Abstract
An increasing amount of evidence supports pleiotropic metabolic roles of the cannibinoid-1 receptor (CB1R) in peripheral tissues such as adipose, liver, skeletal muscle and pancreas. To further understand the metabolic consequences of specific blockade of CB1R function in peripheral tissues, we performed a 10-week-study with an anti-sense oligonucleotide directed against the CB1R in diet-induced obese (DIO) AKR/J mice. DIO AKR/J mice were treated with CB1R ASO Isis-414930 (6.25, 12.5 and 25 mg/kg/week) or control ASO Isis-141923 (25 mg/kg/week) via intraperitoneal injection for 10 weeks. At the end of the treatment, CB1R mRNA from the 25 mg/kg/week CB1R ASO group in the epididymal fat and kidney was decreased by 81% and 63%, respectively. Body weight gain was decreased in a dose-dependent fashion, significantly different in the 25 mg/kg/week CB1R ASO group (46.1±1.0 g vs veh, 51.2±0.9 g, p<0.05). Body fat mass was reduced in parallel with attenuated body weight gain. CB1R ASO treatment led to decreased fed glucose level (at week 8, 25 mg/kg/week group, 145±4 mg/dL vs veh, 195±10 mg/dL, p<0.05). Moreover, CB1R ASO treatment dose-dependently improved glucose excursion during an oral glucose tolerance test, whereas control ASO exerted no effect. Liver steatosis was also decreased upon CB1R ASO treatment. At the end of the study, plasma insulin and leptin levels were significantly reduced by 25 mg/kg/week CB1R ASO treatment. SREBP1 mRNA expression was decreased in both epididymal fat and liver. G6PC and fatty acid translocase/CD36 mRNA levels were also reduced in the liver. In summary, CB1R ASO treatment in DIO AKR/J mice led to improved insulin sensitivity and glucose homeostasis. The beneficial effects of CB1R ASO treatment strongly support the notion that selective inhibition of the peripheral CB1R, without blockade of central CB1R, may serve as an effective approach for treating type II diabetes, obesity and the metabolic syndrome.
Introduction
It has been well established that the endocannabinoid system consisting of CB1R and CB2R and their endogenous ligands (anandamide and 2-arachidonoylglycerol) play a significant role in regulating multiple metabolic pathways [1], [2], [3]. Initially, it was believed that CB1 receptor was predominantly localized in the central nervous system, while CB2 receptor was mainly expressed in peripheral cells and tissues from the immune system. Recently, CB1 receptors were also found in peripheral tissues such as adipose, liver, gastrointestinal tract (e.g., vagal afferent neurons, ileum longitudinal smooth muscle), skeletal muscle, and pancreas [4], [5], [6], [7], [8], [9]. Activation of CB1 receptors triggers many physiological processes, both centrally and peripherally [10], [11], [12]. CB1 receptors in the hypothalamus play a key role in food intake and energy homeostasis [13], [14]. Early work by Di Marzo et al demonstrated that defective leptin signaling pathway was associated with elevated endocannibinoids level in the hypothalamus which in turn over-stimulated CB1 receptors and increased food intake [14]. Moreover, overactivation of the endocannabinoid system in peripheral tissues such as adipose, pancreas and liver has been linked to obesity and the metabolic syndrome in both obese animals [15], [16] and humans [15], [17], [18], [19]. In recent years, emerging evidence has supported the notion that blockade of CB1 receptors with antagonists in peripheral tissues may provide sufficient metabolic benefits in feeding through gut-brain signaling [20], [21], [22], adipose tissue metabolism [23], [24], hepatic lipogenesis [23], glucose homeostasis, insulin release in the pancreas [8], [25], [26], cholesterol metabolism in macrophages [27] and metabolic control in skeletal muscle [28]. Since CB1 receptors are detected in many other central nervous regions influencing key functions, such as mood, motor coordination, and cognition [29], [30], administration of centrally penetrant CB1 receptor antagonists such as rimonabant has been associated with psychiatric risks [10], [11]. Therefore, targeting CB1 receptors in peripheral tissues has emerged to be a promising therapeutic approach to treat obesity, diabetes and the metabolic syndrome (for review, see [31]). To this end, we utilized the anti-sense oligonucleotide approach to evaluate the metabolic effects upon blockade of peripheral CB1R in diet-induced obesity AKR/J mouse model.
Methods
CB1R ASO and ASO Control
CB1R-ASO used in this study was Isis-414930; scrambled control ASO was Isis-141923. To identify mouse CB1R ASO inhibitors, rapid throughput screens were performed in vitro and several potent and specific ASOs were identified, all of which targeted a binding site within the coding region of the CB1R. After extensive dose response characterization, the most potent ASO from the screen was chosen: ISIS-414930, with the following sequence: 5- AGGTAGCTTAACGCACACAT -3. The control ASO, ISIS-141923, has the following sequence, 5 -CCTTCCCTGAAGGTTCCTCC-3, and does not have perfect complementarily to any known gene in public data bases. All ASOs were made in saline at appropriate concentrations.
10-week Study with DIO Male AKR/J Mice
All of the procedures for animal studies were approved by the Janssen Pharmaceutical Companies Institutional Animal Care and Use Committee. Food and water were supplied ad libitum. Room temperature was maintained at 68–72 F and humidity at 50–65%. Room lighting was on a 12-h light/12-h dark cycle. Male AKR/J mice from the Jackson Lab were single-housed and fed D12451 (45% high fat, Research Diets, New Brunswick, NJ) at 7–8-week old. At the initiation of study, mice were on D12451 for 10-week and at the age of 17–18-week. Age-matched lean mice were fed with standard rodent diet (LabDiet 5001, PMI Nutrition Int’l, St. Louis, MO). Mice (n = 10 per group) were treated subcutaneously with CB1R ASO (Isis-414930) and control ASO Isis-141923 dissolved in saline twice a week via i.p. injection for 10 weeks. For CB1R ASO, the mice were treated at 6.25, 12.5 or 25 mg/kg/week. Such dosing scheme provides sustained coverage based on our experience with these ASOs (data not shown). For scrambled control ASO, the mice were treated at 25 mg/kg/week. Body weight and food intake was monitored weekly. Fed blood glucose was obtained in the postprandial state at 9∶00–10∶00 AM. Body composition was assessed by mouse whole body Bruker magnetic resonance analyzer (Woodlands, TX). Oral glucose tolerance test (OGTT) was performed during week 9. For OGTT, all mice were fasted overnight and orally dosed with 2 g/kg glucose (20% glucose at 10 mL/kg). Blood glucose was measured at 0, 30, 60, 120 min after glucose dosing. Necropsy was performed during week 10. The mice were treated with the last dose 18-hour prior to the necropsy. Food was removed for 4-hour prior to the necropsy. Retro-orbital bleeding after 70% CO2/30% O2 anesthesia was performed to collect plasma for determination of triglycerides, total cholesterol, adiponectin, insulin, and leptin. Liver, spleen, epididymal fat, and kidney were dissected for total weight and/or gene expression analysis. Plasma glucose, triglycerides and total cholesterol levels were analyzed on Cobas Mira clinical chemistry analyzer (Roche Molecular Diagnostics, Pleasanton, CA). Clinical chemistry parameters such as creatinine, blood urea nitrogen (BUN), lipase, alanine aminotransferase (ALT), aspartate aminotransferase (AST) were also measured on Cobas Mira clinical chemistry analyzer. Total adiponectin was measured using mouse total adiponectin Elisa kit (Alpco Diagnostics, Salem, NH). Leptin was measured using mouse leptin ELISA kit Insulin was measured with HTRF insulin assay (Cis-bio, Bedford, MA). Mouse cytokine kit MADPK-71 K from Millipore was used to measure plasma total plasminogen activator inhibitor 1 (PAI-1), TNF alpha and IL-6 levels on Luminex 200™ (Millipore, Billerica, MA). For liver TG determination, liver was homogenized and lipid was extracted. Liver TG was then determined with WAKO L-type TG-M assay (WAKO, Richmond, VA) and normalized with tissue weight.
