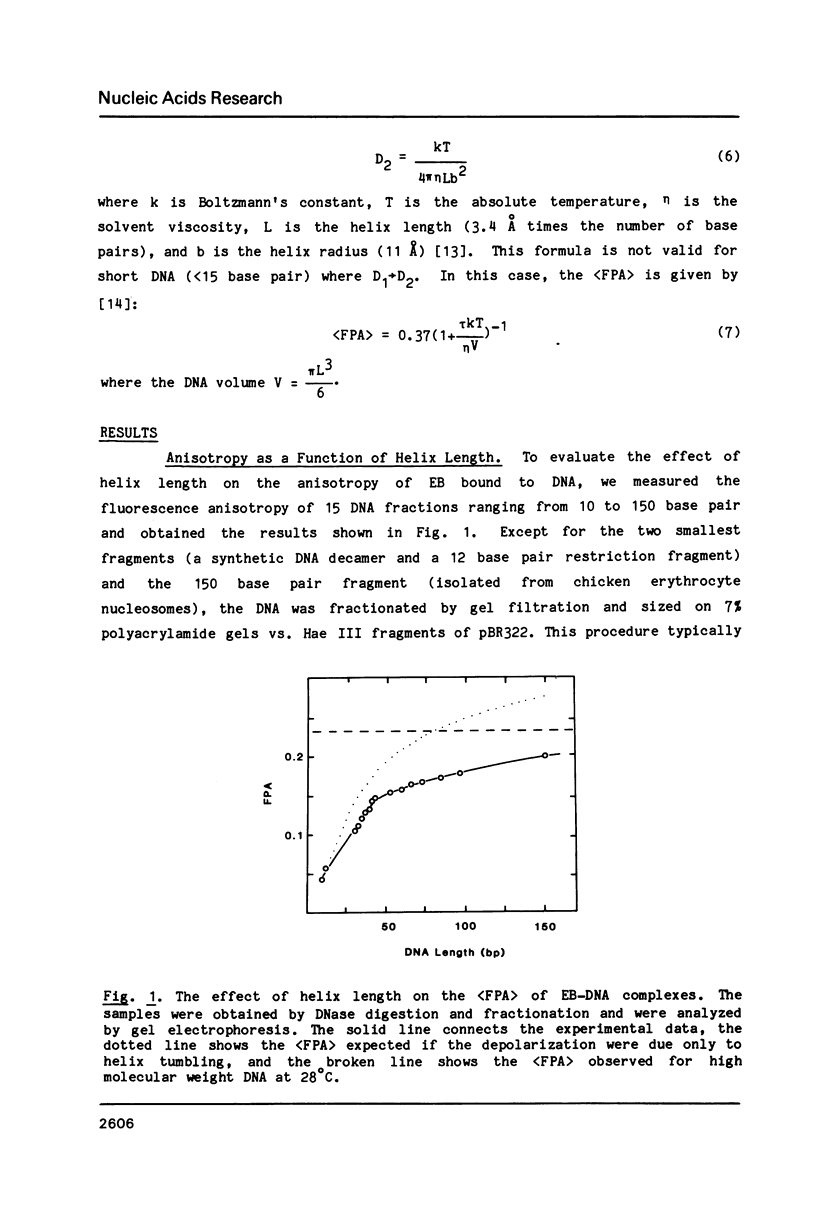
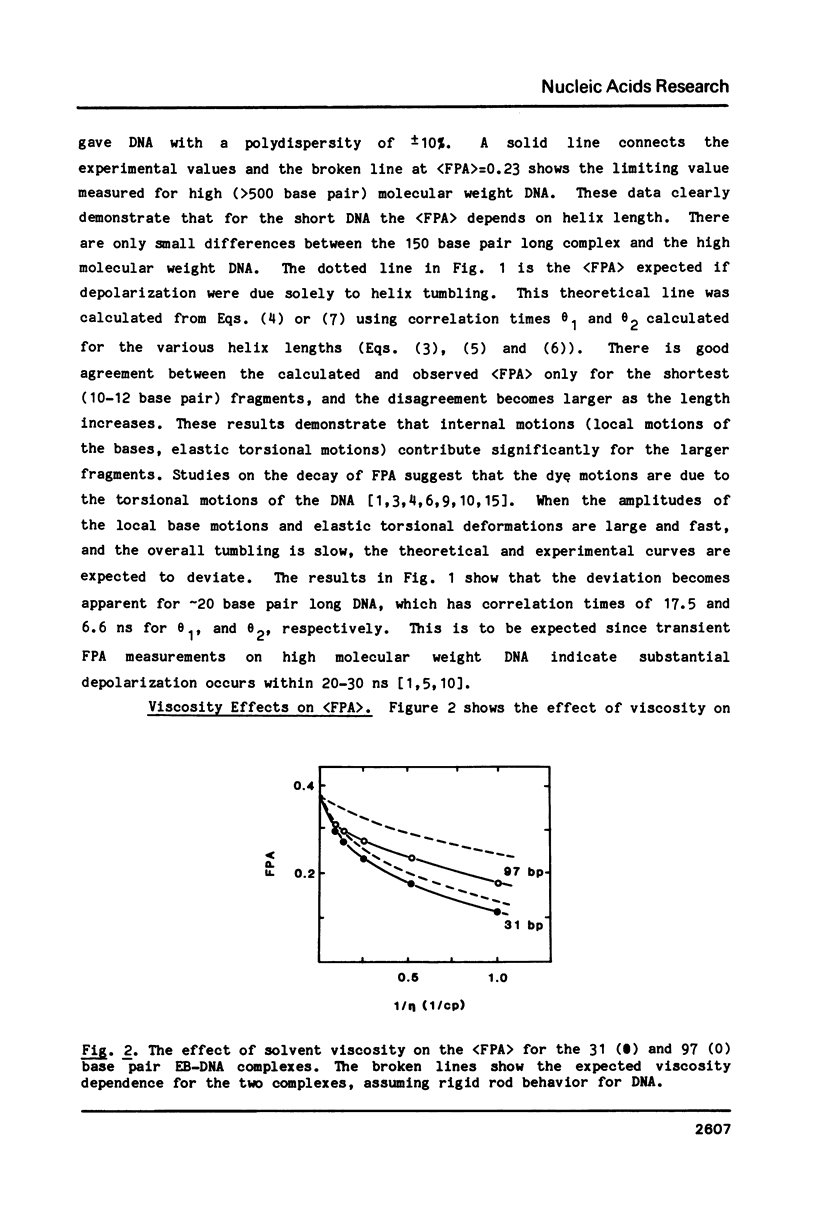
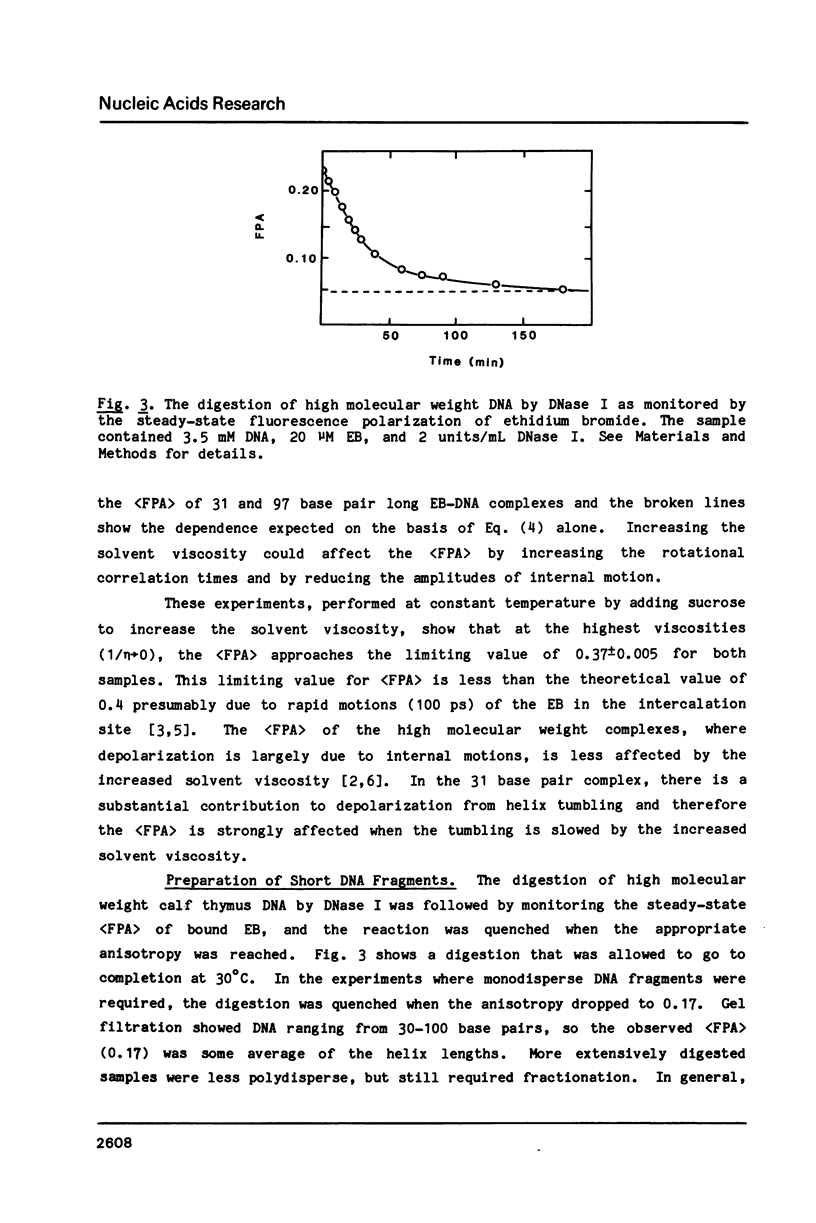
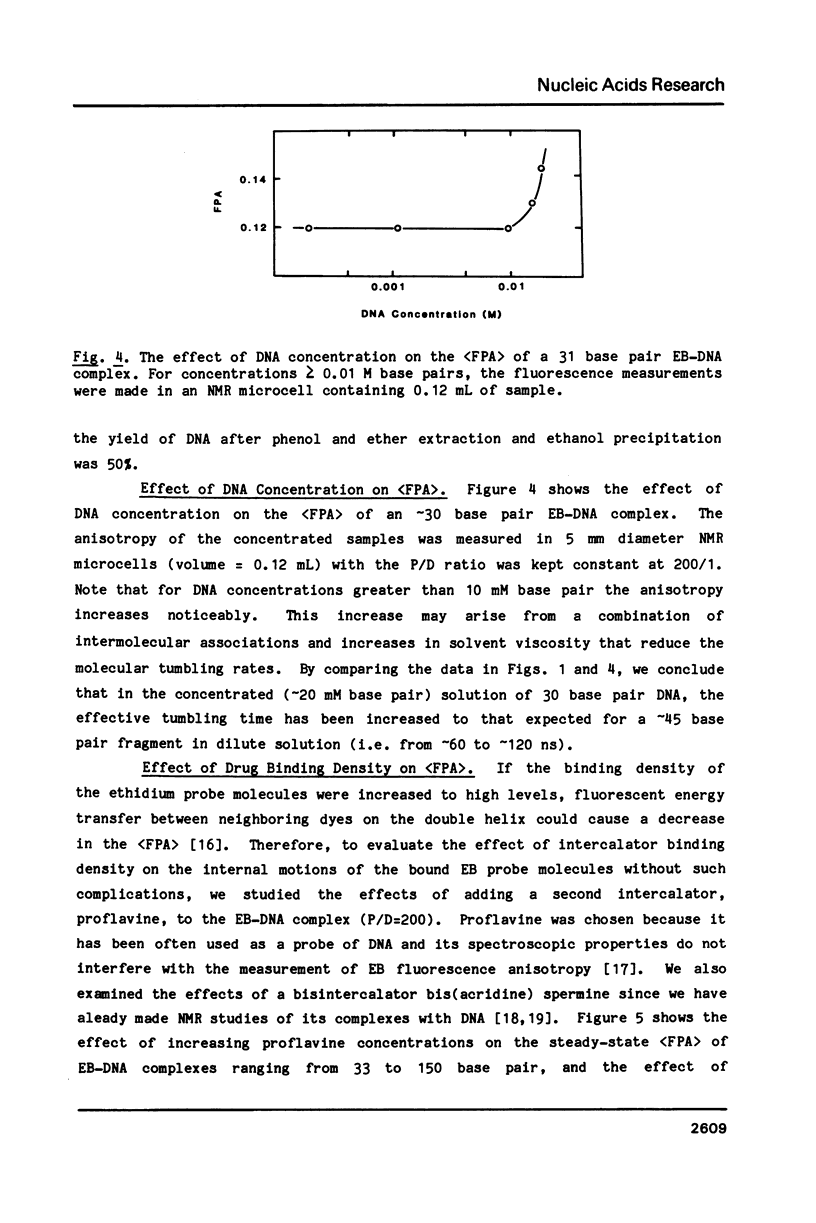
Abstract
We have used steady-state fluorescence polarization anisotropy (FPA) of ethidium probe molecules bound to DNA to investigate DNA-DNA interactions and the effect of high densities of intercalating drugs on the internal motions of DNA responsible for depolarization of the ethidium fluorescence. To calibrate the method, we examined the effect of DNA length on (FPA) using DNA varying in size from 10-150 base pair. The association of approximately 30 base pair DNA at high concentrations was then detected by its effect on (FPA). With sample concentrations approaching those commonly used in various physical experiments (NMR, Raman) significant DNA-DNA interactions are observed. With high molecular weight DNA (greater than 500 base pair), the limiting value of the (FPA) (0.23) is due to internal motions of the DNA (and bound chromophores). The (FPA) of ethidium probe molecules (1 drug/200 base pair) is unaffected by the addition of high levels (1 drug/2 base pair) proflavine. This indicates that either the elastic properties of DNA are unaffected by high densities of intercalated drug or that the depolarization of the ethidium fluorescence is due to highly localized motions of the base pairs that are unperturbed by binding of drugs at neighboring sites.