Abstract
There remains considerable controversy in the management of eosinophilic disorders, mainly due to a paucity of information regarding the clinical interpretation of total blood eosinophil counts versus surface activation markers versus eosinophil-derived or eosinophil-influencing mediator levels. Regrettably, few tests have been validated that define a unique clinical or prognostic phenotype that is more useful than simply monitoring total blood eosinophil counts. In this manuscript, phenotypic (cell surface) markers, along with serum and tissue-based markers that have been examined in the context of disease activity, are reviewed. We also report the development of a novel assay for detecting soluble Siglec-8 (sSiglec-8), a protein likely derived largely from eosinophils, as a potential serum biomarker. The assay consists of a competitive ELISA using a recombinant Siglec-8-Fc fusion protein. The goal of this preliminary study was to determine if sSiglec-8 is a useful biomarker that differentiates among patients with various eosinophil-associated diseases. In the final analysis, it is fair to say that further research is sorely needed to fully understand and validate the utility of various biomarkers, including sSiglec-8, before their use in clinical practice can be recommended with confidence.
Keywords: Eosinophil, granule proteins, ELISA, soluble Siglec-8
1. Overview of involvement of eosinophils in allergic diseases and asthma
Eosinophils are important effector cells in human allergic diseases. Eosinophil numbers generally increase or decrease in association with allergic and asthmatic disease exacerbations and resolution, respectively (Baigelman et al., 1983; Gibson et al., 1990; Zimmerman et al., 1993). Allergic subjects have a higher number and a more activated state of eosinophils compared to non-atopic control subjects. Increased eosinophils in the tissues, blood, and bone marrow are a hallmark of many asthma phenotypes and, in general, elevated numbers of eosinophils correlate with disease severity (Douwes et al., 2002).
The accumulation of and increase in eosinophils are by no means unique to allergies and asthma. Blood and tissue eosinophilia is a common feature of numerous disorders, such as drug reactions, helminth infections, atopic dermatitis, Churg Strauss syndrome, some malignancies, Addison's disease, hypereosinophilic syndrome (HES), eosinophilic gastrointestinal disorders (EGID) and others (Rothenberg and Hogan, 2006; Hogan et al., 2008). In some of these conditions, such as eosinophilic esophagitis, localized tissue eosinophilia can occur without peripheral blood eosinophilia (Simon et al., 2010), and eosinophil-related phenotypic and serum markers tend to be less helpful for diagnosis and in tracking disease activity.
Human studies with selective eosinophil-depleting biologics, such as anti-IL-5 (mepolizumab, reslizumab) and anti-IL-5 receptor (benralizumab) show rapid, profound and sustained reductions of blood eosinophils with a concomitant, but not necessarily as effective tissue depletion from, for example, the upper and lower airways, skin, gastrointestinal tract and bone marrow (Flood-Page et al., 2003a; Flood-Page et al., 2003b; Menzies-Gow et al., 2003; Phipps et al., 2004; Gevaert et al., 2006; Stein et al., 2006; Rothenberg et al., 2008; Stein et al., 2008; Haldar et al., 2009; Nair et al., 2009; Straumann et al., 2009; Busse et al., 2010; Kahn et al., 2010; Kim et al., 2010; Castro et al., 2011; Gevaert et al., 2011; Spergel et al., 2011). This represents a marked effect on their normal turnover, as eosinophils are felt to circulate in blood for about 12 hours before entering tissues, where they persist for about 8-12 days. These therapeutic tools are gradually helping to define the specific contribution of the eosinophil in a range of disorders (Bochner and Gleich, 2010).
1.1. Phenotypic markers of eosinophil activation
Eosinophils are known to display a number of characteristic alterations when activated in vitro or in vivo. Among the first to be identified were those involving microscopic morphology, such as those associated with the so-called “hypodense” phenotype. Such cells frequently appear in the circulation of patients with eosinophilia, including HES and have a characteristic cytoplasmic appearance in which the granules appear condensed and give the false impression that the cells are partially degranulated (Caulfield et al., 1990). They are called hypodense eosinophils because, on density gradient centrifugation, they are lighter than the 1.080 gm/L specific gravity normally used to separate granulocytes from mononuclear cells and therefore tend to float up into the mononuclear cell layers in such gradients. This microscopic phenotype can be reproduced in vitro under activation and priming conditions, especially those associated with exposure to eosinophil survival cytokines (Rothenberg et al., 1988).