Real-Time Quantitative PCR Analysis of Gene Expression
RNA was extracted from tissues using Qiazol lysis buffer according to the manufacturer’s instructions (Qiagen, Valencia, CA). Real-time quantitative PCR reactions were carried out using the TaqMan Gene Expression Assay Universal PCR Master Mix and an ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA). Comparative CT method (ΔΔCT method) was used following the instructions of the manufacturer (Applied Biosystems). Briefly, average CT value from three reactions for a particular gene mRNA of each animal was normalized to the level of its own beta actin to generate ΔCT, followed by ΔΔCT calculation (ΔΔCT = ΔCT-average ΔCT of vehicle group). Fold change of a particular gene mRNA of each animal from that of vehicle group was then obtained by calculating 2−ΔΔC T. Subsequently, an average of fold change and standard error were derived. All primers were purchased from Applied Biosystems (Mm00432621_s1 for CB1R, Mm00438286_m1 for CB2R, Mm01138344_m1 for SREBP1, Mm00839363_m1 for G6PC, Mm00432403_m1 for CD36).
Statistical Analysis
Statistic analysis was performed using Graphpad Prism (Monrovia, CA, USA) with either one-way ANOVA Dunnett’s multiple comparison test or student t-test two-tailed distribution homoscedastic analysis.
Results
CB1R ASO Treatment Decreased CB1R mRNA Levels in WAT and Kidney of DIO AKR/J Mice
In the current study, we treated DIO AKR/J mice with a specific and potent CB1R ASO Isis-414930 at 6.25, 12.5 and 25 mg/kg/week for about ten-week. Control ASO was administered at 25 mg/kg/week. Necropsy was performed 18-hr after the last dose of ASO. Liver, kidney and epididymal fat was dissected and extracted for RNA. The RNA was used to prepare cDNA by reverse transcription. Real-time quantitative RT-PCR (TaqMan) analysis demonstrated 81% and 63% reduction of CB1R mRNA in epididymal fat and kidney, respectively, in the 25 mg/kg/week group (Table 1). 12.5 mg/kg/week CB1R ASO treatment also significantly decreased CB1R mRNA in the epididymal fat (57% reduction), but not in the kidney. We detected very low level of CB1R mRNA in the liver and could not obtain any meaningful comparison between vehicle and CB1R ASO treated groups (data not shown). We also determined CB2R mRNA levels in epididymal fat and kidney and there were no significant changes (Table 1), confirming specificity of CB1R ASO.
Table 1. Relative CB1R and CB2R mRNA level after 10-week ASO Treatment.
| Treatment | CB1R | CB2R | ||
| Epididymal fat | Kidney | Epididymal fat | Kidney | |
| DIO Veh | 1.16±0.25 | 1.08±0.18 | 0.91±0.10 | 1.03±0.10 |
| CB1R ASO 25 mg/kg/wk | 0.19±0.11* | 0.37±0.02* | 1.24±0.11 | 1.34±0.10 |
| CB1R ASO 12.5 mg/kg/wk | 0.43±0.16* | 0.72±0.09 | 0.99±0.12 | 1.31±0.13 |
| CB1R ASO 6.25 mg/kg/wk | 0.88±0.29 | 0.66±0.04 | 0.84±0.10 | 1.32±0.10 |
| Control ASO 25 mg/kg/wk | 0.75±0.23 | 0.94±0.16 | 1.21±0.10 | 1.27±0.11 |
Values shown are fold change from DIO veh group for the same gene and tissue. Student t-test, compared to DIO Veh, *p<0.05.
CB1R ASO Treatment Dose-dependently Reduced Body Weight Gain
The effects of CB1R ASO on body weight and food intake were observed after about 4-week treatment in a dose-dependent manner (Table 2). For veh-treated group, about 10% body gain was obtained during the study, while CB1R ASO 25 mg/kg/week group lost 2–3% of the starting body weight. The decreased body weight gain occurred concurrently with food intake decrease (Table 3). For instance, during week 4, food intake of CB1R ASO 25 mg/kg/week group was 20.0±0.6 g, compared to 22.3±0.6 g of veh-treated group, p<0.05. The extent of food intake reduction maintained to the end of the study. Neither body weight gain nor food intake was affected in control ASO treated group. Body fat mass was also evaluated on day 1 and the end of the study. CB1R ASO treatment produced a dose-dependent fat mass reduction with only 25 mg/kg/week group achieving statistic significance (Table 4).
Table 2. A. Body weight during the treatment.
