Abstract
Graft-versus-host disease (GvHD) remains the most significant complication after allogeneic stem cell transplantation (allo-SCT). Previously, acute GvHD had been considered to be mediated predominantly by Th1 polarized T cells. Recently, investigators have identified a second pro-inflammatory lineage of T cells termed Th17 that is critically dependent on the transcription factor RORγt. Here, we have evaluated the role of Th17 cells in murine acute GvHD by infusing donor T cells lacking RORC and as a consequence the isoform RORγt. Recipients given donor CD4+ and CD8+ T cells lacking RORC had significantly attenuated acute GvHD and markedly decreased tissue pathology in the colon, liver, and lung. Using a clinically relevant haploidentical murine transplantation model, we showed that RORC−/− CD4+ T cells alone diminished the severity and lethality of aGvHD. This was not found when CD4+ T cells from RORC−/− mice were given to completely mismatched BALB/c mice, and correlated with absolute differences in the generation of TNF in the colon post transplant. Thus, CD4+ T cell expression of RORC is important in the pathogenesis of acute GVHD.
Introduction
Allogeneic stem cell transplantation (allo-SCT) is a common treatment for patients with high-risk leukemia, recurrent low-grade lymphomas, aplastic anemia, and congenital bone marrow failure syndromes (1-3). The effectiveness of allo-SCT is limited by the development of acute graft-versus-host disease (aGvHD). aGvHD, a disease characterized by selective epithelial damage to target organs, is mediated by mature T cells present in the stem cell or bone marrow inoculums (4-7). Interactions of donor T cells with predominantly host antigen presenting cells (APC) leads to activation and differentiation of donor T cells ultimately resulting in inflammation in GvHD target organs, which includes primarily the skin, liver, and gastrointestinal tract (8).
Previous GvHD research has focused on cytokine production in T cell subsets. High levels of interferon γ (IFN-γ) and interleukin-2 (IL-2) found in patients after allo-SCT led investigators to conclude that GvHD was mediated predominantly by proinflammatory Th1 cells (9, 10). However and conversely, inhibition of Th1 cytokines leads to disease exacerbation in GvHD (11, 12). As both protective and detrimental effects are seen with Th1 cytokines the exact role of these cytokines in GvHD remains elusive (13). More recent investigations of T cell subsets in GvHD have been directed towards a new subset of CD4+ T cells, Th17 cells. Th17 cell differentiation and expansion requires TGF-β1, IL-6, IL-23, TNF, and IL-1β (14-16). The development of Th17 cells is dependent on the transcription factors retinoid-related orphan receptor (ROR)γt, RORα, IRF-4 and STAT3 (17, 18). Th17 cells produce proinflammatory cytokines such as TNF, IL-21, and IL-22 (19-21) in addition to IL-17A and IL-17F. IL-21 has been found by our group to be critical for blocking the generation of inducible Treg cells (19) while IL-22 has been found to be important for the induction of psoriasis in experimental models (22). IL-17A and IL-17F bind to the IL-17 receptor found on leukocytes, epithelial cells, mesothelial cells, endothelial cells, keratinocytes, and fibroblasts. Binding of IL-17A and IL-17F to the IL-17 receptor enhances production of g-CSF, IL-6, and chemokines that recruit neutrophils such as CXCL1 and CXCL8 (23).
Keppel et al using IL-17A knockout (−/−) CD4+ T cells demonstrated that IL-17 contributes to aGVHD (24). In contrast, Yi et al has shown that IL-17A−/− T cells exacerbated aGVHD due to augmented release of IFN-γ (25). Recent studies in our laboratory demonstrated that in vitro differentiated Th17 cells generated substantial cutaneous and pulmonary pathology in murine models of aGvHD (26) but multiple pathways may have been involved, with IL-17A and TNF being dominant. To better understand the effects of Th17 cells that are differentiated or activated in vivo, we elected not to focus on a particular cytokine effector pathway such as IL-17A itself, which would limit conclusions that can be drawn regarding Th17 cells. Instead, we performed studies using RORC−/− donor T cells that are incapable of producing the array of cytokines generated by Th17 cells including IL-17A, IL-17F, IL-21, IL-22 and TNF. In the absence of RORC conventional T cells attenuated GvHD in a haploidentical, minor, and complete mismatched model. The absence of RORC expression by CD4+ T cells alone was sufficient to attenuate GvHD in the haploidentical model, but had little impact on GvHD in a complete mismatched model. Interestingly, we found increased generation of IL-17 from lesional tissue in BALB/c recipient mice even when transplanted with donor T cells lacking RORC. These data indicate that T cell generation of RORγt is important to the pathogenesis of acute GvHD.
Methods
Mice
C57BL/6J (H2b) (termed B6), BALB/cJ (H2d), C.B10-H2b/LiMcdJ (termed BALB.b), B6.129S6-Tbx21tm1Glm/J (termed T-bet−/−), B6 x DBA/2 F1 (B6D2 F1: H2bxd), and B10.BR-H2k H2-T18a/SjSnJJrep mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6 RORC−/− mice were generated as described (27). Donor and recipient mice were age-matched males between 8 and 16 weeks. All experiments were performed in accordance with protocols approved by the University of North Carolina Institutional Animal and Care Use Committee.
Transplantation Models
Total T cells or CD4+ T cells were isolated using Cedarlane T recovery column kit or CD4+ T cell recovery kit (Cedarlane, Burlington, NC) respectively, followed by antibody depletion using phycoerythrin (PE) conjugated anti-mouse B220 and anti-mouse CD25 antibodies (Ebioscience, San Diego, CA) and magnetic bead selection using anti-PE beads (Miltenyi Biotec, Cambridge, MA). Isolated CD4+ T cell were further purified using anti-mouse CD8 PE antibody. T cell depleted bone marrow bone marrow (TCD BM) and conventional T cells were prepared using previously described methods (28). Histopathology specimens were generated as described (29) and analyzed by one of us (APM) blinded to the genotype of donor used. Scoring of tissues was performed per our previous method (30).