A variety of laboratories have described other phenotypic cell surface markers associated with eosinophil activation. Again, similar approaches have been used, such as comparing eosinophil surface markers on cells from normal donors versus those with allergic, parasitic or other disorders with peripheral blood eosinophilia. Table 1 lists a variety of surface markers that have been examined in activated eosinophils. Some of these surface proteins are decreased or shed, such as L-selectin (CD62L) (Matsumoto et al., 1998), CD23, CD31 and PSGL-1 (CD162) (Davenpeck et al., 2000), while the others, including CD35, CD11b, CD66, CD69 and CD81, are increased (Mawhorter et al., 1996; Pignatti et al., 2002; Wedi et al., 2002; Yoon et al., 2007; Suzukawa et al., 2008). In one study, Matsumoto et al used an extensive panel of monoclonal antibodies previously used to define leukocyte phenotypes including eosinophils (Ebisawa et al., 1995) and determined that CD44, which is normally expressed on eosinophils, is increased about two-to-three-fold during activation, whereas CD69 goes from essentially undetectable to relatively high levels (Matsumoto et al., 1998). Many other molecules, such as CD9 (Fernvik et al., 1999) and CD49d/CD29 (VLA-4), which are expressed on eosinophils but not neutrophils, do not appear to change in level of surface expression very much, if at all, during in vitro activation. However, VLA-4 is reportedly lower on eosinophils of patients with eosinophilia (Kayaba et al., 2001), while activation of eosinophils enhances functional beta 1 integrin binding affinity that can be detected by epitope-specific activation antibodies (Werfel et al., 1996).
Table 1.
Examples of changes in eosinophil surface markers after activation
| Decreased | Increased | No change |
|---|---|---|
| CD31 | CD11b | CD9 |
| CD62L | CD35 | CD11a/CD18 |
| CD125 | CD44 | CD49d/CD18 |
| CD162 | CD66 | Siglec-8 |
| CD69 | ||
| CD81 |
In vivo, cells that have undergone migration into tissues often display phenotypic evidence of activation. For example, following endobronchial allergen provocation in allergic individuals, eosinophils harvested from the lung by lavage have higher levels of CD11b and CD69 with reduced levels of CD62L (L-selectin) (Georas et al., 1992; Julius et al., 1999). The same pattern has been found on lung cells in eosinophilic pneumonia (Nishikawa et al., 1992). In peripheral blood from patients with parasitic diseases, most of the markers mentioned above are indeed altered, as well as in patients with HES and Churg-Strauss syndrome (CSS). Probably most consistent and most readily detectable and useful is surface expression of CD69, since it is not normally expressed on the surface of eosinophils except following activation. Otherwise, alteration of these phenotypic markers is more of a subtle change in the level of surface expression rather than presence versus absence. The same can be said in vitro in models of transendothelial and transepithelial migration, all of which tend to reproduce these phenotypic changes in cells undergoing the transmigration process (Ebisawa et al., 1997). Other antibodies can detect novel priming-related epitopes on eosinophils that can detect changes occurring on eosinophils in vivo (Luijk et al., 2005). Whether any of these changes are due exclusively to cytokine or chemokine activation in vitro or in vivo or other aspects of the migration process per se is difficult to determine. Also reported is the observation that markers of eosinophil activation, including reduced levels of IL-5 receptor and enhanced expression of the activated conformation of integrins such as CD49d/CD29 (so called VLA-4 or very late activation antigen-4) also accompanies diseases such as asthma and reverts to the non-activated forms in subjects treated with anti-eosinophil therapies such as mepolizumab (Johansson et al., 2006; Johansson et al., 2008; Stein et al., 2008; Johansson et al., 2012).
1.2. Serum markers related to eosinophil activation and disease activity
For decades, laboratories have utilized sensitive and specific ELISAs to measure levels of eosinophil-derived granule proteins, including eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), eosinophil peroxidase (EPO), and major basic protein (MBP) (Kita et al., 2003). Because these measurements are performed on serum, it is possible that platelet activation and clotting per se could influence some of these measurements. Nevertheless, there is a fairly extensive literature in which these assays were used to measure levels in a variety of biological fluids. There are numerous examples of experimental and disease conditions correlating levels of various granule proteins with situations such as allergic disease, severity in asthma, and atopic dermatitis, as well as bronchial hyperreactivity. In an extensive literature review of ECP (Koh et al., 2007), the authors state that it can be quantified in many types of biological fluids, where it may, for example, correlate with airway inflammation but not airway hyper-responsiveness. However, in familial eosinophilia, EDN levels (and other measures of eosinophil activation such as CD69) did not correlate with total eosinophil count, but rather with clinical disease activity (Kay and Klion, 2004).