| A | BW (g) | ||||||||
| Treatment | Day 1 | Day 12 | Day 19 | Day 26 | Day 33 | Day 40 | Day 47 | Day 54 | Day 61 |
| Lean Veh | 35.8±0.3* | 35.2±0.4* | 35.5±0.4* | 36.1±0.4* | 36.0±0.4* | 36.0±0.5* | 35.5±0.4* | 37.4±0.3* | 36.3±0.4* |
| DIO Veh | 46.9±1.0 | 46.8±1.0 | 46.6±1.6 | 48.0±1.1 | 47.6±1.1 | 49.9±1.1 | 49.9±0.9 | 52.0±0.8 | 51.2±0.9 |
| CB1 ASO 25mg/kg/wk | 47.3±0.9 | 47.5±0.9 | 47.6±0.9 | 47.1±1.0 | 46.5±1.0 | 46.6±0.8* | 45.9±1.0* | 47.3±0.9* | 46.1±1.0* |
| CB1 ASO 12.5mg/kg/wk | 47.9±1.2 | 47.9±1.3 | 48.0±1.3 | 48.2±1.4 | 48.0±1.5 | 48.2±1.7 | 48.3±1.6 | 50.4±1.5 | 49.4±1.4 |
| CB1 ASO 6.25mg/kg/wk | 47.6±1.1 | 47.4±1.3 | 48.6±1.1 | 48.6±1.1 | 48.9±1.2 | 49.2±1.2 | 49.2±1.1 | 50.9±1.2 | 49.1±1.3 |
| Control ASO 25mg/kg/wk | 47.3±1.0 | 47.3±1.0 | 47.8±1.0 | 48.4±1.1 | 48.8±1.2 | 49.3±1.2 | 49.9±1.2 | 51.9±1.2 | 50.5±1.3 |
| B | BW (% of starting) | ||||||||
| Treatment | Day 1 | Day 12 | Day 19 | Day 26 | Day 33 | Day 40 | Day 47 | Day 54 | Day 61 |
| DIO Veh | 100±2.2 | 99.9±2.0 | 99.4±3.5 | 102.4±2.4 | 101.4±2.4 | 106.5±2.2 | 106.5±1.8 | 111.0±1.8 | 109.2±1.9 |
| CB1 ASO 25mg/kg/wk | 100±1.8 | 100.4±1.9 | 100.7±1.9 | 99.5±2.1 * | 98.3±2.0* | 98.5±1.7* | 97.0±2.1 * | 100.1±2.0* | 97.6±2.0* |
| CB1 ASO 12.5mg/kg/wk | 100±2.5 | 100±2.7 | 100.1±2.7 | 100.6±2.9 | 100.0±3.1 | 100.5±3.5 | 100.7±3.4 | 105.0±±3.2 | 102.9±2.9 |
| CB1 ASO 6.25mg/kg/wk | 100±2.4 | 99.5±2.8 | 100.2±2.2 | 102.1±2.4 | 102.6±2.5 | 103.4±2.4 | 103.3±2.4 | 106.9±2.4 | 103.3±2.8 |
| Control ASO 25mg/kg/wk | 100±2.0 | 100±2.1 | 101.0±2.1 | 102.3±2.3 | 103.2±2.5 | 104.3±2.5 | 105.4±2.6 | 109.8±2.5 | 106.8±2.7 |
B. Body weight change (% of the beginning body weight) during the treatment. One way ANOVA Dunnett’s multiple test compared to DIO Veh, *p<0.05.
Table 3. Food intake during the study.
| Weekly FI (g, mean ± se) | ||||||||
| Treatment | Wk 1&2 | Wk 3 | Wk 4 | Wk 5 | Wk 6 | Wk 7 | Wk 8 | Wk 9 |
| DIO Veh | 23.7±0.5 | 23.4±0.6 | 22.3±0.6 | 22.9±0.5 | 22.6±0.3 | 25.1±0.7 | 22.3±0.4 | 22.0±0.5 |
| CB1 ASO25 mg/kg/wk | 22.7±0.6 | 21.1±0.6 | 20.0±0.6* | 20.5±0.6 | 19.7±0.5* | 22.3±0.6* | 19.5±0.4* | 19.4±0.4* |
| CB1 ASO12.5 mg/kg/wk | 23.6±0.6 | 22.7±0.6 | 22.2±0.6 | 21.7±0.9 | 21.5±1.0 | 24.3±0.7 | 21.7±0.7 | 21.1±0.6 |
| CB1 ASO6.25 mg/kg/wk | 24.6±0.6 | 21.9±1.1 | 22.2±0.5 | 22.6±0.7 | 22.3±0.4 | 24.4±0.6 | 21.0±0.5 | 21.5±0.4 |
| Control ASO25 mg/kg/wk | 24.2±0.6 | 23.4±0.5 | 22.8±0.5 | 23.4±0.6 | 22.1±0.5 | 26.8±0.7 | 22.7±0.6 | 21.9±0.6 |
One way ANOVA Dunnett’s multiple test compared to DIO Veh, *p<0.05.
Table 4. Fat mass change before and after 10-week treatment.
| Treatment | Fat (Day 1, % of total body mass,mean ± se) | Fat (Week 10, % of total body mass,mean ± se) | Fat Mass % change(mean ± se) |
| Lean Veh | 19.3±0.6* | 20.6±0.5* | 1.4±1.0 |
| DIO Veh | 37.7±0.6 | 38.6±0.3 | 0.2±0.6 |
| CB1R ASO 25 mg/kg/wk | 38.8±0.6 | 34.0±0.7* | −4.5±0.8* |
| CB1R ASO 12.5 mg/kg/wk | 39.3±0.5 | 37.5±0.5 | −1.8±0.3 |
| CB1R ASO 6.25 mg/kg/wk | 39.4±0.8 | 38. 5±0.5 | −1.0±1.1 |
| Control ASO 25 mg/kg/wk | 39.9±0.6 | 40.2±0.5 | 0.3±0.7 |
One way ANOVA Dunnett’s multiple test compared to DIO Veh, *p<0.05.