Serum and Organ Cytokine Analysis
Transplant recipient animals were anesthetized and perfused with phosphate-buffered saline. Whole organs were removed and homogenized. Cytokine levels were measured using enzyme-linked immunosorbent assay (ELISA) kits against IFN-γ, IL-17A, and TNF (Biolegend, San Diego CA).
Intracellular Cytokine Staining
Single cells suspensions of livers were digested using collagenase A and DNAse I. Liver cells were stimulated with phorbol myristate acetate (PMA), ionomycin and brefeldin A for 4 hours. Cells were harvested and stained for anti-mouse TNF (Ebioscience, San Diego, CA). Flow cytometry analyses were conducted using FlowJo analysis software (Ashland, OR).
Real Time PCR Analysis
RNA was extracted from organs using TRIzol reagent (Invitrogen, Carlsbad CA) according to the manufacturer’s recommendations. First strand cDNA synthesis was performed with 1μg RNA as previously described (26). Equal amounts of cDNA were analyzed by real time quantitative PCR, in triplicate, using TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA) and the ABI 7300 Real Time PCR System with primer specific standard curves. The expression level of each gene was normalized to the housekeeping gene, GusB, using the standard curve method before fold activation was determined. TaqMan gene expression assay probes for interferon gamma, tumor necrosis factor, and interleukin 17A/F were purchased from Applied Biosystems.
GvHD Scoring
Mice were observed twice weekly for clinical GvHD signs and symptoms based on a previously established clinical scoring system (31).
GvL Analysis
Recipient mice were infused with 1 × 104 P815 murine mastocytoma cells (ATCC: TIB-64) on the transplantation. Weight loss and survival were monitored bi-weekly. Necropsies were performed on mice to confirm death by tumor infiltration.
Statistical Analysis
Survival differences were evaluated using Mantel-Cox log rank test. Survival curves were generated using the method of Kaplan and Meier (32). Differences in GvHD clinical and pathology scores were determined using Mann-Whitney test. P values ≤ 0.05 were considered statistically significant.
Results
Attenuated GvHD in the Absence of RORC
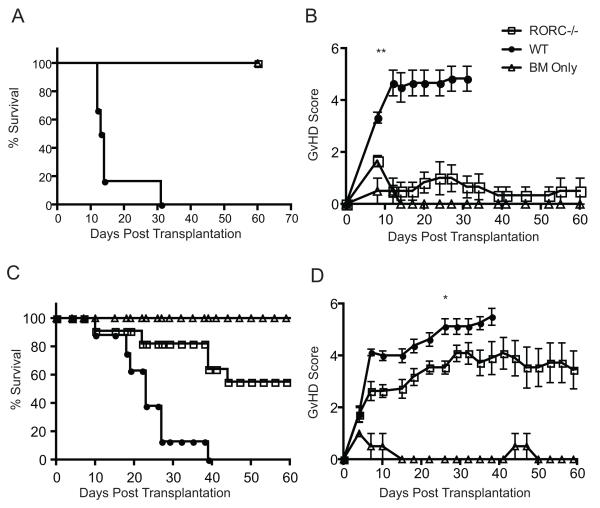
Previous work demonstrated that blocking IFN-γ exacerbated aGvHD suggesting that another T cell lineage may be important in GvHD pathology (12). As our previous work using in vitro differentiated Th17 cells demonstrated their ability to induce lethal aGvHD, we used mice in which the RORC locus (RORC−/−) was altered using homologous recombination to further clarify the contribution of the Th17 subset to GvHD induction under non-polarizing conditions. These mice lack both RORγ and RORγt isoforms generated from this locus. CD25-negative (CD25−) naïve whole T cells (comprised of CD4+ T cells and CD8+ T cells; termed Tconv) from WT C57BL/6 (WT) or RORC−/− donors were transferred into lethally irradiated B6D2 F1 recipients. In addition to T cells, mice were injected with T cell depleted bone marrow (TCD BM) cells from WT donors. Recipient mice given RORC−/− Tconv had a substantial improvement in survival with all B6D2 F1 recipient mice surviving until day 60 post transplantation (Fig 1a). Using a semi-quantitative scoring system we evaluated the clinical manifestations of aGvHD (33) in B6D2 F1 recipient mice. A significant difference in the aGvHD score starting on day 10 and continuing through the completion of the experiment was found in irradiated B6D2 F1 recipient mice transplanted with RORC−/− Tconv compared to WT Tconv (Fig 1b).
Figure 1. Survival and GvHD scores of B6D2 recipients.
(A-B) B6D2 recipients were lethally irradiated (950 cGy) on day -1. One day following irradiation, 4 × 106 WT or RORC−/− CD25-Tconv cells supplemented with 3 × 106 TCD BM were injected intravenously into recipient mice. Recipient mice were monitored and scored weekly. Control mice received TCD bone marrow cells alone (C-D) BALB/c recipients were lethally irradiated (800 cGy) on day -1. One day after irradiation, 5 × 105 WT or RORC−/− CD25-T cells supplemented with 5 × 106 WT TCD BM cells were injected intravenously into irradiated recipients. Survival was determined using the method of Kaplan-Meier. Statistics determined using log-rank test for survival and Mann-Whitney for scores. *p<0.05, **p<0.001. A-B n = 13 B6D2 F1 recipients transplanted with RORC−/− or WT Tconvs; n = 4 bone marrow controls; C-D n = 11 Balb/c recipients given RORC−/− Tconvs and 8 Balb/c recipients given WT Tconvs; n = 3 BM controls. Data are combined from 2 individual experiments.
