. Phillips KM, Ruggio DM, Horst RL, Minor B, Simon R, et al. (2011) Vitamin D and sterol composition of ten types of mushrooms from retail suppliers in the United States. J Agric Food Chem 59: 7841–7853. [DOI] [PubMed] [Google Scholar]
- 15. Weete JD, Abril M, Blackwell M (2010) Phylogenetic distribution of fungal sterols. PLoS One. Available: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0010899. Accessed 2011 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mattila PH, Piironen VI, Uusi-Rauva EJ, Koivistoinen PE (1994) Vitamin D contents in edible mushrooms. J Agric Food Chem 42: 2449–2453. [Google Scholar]
- 17. Mattila P, Könkö K, Eurola M, Pihlava J-M, Astola J, et al. (2001) Contents of vitamins, mineral elements and some phenolic compounds in cultivated mushrooms. J Agric Food Chem 49: 2343–2348. [DOI] [PubMed] [Google Scholar]
- 18. Mattila P, Lampi A-M, Ronkainen R, Toivo J, Piironen V (2007) Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chem 76: 293–298. [Google Scholar]
- 19. Rangel-Castro JI, Staffas A, Danell E (2001) The ergocalciferol content of dried pigmented and albino Cantharellus cibarius fruit bodies. Mycol Res 106: 70–73. [Google Scholar]
- 20. Aburjai T, Al-Khalil S, Abuirjeie M (1998) Vitamin D3 and its metabolites in tomato, potato, eggplant and zucchini leaves. Phytochem 49: 2497–2499. [Google Scholar]
- 21. Boland R, Skliar M, Curino A, Milanesi L (2003) Vitamin D compounds in plants. Plant Sci 164: 357–369. [Google Scholar]
- 22. Byford V, Strugnell S, Coldwell R, Schroeder N, Makin HLJ, et al. (2002) Use of vitamin D4 analogues to investigate differences in hepatic and target cell metabolism of vitamins D2 and D3 . Biochim Biophys Acta 1583: 151–166. [DOI] [PubMed] [Google Scholar]
- 23. Horst RL, Reinhardt TA, Russell R, Napoli JL (1984) The isolation and identification of vitamin D2 and vitamin D3 from Medicago sativa (alfalfa plant). Arch Biochem Biophys 231: 67–71. [DOI] [PubMed] [Google Scholar]
- 24. Brooks CJW, Horning EC, Young JS (1968) Characterization of sterols by gas chromatography-mass spectrometry of the trimethylsilyl ethers. Lipids 3: 391–402. [DOI] [PubMed] [Google Scholar]
- 25. Kenny PTM, Wetzel JM (1995) Fragmentation studies of ergosterol. The formation of the fragment ion at m/z 337. Eur Mass Spectrom 1: 411–413. [Google Scholar]
- 26. San Diego Gas & Electric (2009) Fickle fungi flourish in energy-sparing indoor farm. Progress Through Design Summer. 2–3. [Google Scholar]
- 27. Horwitz W, Kamps LR, Boyer DW (1980) Quality assurance in the analysis of foods for trace constituents. J Assoc Off Anal Chem Int 63: 1344–1354. [PubMed] [Google Scholar]
- 28. Wang T, Bengtsson G, Kärnefelt I, Björn LO (2001) Provitamins and vitamins D2 and D3 in Cladina spp. over a latitudinal gradient: possible correlation with UV levels. J Photochem Photobiol B: Biol 62: 118–122. [DOI] [PubMed] [Google Scholar]
- 29. Simon RR, Phillips KM, Horst RL, Munro IC (2011) Vitamin D mushrooms: comparison of the composition of button mushrooms (Agaricus bisporus) treated post harvest with UVB light or sunlight. J Agric Food Chem 59: 8724–1832. [DOI] [PubMed] [Google Scholar]
- 30. Moss GP (1989) Nomenclature of steroids. Pure and Applied Chem 61: 1783–1822. [Google Scholar]
- 31. Shao S, Hernandez M, Kramer JKG, Rinker DL, Tsao R (2010) Ergosterol profiles, fatty acid composition, and antioxidant activities of button mushrooms as affected by tissue part and developmental stage. J Agric Food Chem 58: 11616–11625. [DOI] [PubMed] [Google Scholar]
- 32.Feldman D, Malloy PJ, Krishnan AV, Balin E (2008) Vitamin D: biology, action, and clinical implications. In: Marcus R, Feldman D, Rosen CJ, Nelson D, editors. Osteoporosis, 3rd edition, Burlington, MA: Elsevier Academic Press. 317–382. [Google Scholar]