CB1R ASO Treatment Improved Insulin Sensitivity and Glucose Homeostasis and Ameliorated Liver Steatosis
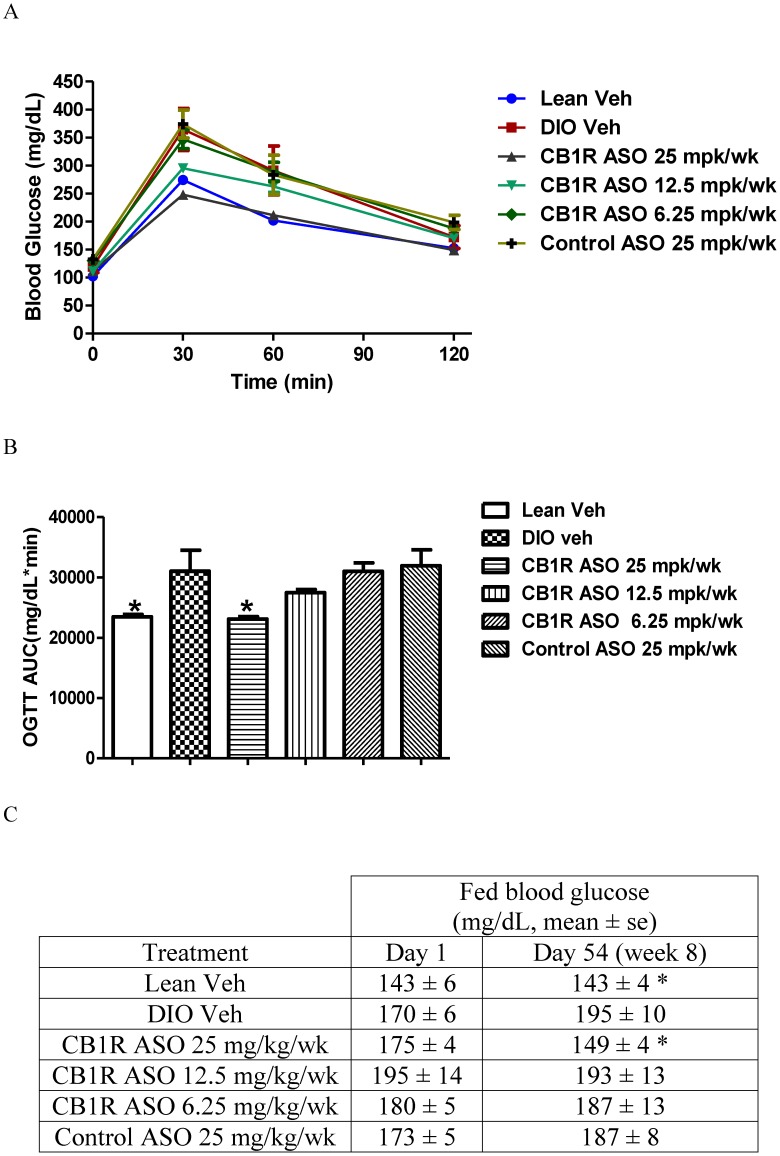
Ad lib fed blood glucose was measured on day 54 (Figure 1). CB1R ASO treatment at 25 mg/kg/week reduced blood glucose from 195±10 mg/dL (veh-treated) to 149±4 mg/dL (p<0.05), while age-matched lean control group was at 143±4 mg/dL. Control ASO at 25 mg/kg/week and CB1R ASO at 12.5 and 6.25 mg/kg/week did not affect ad lib fed glucose level. Oral glucose tolerance was performed on day 64 (Figure 1). CB1R ASO 25 mg/kg/week treatment significantly improved glucose excursion to the level of age-matched lean control group (16±1% glucose AUC reduction for 25 mg/kg/week group from DIO veh group, p<0.05; lean control, 15±1% reduction from DIO veh, p<0.05). CB1R ASO 12.5 mg/kg/week administration tended to decrease glucose AUC, however to a lesser extent, 12±1% reduction from DIO veh group. Consistent with findings from OGTT, insulin level measured at the end of the study (week 10) clearly indicated the improved insulin sensitivity upon CB1R ASO treatment (Table 5). After CB1R ASO 25 mg/kg/week treatment, insulin level was normalized from 2.92±0.46 ng/mL to 0.69±0.09 ng/mL, a level very close to that of lean mice (0.51±0.08 ng/mL, despite the fact that body weight of CB1R ASO 25 mg/kg/week group was significantly higher than that of lean control group (46.1±1.0 g vs lean 36.3±0.4 g). Therefore, CB1R ASO treatment produced body-weight independent improvement in insulin sensitivity and glucose homeostasis. Liver TG was also determined at the end of the study (Table 6). CB1R ASO treatment led to dose-dependent reduction of liver TG content which may have in turn improved, at least in part, insulin sensitivity and glucose homeostasis.
Figure 1. Oral glucose tolerance test (A and B).
On Day 64, overnight fasted mice were challenged with oral glucose dosing at 2 g/kg. Blood glucose was measured at 0, 30, 60 and 120 minute after glucose dosing. Glucose area under curve was also calculated. C. Fed blood glucose was measured on day 1 and day 54. Student t-test vs the vehicle group, *, P<0.05.
Table 5. Plasma insulin, adiponectin, leptin, TG and cholesterol levels at the end of treatment.
| Treatment | Insulin (ng/mL) | AdipQ (µg/mL) | Leptin (ng/mL) | TG (mg/dL) | Total cholesterol (mg/dL) |
| Lean | 0.51±0.08* | 24.0±1.3 | 7.21±0.51* | 273±15* | 92±2* |
| DIO Veh | 2.92±0.46 | 21.2±0.9 | 32.47±1.20 | 178±9 | 158±4 |
| CB1 ASO 25 mg/kg/wk | 0.69±0.09* | 25.9±1.1* | 21.95±1.88* | 189±18 | 111±4* |
| CB1 ASO 12.5 mg/kg/wk | 1.73±0.35 | 23.9±1.1 | 30.73±1.34 | 196±13 | 141±5* |
| CB1 ASO 6.25 mg/kg/wk | 2.04±0.38 | 21.4±1.0 | 30.93±1.73 | 193±21 | 147±6 |
| Control ASO 25 mg/kg/wk | 2.13±0.45 | 25.5±0.4* | 32.80±1.11 | 195±16 | 170±4 |
Values shown are mean ± se. One way ANOVA Dunnett’s multiple test compared to DIO Veh, *p<0.05.
Table 6. Liver, spleen and epididymal fat weights, liver TG and plasma total PAI-1 at the end of treatment.
| Treatment | Liver weight (g) | Liver TG (mg/g tissue) | Epididymal fat (g) | Spleen weight (g) | Plasma total PAI-1(pg/mL) |
| Lean Veh | 1.82±0.05* | 15±1* | 1.17±0.07* | 0.080±0.007 | 837±93* |
| DIO Veh | 2.39±0.16 | 87±5 | 2.17±0.21 | 0.087±0.004 | 1722±244 |
| CB1R ASO25 mg/kg/wk | 1.97±0.09* | 40±6* | 1.99±0.16 | 0.138±0.009* | 2765±327* |
| CB1R ASO12.5 mg/kg/wk | 2.26±0.12 | 71±8 | 1.94±0.22 | 0.121±0.024 | 2145±278 |
| CB1R ASO6.25 mg/kg/wk | 2.21±0.10 | 81±12 | 2.08±0.15 | 0.093±0.003 | 1357±96 |
| Control ASO25 mg/kg/wk | 2.06±0.11 | 69±4 | 2.18±0.13 | 0.076±0.004 | 1180±83 |
Values shown are mean ± se. One way ANOVA Dunnett’s multiple test compared to DIO Veh, *p<0.05.
Effects of CB1R ASO Treatment on Plasma Adiponectin, Leptin, TG, Total Cholesterol, Total PAI-1 and Tissue Weights
Consistent with reduced adiposity demonstrated by fat mass measurement with NMR, plasma leptin level was decreased upon CB1R ASO 25 mg/kg/week treatment, indicating reduced hyperleptinemia induced by high fat diet (Table 5). Total cholesterol level was decreased as well. However, plasma TG level was not changed. Plasma total adiponectin level was slightly increased upon CB1R ASO 25 mg/kg/week treatment (25.9±1.1 µg/mL vs. DIO veh group, 21.2±0.9 µg/mL, p<0.05). However, plasma adiponectin level of control ASO 25 mg/kg/week treatment group was similar to that of CB1R ASO 25 mg/kg/week group. In addition, CB1R ASO 25 mg/kg/week treatment led to reduction of liver weight which is consistent with improved liver steatosis (Table 6). Epididymal fat weight was not significantly altered upon CB1R ASO treatment (Table 6). Spleen weight was increased upon CB1R ASO 25 mg/kg/week treatment (Table 6). This phenomenon is often observed upon ASO treatment and related to induction of pro-inflammatory state. Therefore, we also measured plasma TNFα, IL-6 and total plasminogen activator inhibitor 1 (PAI-1) levels. There were no significant changes for plasma TNFα and IL-6 under the circumstances that levels of both cytokines in DIO veh group were at the very low end of the standard curves (data not shown). However, plasma total PAI-1 level was elevated which seemingly correlated to the spleen weight. Plasma PAI-1 level tends to be increased under inflammatory conditions and its production was shown to be increased by TNF-α [32], [33].