To determine whether the reduced aGVHD lethality observed with the infusion of RORC−/− Tconv vs WT Tconv was model dependent, we evaluated two additional transplantation models. Lethally irradiated BALB/c mice given CD25-depleted donor Tconv from either WT or RORC−/− donors with WT TCD BM had improved median survival (Fig 1c) with a diminished GvHD score (Fig 1d) when receiving RORC−/− compared to WT Tconv. Similarly, the median survival was improved when BALB.B mice were administered RORC−/− Tconv compared to WT Tconv (Supp Fig 1). However, in BALB.B recipients, there was only a transient improvement in GvHD score from days 10-17 post transplant. Thus, in three different GvHD models using CD25-depleted Tconv, the absence of RORC in donor T cells improved survival.
Decrease Tissue Pathology in GvHD Target Organs using RORC−/− Donor T Cells
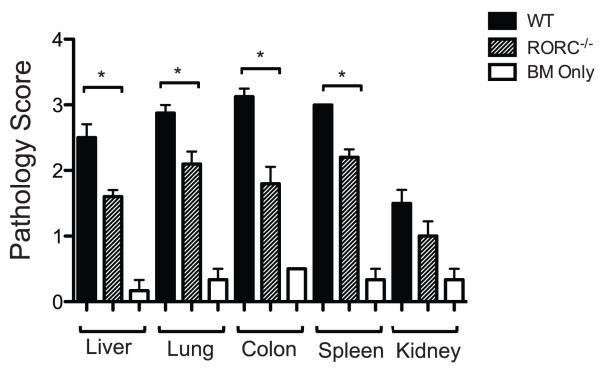
Clinically, multiple organs can be affected in aGvHD including the skin, liver, GI tract and the lung. To determine if RORC−/− Tconv affected aGvHD at a specific site we evaluated the tissue pathophysiology in the liver, GI tract, lung and spleen of RORC−/− Tconv recipients compared to WT Tconv recipients. Fifteen days post transplantation the organs of recipient animals were harvested and pathology analyses conducted. Recipients of RORC−/− Tconv displayed significantly less pathology in the liver, colon, lung, and spleen compared to WT Tconv recipients (p< 0.05, Fig 2). Decreased pathology in recipient mice transplanted with RORC−/− donor Tconv was specific to GvHD target organs as minimal GvHD pathology was detected in the kidney of WT and RORC−/− Tconv recipients. The aggressive nature of GI tract GvHD precluded the development of significant cutaneous GvHD in this model, and therefore cutaneous tissue was not evaluated. These data demonstrate that the function of RORC in the pathophysiology of aGvHD is not limited to a specific organ site.
Figure 2. Decreased tissue pathology in recipient mice given RORC−/− donor T cells.
4 × 106 (CD25−) Tconv cells from RORC−/− or WT mice with WT TCD BM were transplanted into lethally irradiated (950 cGy) B6D2 F1 recipients. Organs were harvested on day 15 post-transplantation and processed as described. Tissues were evaluated by one of us (APM) blinded to the treatment group and scored using a semi-quantitative GvHD scoring system. Shown are the mean scores with error bars indicating SEM. Statistical significance was determined using Mann-Whitney test, *p<0.05. n = 5 mice analyzed given WT or RORC−/− T cells. n=4 for bone marrow controls. Data pooled from an individual transplant using RORC−/− or WT Tconv.
In Vivo Cytokine Production Using RORC−/− Tconv Cells
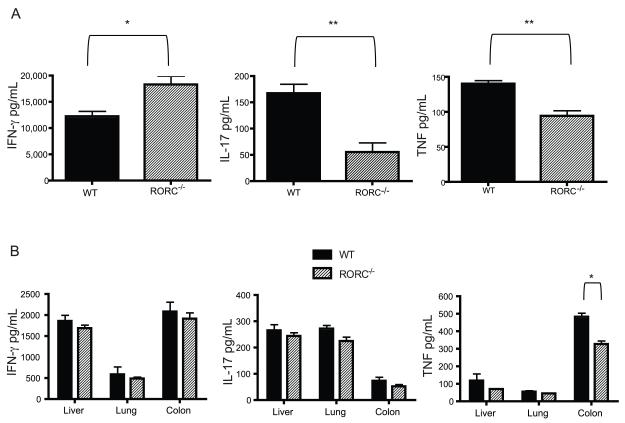
Th17 cells generate a number of cytokines that may be important to the pathogenesis of aGvHD such as TNF, IL-17F, IL-21, and/or IL-22. Cytokine analyses were performed on serum and organ samples from RORC−/− Tconv vs WT Tconv in B6D2 F1 recipients on day 14 post transplantation. Interestingly, the administration of donor T cells unable to express RORC was associated with a modest increase in the production of IFN-γ in the serum of recipient mice compared to those receiving WT Tconv (Fig 3a). A substantial decrease in IL-17 and TNF were seen in the serum of recipients RORC−/− Tconv compared to WT Tconv recipients (Fig 3a). The decrease in TNF production in the serum was associated with statistically significant decreased production of TNF in the colon however no differences were seen in cytokine production in other organs (Fig 3b).
Figure 3. Increased serum IFN-γ and decreased TNF expression RORC−/− recipients.
WT TCD BM and RORC−/− or WT Tconv were transplanted into lethally irradiated B6D2 mice. 14 days post transplantation (A) serum and (B) organs were collected from B6D2 F1 recipients and analyzed by ELISA for the expression of IL-17, TNF and IFN-γ. Shown are the mean values with error bars representing SEM. Data are pooled from 5 individual B6D2 receiving mice RORC−/− or WT Tconv. Statistical analyses were conducted using Mann-Whitney test. Data are combined from 2 individual experiments *p<0.05, **p<0.01
To determine if the lack of differences in pro-inflammatory cytokines outside of the difference in the generation of TNF in the colon was due to the time point we evaluated, we analyzed mRNA expression of IFN-γ, and IL-17A from lesional tissue on days 10 and 18 post transplantation. No difference was found in the expression of these cytokines in the colon, liver or spleen of recipients of WT compared to RORC−/− T cells plus TCD B6 bone marrow. Thus, the absence of RORC in donor T cells led to a marked decrease in the generation systemically of the pro-inflammatory cytokines TNF and IL-17A, and of TNF specifically in the colon.