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. Interaction of closed circular DNA with intercalative dyes. II. The free energy of superhelix formation in SV40 DNA. J Mol Biol. 1970 Feb 14;47(3):419–435. doi: 10.1016/0022-2836(70)90312-8. [DOI] [PubMed] [Google Scholar]
- Chaires J. B., Dattagupta N., Crothers D. M. Binding of daunomycin to calf thymus nucleosomes. Biochemistry. 1983 Jan 18;22(2):284–292. doi: 10.1021/bi00271a009. [DOI] [PubMed] [Google Scholar]
- Crothers D. M. Calculation of binding isotherms for heterogenous polymers. Biopolymers. 1968 Apr;6(4):575–584. doi: 10.1002/bip.1968.360060411. [DOI] [PubMed] [Google Scholar]
- Diekmann S., Hillen W., Morgeneyer B., Wells R. D., Pörschke D. Orientation relaxation of DNA restriction fragments and the internal mobility of the double helix. Biophys Chem. 1982 Jul;15(4):263–270. doi: 10.1016/0301-4622(82)80009-4. [DOI] [PubMed] [Google Scholar]
- Early T. A., Kearns D. R. 1H nuclear magnetic resonance investigation of flexibility in DNA. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4165–4169. doi: 10.1073/pnas.76.9.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigon J., Leupin W., Denny W. A., Kearns D. R. Two-dimensional proton nuclear magnetic resonance investigation of the synthetic deoxyribonucleic acid decamer d(ATATCGATAT)2. Biochemistry. 1983 Dec 6;22(25):5943–5951. doi: 10.1021/bi00294a038. [DOI] [PubMed] [Google Scholar]