Reduced mRNA Levels for Genes Involved in Gluconeogenesis, De Novo Lipogenesis and Fatty Acid Transport
In order to further understand the mechanisms underlying improved glucose homeostasis and reduced hepatic steatosis, we examined the mRNA levels of several genes that play key roles in gluconeogenesis and lipogenesis in the liver and epididymal fat tissue. As shown in Table 7, for the master regulator of lipid synthesis SREBP-1, its mRNA level was significantly reduced in both liver and epididymal fat upon CB1R ASO 25 mg/kg/week treatment. mRNA level of glucose 6-phosphatase catalytic unit (G6PC) was also decreased in the liver. In addition, fatty acid translocase/CD36 was also reduced in liver, consistent with improved hepatic steatosis.
Table 7. mRNA levels of several genes in epididymal fat and liver at the end of the study.
| Tissue | Gene | DIO Veh | CB1R ASO25 mg/kg/week | CB1R ASO12.5 mg/kg/week | CB1R ASO6.25 mg/kg/week | Control ASO25 mg/kg/week |
| Liver | SREBP-1 | 1.02±0.06 | 0.74±0.06* | 0.73±0.08* | 0.90±0.05 | 0.95±0.11 |
| Liver | G6PC | 1.15±0.23 | 0.43±0.09* | 0.68±0.13 | 0.91±0.13 | 0.99±0.13 |
| Liver | CD36 | 1.20±0.30 | 0.20±0.02* | 0.44±0.10* | 0.86±0.13 | 0.53±0.13 |
| Epididymal fat | SREBP-1 | 1.03±0.10 | 0.57±0.11* | 0.73±0.13 | 0.83±0.15 | 0.75±0.13 |
Values shown are mean ± se. Student t- test, compared to DIO Veh, *p<0.05.
Clinical Chemistry Parameters Unchanged upon CB1 ASO Treatment
Plasma levels of creatinine, BUN, lipase and liver enzymes (ALT and AST) were measured at the end of the 10-week study to collectively monitor any treatment-induced adverse renal, pancreatic and hepatic effects. As shown in Table 8, there was no significant difference between lean veh and DIO veh groups with regard to plasma creatinine, BUN and lipase levels. ASO treatment did not lead to any changes in these parameters, either. For liver enzymes, both ALT and AST levels were elevated in DIO veh group compared to that of lean veh group, which is a general phenomenon related to high fat diet-induced steatosis. ALT level appeared to be reduced upon CB1 ASO 25 mg/kg/wk treatment. However, it’s unclear why the control ASO group exhibited reduced plasma ALT and AST levels. In summary, the overall well-being of the mice was not significantly affected by 10-week CB1R or control ASO treatment.
Table 8. Plasma creatinine, BUN, lipase, ALT and AST levels at the end of treatment.
| Treatment | Creatinine (mg/dL) | BUN (mg/dL) | Lipase (U/L) | ALT (U/L) | AST (U/L) |
| Lean Veh | 0.32±0.01 | 18.5±0.7 | 76±12 | 54±14* | 64±6* |
| DIO Veh | 0.35±0.01 | 16.1±0.9 | 86±2 | 125±11 | 106±12 |
| CB1 ASO 25 mg/kg/wk | 0.33±0.00 | 14.9±0.9 | 70±4 | 80±6* | 103±12 |
| CB1 ASO 12.5 mg/kg/wk | 0.36±0.02 | 17.5±1.5 | 89±5 | 122±10 | 138±32 |
| CB1 ASO 6.25 mg/kg/wk | 0.33±0.01 | 15.9±0.3 | 97±10 | 103±15 | 85±9 |
| Control ASO 25 mg/kg/wk | 0.47±0.13 | 17.1±0.3 | 90±2 | 73±7* | 71±5* |
Values shown are mean ± se. One way ANOVA Dunnett’s multiple test compared to DIO Veh, *p<0.05.
Discussion
Since anti-sense oligonucleotides do not cross the blood brain barrier, we utilized this approach to investigate the metabolic consequencies of inhibiting CB1R function in peripheral tissues. High-fat diet has been shown to increase plasma and/or tissue endocannaibinoid levels which over-stimulates CB1R in the peripheral tissues [15], [16], [34], leading to dysregulation of many metabolic pathways. Therefore, we performed the 10-week study with a specific CB1R ASO in diet-induced obese AKR/J mice. We demonstrated that CB1R ASO treatment improved glycemic control and reversed hepatic steatosis. CB1R was found to express at highest level in the CNS [30]. Although CB1R expression at the peripheral tissues was much lower than that of CNS, its functional relevance was well demonstrated (for review, see [1], [12], [23], [31] ). In the present study, the rank order of CB1R mRNA level in diet-induced obese AKR/J mice was adipose>kidney>liver. In both pre-clinical animal models and clinical trials, inhibition of CB1R by antagonists such as rimonabant led to initial weight loss caused by transient reduction in food consumption (for review, see [11], [12]). Although tolerance to anorectic effect of brain penetrant CB1R antagonists quickly developed, body weight loss was sustained [35], [36]. Therefore, the long-lasting metabolic effects of CB1R antagonists such as rimonabant cannot be attributed entirely to the reduced caloric intake and weight loss. Instead, an increase in energy expenditure and improvement in lipid metabolism and glucose homeostasis may have occurred in peripheral tissues. Here we observed approximately 10% body weight gain reduction after 4–5 week treatment with CB1R ASO 25 mg/kg/week which sustained to the end of the study. In parallel, there was a small reduction in food intake. Beyond the orexigenic effects CB1R plays in the hypothalamus, CB1R also co-expresses with satiety hormone cholecystokinin in vagal afferent neurons and may influence food intake by mediating satiety signaling in the gut [20]. Similar to findings in DIO mice treated with CB1R antagonists such as rimonabant, body weight gain reduction upon ASO treatment was correlated with fat mass decrease which may be derived from increased lipolysis and fatty acid oxidation [35], [36]. The white adipose tissue has been commonly recognized to be an endocrine organ which plays a central role in many metabolic pathways via secretion of various adipokines including adiponectin and leptin. The physiological roles of CB1R in adipocytes and adipose tissue have been extensively explored and well established. In primary adipocytes, blockade of CB1R led to enhanced secretion of adiponectin and mitochondria biogenesis as well as decrease of lipogenic gene expression (e.g., SCD1 and FAS) [23], [24], [37]. In animal models of obesity and diabetes [38], [39], [40] as well as in obese subjects [41], [42], treatment with CB1R antagonists such as rimonabant has led to increased plasma adiponectin level, in particular the high molecular weight adiponectin. Adiponectin increases glucose uptake and fatty acid oxidation in skeletal muscle, reduces hepatic glucose production and improves overall insulin sensitivity [43]. Here in the present