RORC−/− CD4+ T Cells Mediate GvHD in a Haploidentical Transplantation
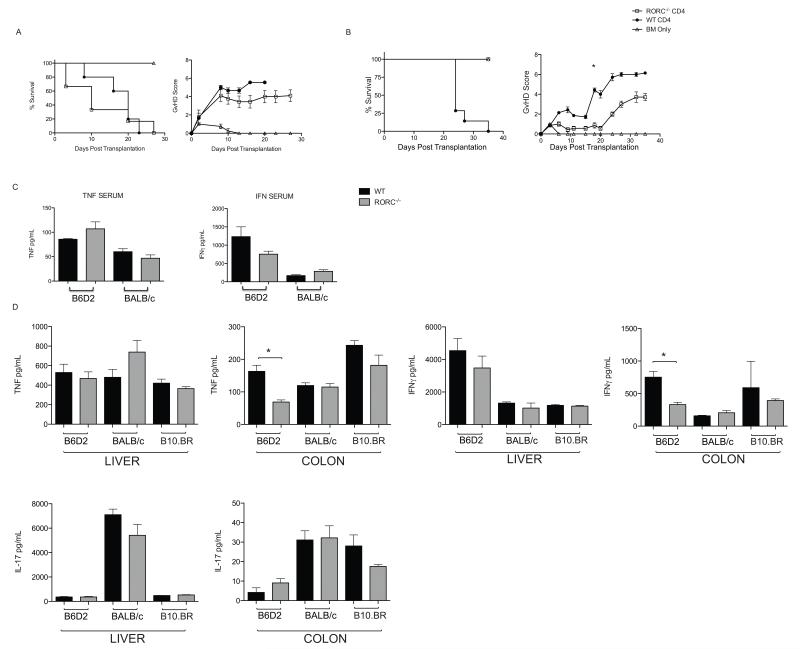
Previous investigators have found that the infusion of donor T cells lacking RORC did not affect the incidence or severity of aGvHD when administered to lethally irradiated BALB/c recipients (34). However, the T cell inoculum for these experiments was comprised exclusively of CD4+ T cells. The difference found by our group in the outcome of BALB/c recipients receiving RORC−/− T cells occurred when infusing CD4+ and CD8+ T cells. To determine if the different T cell inoculums mediate the changes in outcome initially, we confirmed the data from Icozlan et al. BALB/c mice receiving RORC−/− CD4+ T cells did not have improved survival or GvHD scores compared to recipients given WT CD4+ T cells (Fig 4a). Next, we determined if the absence of RORC by donor CD4+ T cells would impact the outcome in the haploidentical B6 into B6D2 model. All B6D2 recipients of RORC−/− CD4+ T cells survived until completion of the experiment with minimal evidence of clinical GvHD, while recipients of WT CD4+ T cells succumbed to disease by day 35 post transplantation (Fig 4b). This indicated that the difference in the outcome of recipient mice given donor RORC−/− CD4+ T cells was model dependent. These data demonstrate a requirement for RORC CD4+ T cell expression for GvHD pathogenesis in the haploidentical transplant setting.
Figure 4. Function of RORC in Donor CD4+ T cells is Model Dependent.
(A) Lethally irradiated BALB/c recipients were injected with 5 × 105 RORC−/− CD4+ or WT CD4+ T cells supplemented with 5 × 106 WT TCD BM. Survival and GvHD scores are shown. n= 9 for RORC−/− recipients. n=9 for WT recipients, n=4 for bone marrow only recipients. (B) Lethally irradiated B6D2 F1 recipients were injected with 2 × 106 RORC−/− CD4+ T cells or WT CD4+ T cells supplemented with 3 × 106 WT TCD BM. n= 7 for RORC−/− CD4+ T cells, n=7 for WT CD4+ T cells, n=3 bone marrow only. p<0.05 for survival. p < 0.05 from day 17 until the completion of the experiment for the difference in GvHD score. Data are combined from 2 individual experiments. (C) Serum and (D) organs were harvested from lethally irradiated BALB/c, B6D2 F1, or B10.BR recipients transplanted with RORC−/− or WT CD4+ T cells 14 days post transplantation. WT B10.BR recipients were harvested 10 days post transplantation. TNF, IFN-γ, and IL-17 production were determined by ELISA. Data pooled from 5 RORC−/− CD4+ T cell BALB/c recipients and 4 WT CD4+ T cell BALB/c recipients, 6 RORC−/− CD4+ T cell B6D2 recipients and 4 WT CD4+ T cell B6D2 recipients, 4 RORC−/− CD4+ T cell B10.BR and 3 WT CD4+ T cell B10.BR recipients. Statistical analysis determined by Mann-Whitney test. * p<0.05.