- Fried M. G., Bloomfield V. A. DNA gelation in concentrated solutions. Biopolymers. 1984 Nov;23(11 Pt 1):2141–2155. doi: 10.1002/bip.360231104. [DOI] [PubMed] [Google Scholar]
- Genest D., Sabeur G., Wahl P., Auchet J. C. Fluorescence anisotropy decay of ethidium bound to chromatin. Biophys Chem. 1981 Feb;13(1):77–87. doi: 10.1016/0301-4622(81)80027-0. [DOI] [PubMed] [Google Scholar]
- Genest D., Wahl P. Fluorescence anisotropy decay due to rotational brownian motion of ethidium intercalated in double strand DNA. Biochim Biophys Acta. 1978 Dec 21;521(2):502–509. doi: 10.1016/0005-2787(78)90292-7. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Investigation of the flexibility of DNA using transient electric birefringence. Biopolymers. 1981 Jul;20(7):1503–1535. doi: 10.1002/bip.1981.360200710. [DOI] [PubMed] [Google Scholar]
- Hogan M. E., Jardetzky O. Internal motions in DNA. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6341–6345. doi: 10.1073/pnas.76.12.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M. E., Jardetzky O. Internal motions in deoxyribonucleic acid II. Biochemistry. 1980 Jul 22;19(15):3460–3468. doi: 10.1021/bi00556a009. [DOI] [PubMed] [Google Scholar]