study, we did observe an apparent dose-dependent total adiponectin increase upon CB1R ASO treatment, although this finding was complicated by the increased adiponection level from the ASO control group. It’s possible that the HMW adiponectin level profile was more reflective of the metabolic benefits upon CB1R ASO treatment since this form of adiponectin has been shown to possess the most potent insulin-sensitizing activity [40], [44]. High-fat diet is also known to trigger a peripheral leptin resistance and hyperleptinemia [45], [46]. Leptin was shown to stimulate fatty acid oxidation by activating AMP-activate protein kinase in skeletal muscle [47]. Here in this study, high-fat diet significantly increased plasma leptin level and CB1R ASO treatment normalized that, indicating amelioration of leptin resistance (Table 5). Our findings are consistent with reported reduction of plasma leptin level upon administration of rimonabant and a peripherally restricted CB1R antagonist AM6545 in DIO C57Bl/6 [48]. An alternative mechanism for CB1R ASO action in white adipose tissue (WAT) is inhibition of CB1R in the sympathetic nerve terminals. CB1R was shown to express at the peripheral sympathetic nerve terminals and inhibit norepinepherine release upon activation [49]. It’s commonly known that norepinepherine activates hormone sensitive lipase and consequently stimulates lipolysis in the adipose tissue via adrenergic receptors [50]. Furthermore, a recent report by Quarta et al demonstrated the key role of sympathetic nervous system (SNS) in rimonabant-mediated increase of lipid utilization and energy expenditure by using a conditional knock-out of CB1R in the forebrain and sympathetic neurons [51]. The authors did recognize that relative contribution from CNS versus SNS endoconnabinoid signaling in the control of peripheral energy balance remained to be understood, possibly by specific knock-out of CB1R in the sympathetic ganglion [51]. In addition to WAT, brown adipose tissue (BAT) was also shown to be under the SNS control and account for rimonabant-stimulated thermogenesis in BAT and possibly in part, the body weight loss [36], [51], [52]. Here in the present study, we showed that fat mass was significantly reduced upon peripheral blockade upon CB1R ASO treatment, implicating enhanced lipolysis and decreased lipogenesis. This was supported by reduced SREBP-1 mRNA level in epididymal fat (Table 7). Similarly, a peripherally restricted CB1R antagonist AM6545 in DIO C57Bl/6 decreased lipogenic gene expression (e.g., SCD1 and FAS) in both subcutaneous and visceral fats [51]. CB1R was also found in human adipose tissue and the endocannabinoid system was up-regulated in obese subjects [17], [18]. Therefore, findings in preclinical animal models may be translated in humans.
The liver plays a pivotal role in glucose homeostasis and lipid metabolism. Liver expresses a low level of CB1R [6], [53], [54], [55]. Nonetheless, activation of CB1 in mice increases hepatic gene expression of the lipogenic transcription factor SREBP-1 and the lipogenic genes acetyl-CoA carboxylase-1 (ACC1) and fatty acid synthase (FAS) [6]. Furthermore, hepatocyte-selective genetic knockout of CB1R in mice led to reduced steatosis, hyperglycemia and insulin and leptin resistance on high-fat diet in the context of similar adiposity when compared to wild type mice on high-fat diet [6], [56]. Other cells in the liver such as stellate cells may also affect lipid metabolism by exerting paracrine effects on hepatocyte CB1R [57]. Here in this report, after CB1R ASO treatment, liver TG was reduced (Table 6), indicating ameliorated hepatic steatosis. Our RT-PCR analysis of liver samples suggested again the very low level of CB1R expression in these mice even after HFD feeding (data not shown). It may be possible that the cross-talk of adipose tissue and liver via altered secretion of adipokines and inflammatory cytokines plays a more important role than modulation of CB1R per se in the liver [39], [40], [58], [59]. CB1R was also found in skeletal muscle and pancreas. Rimonbant treatment led to increased glucose uptake in skeletal muscle [60]. The roles of CB1R play in the pancreas remain to be fully understood. Activation of CB1R in mouse pancreatic islets led to inhibition of insulin secretion [61], while rimonabant treatment in Zucker fa/fa rats increased glucose-dependent insulin secretion index, indicating a protective role of rimonabant in β-cell function [25], [26], [61]. In isolated human islets, CB1R was found to express more densely in the alpha cells than in the beta cells [8]. CB1 stimulation enhanced insulin and glucagon secretion in human islets [8]. The roles CB1R play in the pancreas remains to be fully understood. Since anti-sense oligonucleotides generally don’t distribute to skeletal muscle and pancreas well, the improved metabolic profile here may not be attributed to reduction of CB1R expression in these tissues. Glucose homeostasis and insulin sensitivity was improved upon CB1R ASO treatment as demonstrated by oral glucose tolerance test and reduced plasma insulin level (Figure 1 and Table 5). It’s generally realized that ASO chronic treatment in animals tended to induce pro-inflammatory status. Here we observed the dose-dependent increase of spleen weight and total plasma PAI-1 level upon CB1R ASO treatment, although both spleen weight and PAI-1 level of control ASO group was similar to that of DIO vehicle-group. This seemingly up-regulated pro-inflammatory state may worsen insulin resistance in diet-induced obesity in this study. Nonetheless, overall insulin resistance and glucose homeostasis was improved, strongly suggesting that reduction of CB1R expression exerted a more predominant role in the peripheral tissues. This result is consistent with findings in diet-induced obese mice when treated with CB1 antagonists [34], [39], [59]. Kidney also contributes substantially to total-body glucose homeostasis by regulating gluconeogenesis, filtering and reabsorbing glucose [62], [63]. Here we showed CB1R ASO treatment led to significant reduction of CB1 receptor in the kidney. Whether or not CB1R plays a role in renal glucose release and the consequences of CB1R blockade remain to be explored.