Cytokine Production in RORC−/− CD4+ T Cell Recipients
Differences in outcome using RORC−/− CD4+ T cells in the haploidentical versus the complete mismatch model are likely due to increased genetic disparity and potentially increased GvHD due to the ability of a smaller number of donor T cells to mediate GvHD, or GvHD mediated through different pro-inflammatory pathways. To elucidate the differences in outcome using RORC−/− CD4+ T cells in the B6 into BALB/c transplant model compared to the B6 into B6D2 transplant model, we evaluated cytokine production in the serum and organs from recipient animals. Lethally irradiated B6D2 recipients were transplanted with 3 × 106 RORC−/− or WT CD4+ T cells with 3 × 106 WT TCD BM cells while lethally irradiated BALB/c recipients were infused with 5 × 105 RORC−/− or WT CD4+ T cells supplemented with 5 × 106 WT TCD BM cells. Serum and tissue homogenates from the liver, GI tract, lung and spleen were collected from recipients 14 days post transplantation. We found that B6D2 recipients of RORC−/− CD4+ T cells had increased TNF production in the serum with decreased IFN-γ production compared to B6D2 recipients of WT CD4+ T cell (Fig 4c), however neither of these values reached statistical significance. B6D2 recipients of RORC−/− CD4+ T cells had a significant decrease in the production of TNF and IFN-γ in the colon compared to B6D2 recipients of WT CD4+ T cells (Fig 4d). This was not found in BALB/c recipients given either RORC−/− or WT donor CD4+ T cells. Interestingly, IL-17 production in the liver of BALB/c recipients was 8 times higher than IL-17 production in B6D2 recipients (Fig 4d) and not altered by the infusion of donor T cells lacking RORC. To determine if differences in the production of IL-17A was specific to BALB/c recipients, we analyzed a second MHC mismatched model. Lethally irradiated B10.BR mice were injected with 3 × 106 WT or RORC−/− CD4+ T cells with 3 × 106 TCD BM. TNF and IFN generation in the liver and colon of B10.BR recipients did not differ in the absence of RORC−/−. Interestingly, similar to BALB/c recipients, increased expression of IL-17 was seen in recipient B10.BR mice given either RORC−/− or WT CD4+ T cells (Fig 4d). These data suggest that the generation of IL-17A in the completely mismatched MHC transplant models is more dependent on production by cells other than donor T cells. Moreover, we found that the absence of RORC in donor T cells mediated protection against GvHD only in models in which there was a decrease in the production of TNF systemically and in the colon after the infusion of RORC−/− T cells.
RORC and TNF Production
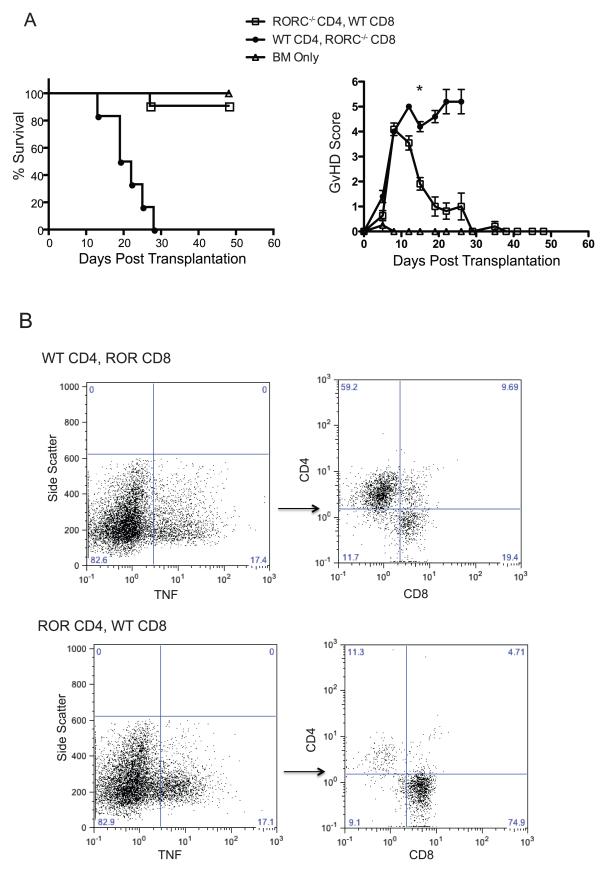
Our data indicate a role for RORC in the function of CD4+ T cells in the haploidentical transplant model. To determine if there was a function for RORC in donor CD8+ T cells, we transplanted mice with either RORC or WT CD4+ or CD8+ T cells. Three cohorts of lethally irradiated B6D2 F1 recipients were used for these experiments. One group received 2 × 106 RORC−/− CD4+ T cells with 2 × 106 WT CD8+ T cells supplemented with 3 × 106 TCD BM cells. A second group received 2 × 106 WT CD4+ T cells with RORC−/− CD8+ T cells supplemented with 3 × 106 WT TCD BM cells. A final group received only 3 × 106 TCD BM cells. Interestingly, more than 80 percent of mice that received RORC−/− CD4+ T cells with WT CD8+ T cells survived until day 50 post transplantation while those receiving WT CD4+ T cells with RORC−/− CD8+ T cells died from GvHD by day 30 post transplantation (Fig 5a). Intracellular cytokine analyses of TNF and IFN-γ production were conducted on T cells isolated from liver of WT CD4+ T, RORC−/− CD8+ T cell and RORC−/− CD4+, WT CD8+ T cell recipients 10 days post transplantation. Overall production of both TNF and IFN-γ were equivalent between the two groups. However in both cohorts independent of whether RORC−/− CD4+ T cells or RORC−/− CD8+ T cells were injected, WT T cells were the primary producers of TNF (Fig 5b). These data suggest that the production of TNF by CD4+ and not CD8+ T cells is critical to the pathogenesis of GvHD in this model.
Figure 5. Attenuated GvHD using RORC−/− Tconv cells is mediated by CD4+ T cells.
(A) Lethally irradiated B6D2 F1 mice were injected with 3 × 106 TCD BM. In addition to BM one group received 2 × 106 RORC−/− CD4+ T cells and 2 × 106 WT CD8+ T cells, one group received 2 × 106 WT CD4+ T cells and 2 × 106 RORC−/− CD8+ T cells, and a final group received only BM cells. Recipients of RORC−/− CD4+ T cells with WT CD8+ Tcells showed less GvHD reaching statistical significance by day 15 post transplantation. n= 11 recipient mice receiving RORC−/− CD4+ T cells and WT CD8+ T cells, n=5 for recipient mice receiving WT CD4+ T cells and RORC−/− CD8+ T cells, n=4 for bone marrow only. Data are combined from 2 individual experiments *p<0.05. (B) 10 days post transplantation the livers of RORC−/− CD4+, WT CD8+ T cell or WT CD4+, RORC−/− CD8+ T cell recipient mice were harvested and T cells isolated. Data are representative from 3 WT CD4, RORC−/− CD8+ recipients and 4 RORC−/− CD4+, WT CD8+ recipients.