- Hogan M., Wang J., Austin R. H., Monitto C. L., Hershkowitz S. Molecular motion of DNA as measured by triplet anisotropy decay. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3518–3522. doi: 10.1073/pnas.79.11.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns D. R. NMR studies of conformational states and dynamics of DNA. CRC Crit Rev Biochem. 1984;15(3):237–290. doi: 10.3109/10409238409102803. [DOI] [PubMed] [Google Scholar]
- Le Bret M., Le Pecq J. B., Barbet J., Roques B. P. A reexamination of the problem of resonance energy transfer between DNA intercalated chromophores using bisintercalating compounds. Nucleic Acids Res. 1977;4(5):1361–1379. doi: 10.1093/nar/4.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar D. P., Robbins R. J., Zewail A. H. Direct observation of the torsional dynamics of DNA and RNA by picosecond spectroscopy. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5593–5597. doi: 10.1073/pnas.77.10.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted J., 3rd, Kearns D. R. Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry. 1977 Aug 9;16(16):3647–3654. doi: 10.1021/bi00635a022. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Lerman L. S., Beth A. H., Frisch H. L., Dalton L. R., Auer C. Analysis of double-helix motions with spin-labeled probes: binding geometry and the limit of torsional elasticity. J Mol Biol. 1980 May 5;139(1):19–44. doi: 10.1016/0022-2836(80)90113-8. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Gilbert S. G., Jain S. C., Sakore T. D. Organization of DNA in chromatin. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3068–3072. doi: 10.1073/pnas.73.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. C., Allison S. A., Appellof C. J., Schurr J. M. Torison dynamics and depolarization of fluorescence of linear macromolecules. II. Fluorescence polarization anisotropy measurements on a clean viral phi 29 DNA. Biophys Chem. 1980 Oct;12(2):177–188. doi: 10.1016/0301-4622(80)80050-0. [DOI] [PubMed] [Google Scholar]
- Thomas J. C., Schurr J. M. Fluorescence depolarization and temperature dependence of the torsion elastic constant of linear phi 29 deoxyribonucleic acid. Biochemistry. 1983 Dec 20;22(26):6194–6198. doi: 10.1021/bi00295a024. [DOI] [PubMed] [Google Scholar]
- Thomes J. C., Weill G., Daune M. Fluorescence of proflavine--DNA complexes: heterogeneity of binding sites. Biopolymers. 1969;8(5):647–669. doi: 10.1002/bip.1969.360080507. [DOI] [PubMed] [Google Scholar]
- Wahl P., Paoletti J., Le Pecq J. B. Decay of fluorescence emission anisotropy of the ethidium bromide-DNA complex. Evidence for an internal motion in DNA. Proc Natl Acad Sci U S A. 1970 Feb;65(2):417–421. doi: 10.1073/pnas.65.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]