We do recognize some important limitations of this study here. Although ASOs are well known not to penetrate blood brain barrier, compensatory mechanism may lead to changes of the endocannabinoid system in the central nervous system (CNS) after prolonged reduction of CB1R in peripheral tissues. Therefore we cannot totally rule out the contribution of the CNS to the observed metabolic benefits here. Based on our and others’ experience, steady state mRNA level after chronic ASO treatment generally correlates well with protein level of the target gene. Due to this belief, we did not perform analysis of CB1R protein level in target tissues in this study. In retrospect, confirmation of CB1R protein level could have made a more compelling case. The results here should be interpretated with caution.
Finally, we also recognize that the endocannabinoid system also contains CB2R. Although CB2R is less understood than CB1R with regard to its roles in metabolic disorders, evidvence has emerged suggesting the potential involvement of CB2R in glucose homeostasis, hepatic steatosis and obesity-associated inflammation [64], [65], [66], [67], [68]. In this study, we also measured the mRNA level of CB2R in epididymal fat, there was no change upon CB1R or control ASO treatment.Nonetheless, since plasma or tissue endocannabinoids levels were not determined in this study, we cannot completely rule out the possible contribution of CB2R to the observed metabolic benefits.
In summary, we demonstrated here that selective knockdown of the peripheral CB1R using an ASO approach led to improved insulin sensitivity and glucose homeostasis in DIO AKR/J mice. Liver steatosis associated with DIO was also ameliorated upon CB1R ASO treatment. These findings support the notion that inhibition of CB1R in peripheral tissues may exert significant beneficial metabolic benefits and may represent a novel therapeutic approach for treating diabetes and related metabolic syndrome.
Acknowledgments
The authors would like to thank Dr. Sanjay Bhanot and Dr. Brett P. Monia from ISIS Pharmaceuticals for providing CB1R-ASO (Isis-414930) and scrambled control ASO (Isis-141923).
Funding Statement
The study was funded by Janssen Pharmaceutical Companies of Johnson and Johnson. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez de Fonseca F (2010) The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacol Biochem Behav 95: 375–382. [DOI] [PubMed] [Google Scholar]
- 2. Scheen AJ (2009) The endocannabinoid system: a promising target for the management of type 2 diabetes. Curr Protein Pept Sci 10: 56–74. [DOI] [PubMed] [Google Scholar]
- 3. Vemuri VK, Janero DR, Makriyannis A (2008) Pharmacotherapeutic targeting of the endocannabinoid signaling system: Drugs for obesity and the metabolic syndrome. Physiol Behav 93: 671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Esposito I, Proto MC, Gazzerro P, Laezza C, Miele C, et al. (2008) The cannabinoid CB1 receptor antagonist rimonabant stimulates 2-deoxyglucose uptake in skeletal muscle cells by regulating the expression of phosphatidylinositol-3-kinase. Mol Pharmacol 74: 1678–1686. [DOI] [PubMed] [Google Scholar]
- 5. Baldassano S, Serio R, Mule F (2008) Cannabinoid CB1 receptor activation modulates spontaneous contractile activity in mouse ileal longitudinal muscle. Eur J Pharmacol 582: 132–138. [DOI] [PubMed] [Google Scholar]
- 6. Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, et al. (2005) Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115: 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, et al. (2000) Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J 346: 835–840. [PMC free article] [PubMed] [Google Scholar]
- 8. Bermudez-Silva FJ, Suarez J, Baixeras E, Cobo N, Bautista D, et al. (2008) Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia 51: 476–487. [DOI] [PubMed] [Google Scholar]
- 9. Pagano C, Pilon C, Calcagno A, Urbanet R, Rossato M, et al. (2007) The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab 92: 4810–4819. [DOI] [PubMed] [Google Scholar]
- 10. Janero DR, Makriyannis A (2009) Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerging Drugs 14: 43–65. [DOI] [PubMed] [Google Scholar]
- 11. Despres J-P (2009) Pleiotropic effects of rimonabant: clinical implications. Curr Pharm Des 15: 553–570. [DOI] [PubMed] [Google Scholar]
- 12. Kunos G, Osei-Hyiaman D, Liu J, Godlewski G, Batkai S (2008) Endocannabinoids and the Control of Energy Homeostasis. J Biol Chem 283: 33021–33025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osei-Hyiaman D, Depetrillo M, Harvey-White J, Bannon AW, Cravatt BF, et al. (2005) Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology 81: 273–282. [DOI] [PubMed] [Google Scholar]
- 14. Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, et al. (2001) Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410: 822–825. [DOI] [PubMed] [Google Scholar]
- 15. Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, et al. (2006) Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91: 3171–3180. [DOI] [PubMed] [Google Scholar]
- 16. Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, et al. (2008) Endocannabinoid Dysregulation in the Pancreas and Adipose Tissue of Mice Fed With a High-fat Diet. Obesity 16: 553–565. [DOI] [PubMed] [Google Scholar]
- 17. Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, et al. (2006) Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 55: 3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engeli S, Boehnke J, Feldpausch M, Gorzelniak K, Janke J, et al. (2005) Activation of the peripheral endocannabinoid system in human obesity. Diabetes 54: 2838–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jumpertz R, Guijarro A, Pratley RE, Piomelli D, Krakoff J (2011) Central and peripheral endocannabinoids and cognate acylethanolamides in humans: association with race, adiposity, and energy expenditure. J Clin Endocrinol Metab 96: 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, et al. (2004) Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 24: 2708–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Croci T, Manara L, Aureggi G, Guagnini F, Rinaldi-Carmona M, et al. (1998) In vitro functional evidence of neuronal cannabinoid CB1 receptors in human ileum. Br J Pharmacol 125: 1393–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, et al. (2002) A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22: 9612–9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cota D, Marsicano G, Tschoep M, Gruebler Y, Flachskamm C, et al. (2003) The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, et al. (2003) The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 63: 908–914. [DOI] [PubMed] [Google Scholar]
- 25. Getty-Kaushik L, Richard AM, Deeney JT, Krawczyk S, Shirihai O, et al. (2009) The CB1 antagonist rimonabant decreases insulin hypersecretion in rat pancreatic islets. Obesity (Silver Spring) 17: 1856–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bermudez-Silva FJ, Suarez Perez J, Nadal A, Rodriguez de Fonseca F (2009) The role of the pancreatic endocannabinoid system in glucose metabolism. Best Pract Res, Clin Endocrinol Metab 23: 87–102. [DOI] [PubMed] [Google Scholar]
- 27. Jiang L-s, Pu J, Han Z-h, Hu L-h, He B (2009) Role of activated endocannabinoid system in regulation of cellular cholesterol metabolism in macrophages. Cardiovasc Res 81: 805–813. [DOI] [PubMed] [Google Scholar]