Tissue Specific Role for T-bet in aGvHD
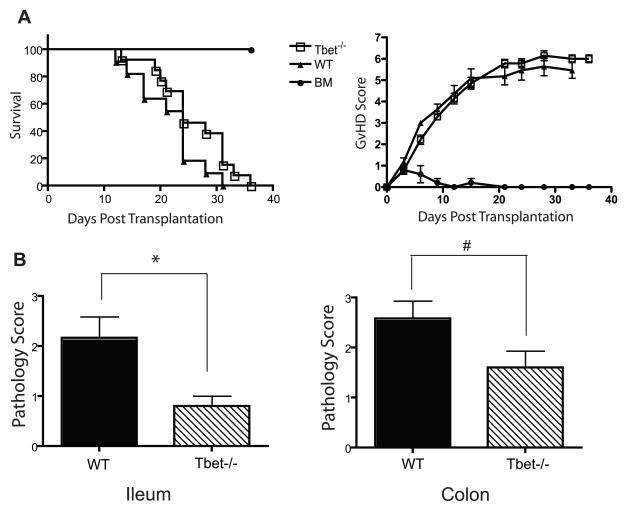
To determine if the inability to produce proinflammatory cytokines was sufficient to attenuate aGvHD we investigated the transcription factor that controls the expression of the Th1 cytokine IFN-γ, Tbx21(T-bet). Donor CD25− Tconv from T-bet−/− or WT mice supplemented with WT TCD BM were transplanted into lethally irradiated B6D2 F1 recipients. Interestingly, in this model, no difference was found in survival or GvHD score in mice receiving WT compared to T-bet−/− Tconv (Fig 6a). However, analysis fifteen days post transplantation revealed statistically significant decreased pathology in the ileum of recipients of T-bet−/− compared to wild type Tconv cells (p< 0.05, Fig 6b). A trend for decreased pathology was also seen in the colon (p = 0.08, Fig 6b). However, we did not find a difference in tissue pathology in other GvHD target organs given WT compared to T-bet−/− T cells (data not shown). These data support the established function for Th1 cells in the pathophysiology of GvHD in the GI tract, but indicate that in this haploidentical transplant model, T cell generation of T-bet was not critical for GvHD lethality (35).
Figure 6. T-bet−/− Tconv cells decrease pathology in the GI tract but do not attenuate GvHD.
(A) B6D2 F1 recipient mice were lethally irradiated (950 cGy) on day -1. Following irradiation on day 0 mice were injected intravenously with 4 × 106 WT or T-bet−/− Tconv cells supplemented with 3 × 106 WT TCD BM. Mice were monitored for survival and scored twice weekly for clinical GvHD. n=14 for T-bet−/− recipients, n=11 for WT recipients, n=4 bone marrow only. All recipient mice receiving BM only cells survived until the completion of the experiment. (B) On day 15 post transplantation organs were harvested from WT and T-bet−/− recipients and evaluated for pathology as described above. Error bars indicate SEM. Statistical significance was determined using Mann-Whitney test. *p<0.05, # p=0.09. Data are combined from 2 individual experiments.
GvL Response in the Absence of RORC
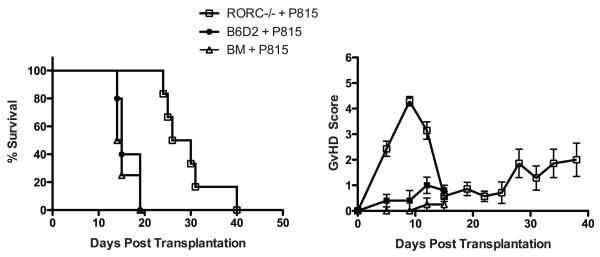
Next, we addressed whether the loss of RORC would impact the anti-tumor activity of SCT. Anti-tumor activity after transplantation was evaluated by adding 1 × 104 P815 cells to the donor bone marrow inoculum on day 0. One group of B6D2 F1 mice received RORC−/− Tconv cells in addition to WT TCD BM cells infused with P815 tumor cells. Since recipients of WT Tconv often succumb to GvHD before anti-tumor properties can be analyzed syngeneic T cells were used as a control. Syngeneic controls were given B6D2 Tconv supplemented with WT TCD BM infused with P815 tumor cells. Control mice received only WT TCD BM infused with P815 tumor cells. All mice receiving only WT TCD BM with P815 tumor cells died by day 20 due to tumor growth. Recipient mice receiving B6D2 Tconv died by day 20 due to tumor infiltration (Fig 7). Interestingly, survival was extended to day 40 in recipient mice given RORC−/− Tconvs and P815 cells indicating that the GvL response remained somewhat intact in mice given T cells lacking RORC. To demonstrate that this difference was not mediated by donor bone marrow cells, we administered RORC−/− TCD BM or WT TCD BM cells plus P815 cells to lethally irradiated B6D2 F1 recipient mice. As expected all recipient mice succumbed to tumor infiltration by day 30 (data not shown).
Figure 7. Improved anti-tumor responses in the absence of RORC.
Lethally irradiated B6D2 F1 mice were injected with 3 × 106 TCD BM with or without 4 × 106 WT B6D2 or RORC−/− Tconv cells. Additionally all recipient mice received 1 × 104 P815 cells with the BM inoculum. Survival was determined by Kaplan and Meier method. An improvement in overall survival was found in B6D2 F1 mice given RORC−/− Tconv cells compared to B6D2 T cells or BM + P815 cells (p < 0.05). n=7 recipients receiving RORC null T cells, n=5 recipients receiving B6D2 T cells, n=4 recipients receiving BM. Data combined from 2 individual experiments.