- 33.Horst RL, Reinhardt TA, Reddy SG (2005) Vitamin D metabolism. In: Pike JW, Glorieux FH, Feldman D, editors. Vitamin D, 2nd edition. San Diego: Academic Press. 15–36. [Google Scholar]
- 34. De Luca HF (2004) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 80: 1689S–96S. [DOI] [PubMed] [Google Scholar]
- 35. Outila TA, Mattila PH, Piironen VI, Lamberg-Allardt CJE (1999) Bioavailability of vitamin D from wild edible mushrooms (Cantharellus tubaeformis) as measured with a human bioassay. Am J Clin Nutr 69: 95–98. [DOI] [PubMed] [Google Scholar]
- 36. Urbain P, Biesalski HK, Bertz H (2010) Bioavailability of vitamin D2 from UVB-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D (24OHD): a randomized-controlled trial. Abstracts of the 32nd ESPEN Congress, Clin Nutr. Suppl. 5 15. [DOI] [PubMed] [Google Scholar]
- 37. De Luca HF, Weller M, Blunt JW, Neville PF (1968) Synthesis, biological activity, and metabolism of 22,23−3H-vitamin D4 . Arch Biochem Biophys 124: 122–128. [DOI] [PubMed] [Google Scholar]
- 38. Napoli JL, Fivizzani MA, Schnoes HK, Deluca HF (1979) Synthesis of vitamin D5: its biological activity relative to vitamins D3 and D2 . Arch Biochem Biophys 197: 119–125. [DOI] [PubMed] [Google Scholar]
- 39. Mehta RG, Moriarty RM, Mehta RR, Penmasta R, Lazzaro G (1997) Prevention of preneoplastic mammary lesion development by a novel vitamin D analogue, 1alpha-hydroxyvitamin D5 . J Natl Cancer Inst 89: 212–218. [DOI] [PubMed] [Google Scholar]
- 40. Mehta RG (2004) Stage-specific inhibition of mammary carcinogenesis by 1alpha-hydroxyvitamin D5 . Eur J Cancer 40: 2331–2337. [DOI] [PubMed] [Google Scholar]
- 41. Murillo G, Mehta RG (2005) Chemoprevention of chemically-induced mammary and colon carcinogenesis by 1alpha-hydroxyvitamin D5 . J Steroid Biochem Mol Biol 97: 129–136. [DOI] [PubMed] [Google Scholar]
- 42. Tachibana Y, Tsuji M (2001) Study on the metabolites of 1α,25-dihydroxyvitamin D4 . Steroids 66: 93–97. [DOI] [PubMed] [Google Scholar]
- 43. Jones G (2010) Vitamin D analogs. Endocrinol Metab Clin North Am 39: 447–472. [DOI] [PubMed] [Google Scholar]
- 44. Patterson GW (1969) Sterols of Chlorella–III. Species containing ergosterol. Comp Biochem Physiol 31: 391–394. [Google Scholar]
- 45. McCorkindale NJ, Hutchinson SA, Pursey BA, Scott WT, Wheeler R (1969) A comparison of the types of sterol found in species of the Saprolegniales and Leptomitales with those found in some other Phycomycetes. Phytochem 8: 861–867. [Google Scholar]
- 46. Mercer EI, Carrier DJR (1976) Ergosterol biosynthesis in Mucor pusillus . Phytochem 15: 283–286. [Google Scholar]
- 47. Nasir H, Noda H (2003) Yeast-like symbiotes as a sterol source in anobiid beetles (Coleoptera, Anobiidae): possible metabolic pathways from fungal sterols to 7-dehydrocholesterol. Arch Insect Biochem Physiol 52: 175–182. [DOI] [PubMed] [Google Scholar]
- 48. Korn ED, Von Brand T, Tobie EJ (1969) The sterols of Trypanosoma cruzi and Crithidia fasciculata . Comp Biochem Physiol 30: 601–610. [DOI] [PubMed] [Google Scholar]
- 49. Karmakar T, Chakraborty DP (1983) 7-dehydrositosterol from Rauwolfia serpentina. . Phytochem 22: 608–609. [Google Scholar]
- 50. Seckbach J, Ikan R (1972) Sterols and chloroplast structure of Cyanidium caldarium . Plant Physiol 49: 457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thompson MJ, Dutky SR, Patterson GW, Gooden EL (1972) NMR spectra of C-24 isomeric sterols. Phytochem 11: 1781–1790. [Google Scholar]
- 52. Matsumoto T, Nakagawa M, Itoh T (1984) 24α-methyl-5α-cholest-7-en-3β-ol from seed oil of Helianthus annuus. . Phytochem 21: 921–923. [Google Scholar]
- 53.U.S. Department of Agriculture, Agricultural Research Service (2011) USDA National Nutrient Database for Standard Reference, Release 24. Beltsville, MD: United States Department of Agriculture Agricultural Research Service. Available: http://www.ars.usda.gov/ba/bhnrc/ndl. Accessed 2011 Jul 1.