- 28. Crespillo A, Suarez J, Bermudez-Silva FJ, Rivera P, Vida M, et al. (2011) Expression of the cannabinoid system in muscle: effects of a high-fat diet and CB1 receptor blockade. Biochem J 433: 175–185. [DOI] [PubMed] [Google Scholar]
- 29. Corbille A-G, Valjent E, Marsicano G, Ledent C, Lutz B, et al. (2007) Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci 27: 6937–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Svizenska I, Dubovy P, Sulcova A (2008) Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures - a short review. Pharmacol, Biochem Behav 90: 501–511. [DOI] [PubMed] [Google Scholar]
- 31. Silvestri C, Ligresti A, Di Marzo V (2011) Peripheral effects of the endocannabinoid system in energy homeostasis: adipose tissue, liver and skeletal muscle. Rev Endocr Metab Disord 12: 153–162. [DOI] [PubMed] [Google Scholar]
- 32. Cigolini M, Tonoli M, Borgato L, Frigotto L, Manzato F, et al. (1999) Expression of plasminogen activator inhibitor-1 in human adipose tissue: a role for TNF-alpha? Atherosclerosis 143: 81–90. [DOI] [PubMed] [Google Scholar]
- 33. Lobo SM, Quinto BM, Oyama L, Nakamichi R, Ribeiro AB, et al. (2012) TNF-alpha modulates statin effects on secretion and expression of MCP-1, PAI-1 and adiponectin in 3T3-L1 differentiated adipocytes. Cytokine. (in press).. [DOI] [PubMed] [Google Scholar]
- 34. Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, et al. (2010) CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes 59: 926–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, et al. (2003) Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284: R345–353. [DOI] [PubMed] [Google Scholar]
- 36. Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, et al. (2005) The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 19: 1567–1569. [DOI] [PubMed] [Google Scholar]
- 37. Tedesco L, Valerio A, Cervino C, Cardile A, Pagano C, et al. (2008) Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes 57: 2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Migrenne S, Lacombe A, Lefevre A-L, Pruniaux M-P, Guillot E, et al. (2009) Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. Am J Physiol 296: R929–R935. [DOI] [PubMed] [Google Scholar]
- 39. Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, et al. (2007) Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology 46: 122–129. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe T, Kubota N, Ohsugi M, Kubota T, Takamoto I, et al. (2009) Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem 284: 1803–1812. [DOI] [PubMed] [Google Scholar]
- 41. Cote M, Mauriege P, Bergeron J, Almeras N, Tremblay A, et al. (2005) Adiponectinemia in visceral obesity: impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J Clin Endocrinol Metab 90: 1434–1439. [DOI] [PubMed] [Google Scholar]
- 42. Despres JP, Golay A, Sjostrom L (2005) Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353: 2121–2134. [DOI] [PubMed] [Google Scholar]
- 43. Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, et al. (2010) Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem 285: 33623–33631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang LC, Huang KC, Wu YW, Kao HL, Chen CL, et al. (2009) The clinical implications of blood adiponectin in cardiometabolic disorders. J Formos Med Assoc 108: 353–366. [DOI] [PubMed] [Google Scholar]
- 45. Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P (2004) CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28: 640–648. [DOI] [PubMed] [Google Scholar]
- 46. Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, et al. (1997) Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, et al. (2002) Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415: 339–343. [DOI] [PubMed] [Google Scholar]
- 48. Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, et al. (2010) Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120: 2953–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, et al. (1996) Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol 118: 2023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buettner C, Muse ED, Cheng A, Chen L, Scherer T, et al. (2008) Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med 14: 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Quarta C, Bellocchio L, Mancini G, Mazza R, Cervino C, et al. (2010) CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab 11: 273–285. [DOI] [PubMed] [Google Scholar]
- 52. Verty ANA, Allen AM, Oldfield BJ (2009) The Effects of Rimonabant on Brown Adipose Tissue in Rat: Implications for Energy Expenditure. Obesity FIELD Full Journal Title:Obesity 17: 254–261. [DOI] [PubMed] [Google Scholar]
- 53. Xu X, Liu Y, Huang S, Liu G, Xie C, et al. (2006) Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet 171: 31–38. [DOI] [PubMed] [Google Scholar]
- 54. Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, et al. (2006) CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 12: 671–676. [DOI] [PubMed] [Google Scholar]
- 55. Moezi L, Gaskari SA, Liu H, Baik SK, Dehpour AR, et al. (2006) Anandamide mediates hyperdynamic circulation in cirrhotic rats via CB(1) and VR(1) receptors. Br J Pharmacol 149: 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, et al. (2008) Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 118: 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Friedman SL, Nieto N (2008) Cannabinoids provoke alcoholic steatosis through a conspiracy of neighbors. Cell Metab 7: 187–188. [DOI] [PubMed] [Google Scholar]
- 58. Evans RM, Barish GD, Wang YX (2004) PPARs and the complex journey to obesity. Nat Med 10: 355–361. [DOI] [PubMed] [Google Scholar]
- 59. Wang Q, Perrard XD, Perrard JL, Mansoori A, Smith CW, et al. (2011) Effect of the cannabinoid receptor-1 antagonist rimonabant on inflammation in mice with diet-induced obesity. Obesity (Silver Spring) 19: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu YL, Connoley IP, Wilson CA, Stock MJ (2005) Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lepob/Lepob mice. Int J Obes 29: 183–187. [DOI] [PubMed] [Google Scholar]
- 61. Nakata M, Yada T (2008) Cannabinoids inhibit insulin secretion and cytosolic Ca2+ oscillation in islet beta -cells via CB1 receptors. Regul Pept 145: 49–53. [DOI] [PubMed] [Google Scholar]
- 62. Marsenic O (2009) Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 53: 875–883. [DOI] [PubMed] [Google Scholar]
- 63. Stumvoll M, Meyer C, Perriello G, Kreider M, Welle S, et al. (1998) Human kidney and liver gluconeogenesis: evidence for organ substrate selectivity. Am J Physiol 274: E817–826. [DOI] [PubMed] [Google Scholar]
- 64. Agudo J, Martin M, Roca C, Molas M, Bura AS, et al. (2010) Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia 53: 2629–2640. [DOI] [PubMed] [Google Scholar]
- 65. Bermudez-Silva FJ, Sanchez-Vera I, Suarez J, Serrano A, Fuentes E, et al. (2007) Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur J Pharmacol 565: 207–211. [DOI] [PubMed] [Google Scholar]
- 66. De Gottardi A, Spahr L, Ravier-Dall’Antonia F, Hadengue A (2010) Cannabinoid receptor 1 and 2 agonists increase lipid accumulation in hepatocytes. Liver Int 30: 1482–1489. [DOI] [PubMed] [Google Scholar]
- 67. Deveaux V, Cadoudal T, Ichigotani Y, Teixeira-Clerc F, Louvet A, et al. (2009) Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS One 4: e5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mendez-Sanchez N, Zamora-Valdes D, Pichardo-Bahena R, Barredo-Prieto B, Ponciano-Rodriguez G, et al. (2007) Endocannabinoid receptor CB2 in nonalcoholic fatty liver disease. Liver Int 27: 215–219. [DOI] [PubMed] [Google Scholar]