Discussion
Acute GvHD is mediated by donor T cells that recognize minor or major MHC disparities presented predominantly by host APCs. This process leads to activation, differentiation and T cell effector responses that are critical for the pathophysiology of acute GvHD. Over the past decade multiple investigators have identified new T cell subsets characterized by the activity of canonical transcription factors and the generation of specific cytokines. The T cell subset(s) critical for the pathophysiology of acute GvHD is currently unclear and the focus of this manuscript. Here, we find unexpectedly that the loss of the Th17 transcription factor, RORC, in donor CD25-depleted T cells led to markedly diminished acute GvHD. In three different models, recipient mice given RORC−/− Tconv cells had significantly less GvHD and increased survival compared to recipients given WT Tconv cells. The absence of RORC was associated with diminished GvHD in all target organs evaluated and correlated with diminished systemic generation of pro-inflammatory cytokines. The difference in pathology of GvHD target organs was not associated with a difference in frequency of regulatory T cells in these organs post transplant (Fulton and Serody unpublished). As was previously found, the absence of RORC on CD4+ T cells had no effect on GvHD outcome in a completely mismatched B6 into BALB/c model. Interestingly, in the B6 into B6D2 model, the absence of T-bet in donor T cells led to diminished pathology in the GI tract but no overall survival benefit. When challenged with P815 tumor cells, recipient mice receiving donor T cells lacking RORC survived longer than mice receiving bone marrow alone, indicating the presence of an anti-tumor GvL response. However, in both instances recipient mice succumbed eventually to tumor growth indicating that the GvL response is modestly compromised using T cells unable to generate RORC perhaps due to the diminished generation of TNF.
Previous work has clearly indicated a critical role for Th1/Tc1 T cells in the pathophysiology of acute GvHD particularly involving the GI tract. Thus, it was somewhat unexpected that the absence of T-bet alone, while diminishing GvHD in the small bowel and to a lesser extent the colon, was not associated with an improved overall survival. T-bet has been found to be critical for the generation of IFN-γ by CD4+ T cells and NK cells. However, the generation of IFN-γ by CD8+ T cells is not impaired in the absence of T-bet, which may be responsible for the similar survival (36). As we have found that RORC is required in the CD4+ T cell compartment, our data would be consistent with a role for IFN-γ generation by CD8+ T cells and TNF production by CD4+ T cells in the pathogenesis of acute GvHD.
Quite recently, Yu et al evaluated the ability of T cells from mice deficient in RORC or Tbx21 to induce GvHD (37). They found diminished GvHD using T cells from B6 Tbx21−/− donors but no difference in GvHD using CD4+ T cells from RORC−/− donors when given to lethally irradiated BALB/c recipients. Interestingly, they did find a modest survival benefit when infusing CD25-depleted T cells lacking RORC suggesting that the Treg compartment may not function in RORC mice as it does in WT mice. They found that BALB/c recipient mice given T cells from mice deficient in both RORC and Tbx21 had markedly diminished GvHD. This was associated with diminished generation of Th1 and Th17 cells and impaired expression of chemokine receptors important for the trafficking of donor T cells to GvHD target organs. Our data confirm and extend these findings as they relate to the function of RORC by evaluating the mechanism for the decreased GvHD when CD25-depleted donor T cells lacking RORC are given to lethally irradiated recipients. Additionally, we confirmed their previous data regarding the absence of an effect by infusing CD4+ T cells lacking RORC in the B6 into BALB/c model. We found substantially increased IL-17 in the colon and liver of BALB/c compared to B6D2 recipient mice after transfer of B6 T cells and TCD BM. Interestingly, the production of IL-17 was not impacted by the infusion of T cells lacking RORC suggesting that other donor or perhaps host cells generate substantial quantities of IL-17 in BALB/c recipients. Currently, we are evaluating which recipient cells generate IL-17 in BALB/c mice. Nonetheless, these data indicate that the model used may be critically important in interpreting the function of IL-17 after bone marrow transplantation.
We found a substantial difference in the generation of TNF and IL-17A in the serum and TNF in the colon of recipient mice given RORC−/− compared to WT T cells. Our previous data has indicated that TNF is critical for the systemic manifestations of GvHD mediated by Th17 cells. Interestingly, here we found that TNF production by CD4+ and/or CD8+ T cells was markedly reduced when that subset did not express RORC. However, this was compensated for by production of TNF from the WT T cells when both were given. However, GvHD was decreased only when TNF production was diminished by CD4+ T cells and not from CD8+ T cells indicating cell intrinsic differences in the function of TNF post SCT. We found an increase in the generation of dual positive IL-17A/IFN-γ T cells when WT Tconv cells were infused compared to RORC−/− Tconv cells 12 days post transplantation (Supp Fig 2). The generation of these cells, which may eventually become Th1 cells (Carlson and Serody unpublished), may be one mechanism for the decreased incidence and severity of aGvHD after the infusion of T cells unable to generate RORC.
For allogeneic transplantation to be successful requires the elimination of GvHD without compromising the anti-tumor, GvL activity of donor T cells. Here we found that donor T cells lacking RORC still mediated an anti-tumor response against the mastocytoma cell line, P815. Killing of P815 cells is dependent on the generation of IFN-γ and TNF (38). This suggests that the decreased generation of TNF in the absence of RORC is not sufficient to completely lose the anti-tumor activity of donor T cells.
In summary, we have shown that donor T cells lacking RORC do not mediate substantial acute GvHD in three different transplant models. This finding is dependent on the absence of RORC in CD4+ T cells, correlated with reduced generation of TNF and IL-17A systemically and TNF in the colon, and was important for the diminished GvHD that occurred in clinically relevant transplant models.
Supplementary Material
Acknowledgments
This study was supported in part by research funding from the National Institutes of Health R01 AI064363 and R56 AI082219 to J.S.S. and R01 HL56067, AI34495 and CA72669 to B.R.B. M.J.C., J.M.C., and L.E.O. were supported by training awards T32HL007149, K12CA120780, and T32CA009156 respectively. L.M.F. was supported by training award F31AI096900. This work was also supported by the Mary Elizabeth Thomas Endowment fund (JSS).
References
- 1.Forman SJ. Stem cell transplantation in acute leukemia. Current Opinion in Oncology. 1998;10:10–16. doi: 10.1097/00001622-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Khouri IF, Champlin RE. Nonmyeloablative stem cell transplantation for lymphoma. Semin Oncol. 2004;31:22–26. doi: 10.1053/j.seminoncol.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Tsai TW, Freytes CO. Allogenic bone marrow transplantation for leukemias and aplastic anemia. Advances in Internal Medicine. 1997;42:423–451. [PubMed] [Google Scholar]
- 4.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH, Negrin RS. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean RM, Bishop MR. Graft-versus-host disease: emerging concepts in prevention and therapy. Curr Hematol Rep. 2003;2:287–294. [PubMed] [Google Scholar]
- 7.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 9.Carayol G, Bourhis JH, Guillard M, Bosq J, Pailler C, Castagna L, Vernant JP, Pico JL, Hayat M, Chouaib S, Caignard A. Quantitative analysis of T helper 1, T helper 2, and inflammatory cytokine expression in patients after allogeneic bone marrow transplantation: relationship with the occurrence of acute graft-versus-host disease. Transplantation. 1997;63:1307–1313. doi: 10.1097/00007890-199705150-00019. [DOI] [PubMed] [Google Scholar]
- 10.Imamura M, Hashino S, Kobayashi H, Kubayashi S, Hirano S, Minagawa T, Tanaka J, Fujii Y, Kobayashi M, Kasai M, et al. Serum cytokine levels in bone marrow transplantation: synergistic interaction of interleukin-6, interferon-gamma, and tumor necrosis factor-alpha in graft-versus-host disease. Bone Marrow Transplant. 1994;13:745–751. [PubMed] [Google Scholar]
- 11.Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy WJ, Welniak LA, Taub DD, Wiltrout RH, Taylor PA, Vallera DA, Kopf M, Young H, Longo DL, Blazar BR. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102:1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Taylor PA, Mellor AL, Munn DH, Blazar BR. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood. 2008;111:3257–3265. doi: 10.1182/blood-2007-06-096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mus AM, Cornelissen F, Asmawidjaja PS, van Hamburg JP, Boon L, Hendriks RW, Lubberts E. Interleukin-23 promotes Th17 differentiation by inhibiting T-bet and FoxP3 and is required for elevation of interleukin-22, but not interleukin-21, in autoimmune experimental arthritis. Arthritis Rheum. 62:1043–1050. doi: 10.1002/art.27336. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 17.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov BS, II, McKenzie, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Bucher C, Koch L, Vogtenhuber C, Goren E, Munger M, Panoskaltsis-Mortari A, Sivakumar P, Blazar BR. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114:5375–5384. doi: 10.1182/blood-2009-05-221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 22.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, Young DA. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi T, Zhao D, Lin CL, Zhang C, Chen Y, Todorov I, LeBon T, Kandeel F, Forman S, Zeng D. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graftversus-host disease. Blood. 2008;112:2101–2110. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 28.Coghill JM, Carlson MJ, Panoskaltsis-Mortari A, West ML, Burgents JE, Blazar BR, Serody JS. Separation of graft-versus-host disease from graft-versus-leukemia responses by targeting CC-chemokine receptor 7 on donor T cells. Blood. doi: 10.1182/blood-2009-08-239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serody JS, Burkett SE, Panoskaltsis-Mortari A, Ng-Cashin J, McMahon E, Matsushima GK, Lira SA, Cook DN, Blazar BR. T-lymphocyte production of macrophage inflammatory protein-1{alpha} is critical to the recruitment of CD8+ T cells to the liver, lung, and spleen during graft-versus-host disease. Blood. 2000;96:2973–2980. [PubMed] [Google Scholar]
- 30.Blazar BR, Taylor PA, McElmurry R, Tian L, Panoskaltsis-Mortair A, Lam S, Lees C, Wasdschmidt T, Vallera DA. Engraftment of severe combined immune deficient (SCID) mice given allogeneic bone marrow (BM) via in utero or post-natal transfer. Blood. 1998;92:3949–3959. [PubMed] [Google Scholar]
- 31.van Den Brink MR, Moore E, Horndasch KJ, Crawford JM, Hoffman J, Murphy GF, Burakoff SJ. Fas-deficient lpr mice are more susceptible to graft-versus-host disease. J Immunol. 2000;164:469–480. doi: 10.4049/jimmunol.164.1.469. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of American Statistical Association. 1958;53:457. [Google Scholar]
- 33.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, Kirby SL, Luster AD, McKinnon K, Blazar BR, Serody JS. Differential Roles for CCR5 Expression on Donor T Cells during Graft-Versus-Host Disease Based on Pretransplant Conditioning. Journa of Immunology. 2004;173:845–854. doi: 10.4049/jimmunol.173.2.845. [DOI] [PubMed] [Google Scholar]
- 34.Iclozan C, Yu Y, Liu C, Liang Y, Yi T, Anasetti C, Yu XZ. T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol Blood Marrow Transplant. 16:170–178. doi: 10.1016/j.bbmt.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. [PubMed] [Google Scholar]
- 36.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Wang D, Liu C, Kaosaard K, Semple K, Anasetti C, Yu XZ. Prevention of GVHD while sparing GVL by targeting Th1 and Th17 transcription factor T-bet and ROR{gamma}t in mice. Blood. 2011 doi: 10.1182/blood-2011-03-340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hori K, Ehrke MJ, Mace K, Mihich E. Effect of recombinant tumor necrosis factor on tumoricidal activation of murine macrophages: synergism between tumor necrosis factor and gamma-interferon. Cancer Res. 1987;47:5868–5874. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.