It is often the case that similar correlations are detected simply by assessing total eosinophil counts, although this is usually not the case in urinary analyses where soluble mediators are more readily detected. Other serum markers associated with eosinophilia include levels of eosinophil survival-promoting, activity, likely representing IL-5 and/or GM-CSF (Ackerman and Bochner, 2007). Studies have begun to look at levels of IL-5 receptor and a soluble form of its receptor, but unfortunately, these molecules are not measurable in all conditions associated with eosinophilia (Wilson et al., 2011). Another soluble marker whose utility has been explored is the chemokine TARC (CCL17), which has been shown to correlate with disease activity in atopic dermatitis (Hijnen et al., 2004). One sizable retrospective study of hypereosinophilic syndrome suggested that higher levels of TARC were associated with a higher likelihood of steroid responsive disease, meaning that there disease activity was more likely to be well-controlled with oral corticosteroids if they had elevated serum TARC levels before treatment (Ogbogu et al., 2009). In another study, total blood eosinophil counts, EDN and eotaxin-3 levels correlated modestly with the density of esophageal eosinophils in children with eosinophilic esophagitis (Konikoff et al., 2006). Thus, despite some promising data, at this point, without prospective studies, one cannot confidently combine the use of peripheral blood eosinophil counts with any of these assays to make a definitive determination with regard to eosinophil activation and disease activity that would be clinically useful in diagnosis or disease management beyond that of simply following blood eosinophil counts.
1.3. Tissue markers related to eosinophil activation
For a time, it was in vogue to use a particular monoclonal antibody (EG2), which was purported to detect an activated or secreted form of ECP (Tai et al., 1984). This antibody was used to detect extracellular forms of these granule proteins that expressed neo-epitopes recognized by this particular monoclonal antibody. Despite initial enthusiasm, subsequent studies suggested that EG2 histochemical staining could occur under a variety of conditions and therefore this type of analysis and its interpretation are no longer considered reliable (Jahnsen et al., 1994; Nakajima et al., 1999). In performing tissue staining for eosinophils, it remains useful to use antibodies to eosinophil granule proteins, including ECP, as these are relatively specific for eosinophils.
Recent studies in mouse systems have embraced the use of antibodies to Siglec-F, the functional paralog of Siglec-8, as this is highly expressed on eosinophils (Tateno et al., 2005; Voehringer et al., 2007; Wu et al., 2011). In the mouse, unlike in humans, Siglec-F is not expressed on basophils or mast cells. Consequently, eosinophils are the only peripheral blood granulocyte that expresses Siglec-F. In lung tissues, however, the situation is not so simple, as alveolar macrophages in the mouse express Siglec-F (Stevens et al., 2007). Finally, it is important to point out that inducing eosinophil degranulation has been difficult to accomplish in vitro. Several laboratories have reported conditions for inducing eosinophil degranulation in vitro, including the use of IgA and cytokines, but a relatively modest percentage of total granule contents end up being released (Kita et al., 2003). Eosinophils also secrete sulfidopeptide leukotrienes and a variety of cytokines and chemokines , but again the latter two are generally produced in relatively small quantities compared to other cells. It is thus clear that more information is needed regarding mechanisms of eosinophil degranulation, before we can use levels of such mediators in tissues or other body compartments as useful biomarkers of disease activity.
1.4. Discovery of Siglec-8 and the development of a novel eosinophil-related biomarker assay to measure soluble Siglec-8 levels
In the late 1990's, our lab participated in a collaborative effort to identify novel eosinophil-specific markers. These efforts led to the discovery of Siglec-8, formerly known as sialoadhesin factor-2 or SAF-2 (Floyd et al., 2000; Kikly et al., 2000). Siglecs (sialic acid-binding, immunoglobulin-like lectins) are single-pass transmembrane cell surface proteins found predominantly on leukocytes (Varki and Angata, 2006; von Gunten and Bochner, 2008). Siglec-8 was initially thought to be an eosinophil-specific cell surface protein, but once antibodies were made to Siglec-8, it was quickly discovered that it is also expressed on mast cells and weakly on basophils (Kikly et al., 2000). Siglec-8 recognizes the glycan 6'-sulfo-sialyl Lewis X and contains an intracellular ITIM domain (Bochner et al., 2005; Bochner, 2009). Its engagement on eosinophils results in apoptosis; whereas, in mast cells it was shown to inhibit allergic degranulation responses activated via FcεRI (Nutku et al., 2003; Yokoi et al., 2008). While eosinophil surface levels of Siglec-8 do not appear to differ among donors with various allergic or eosinophilic conditions (Bolos-Sy et al., 2000), it was noted that Siglec-8 levels tend to decline as cells are handled in vitro. Indeed, a common paradigm for many siglecs is that their surface levels are reduced following external ligation (Tateno et al., 2007). This occurs as a result of internalization, with the receptors often getting recycled back to the cell surface. We therefore hypothesized that the reduction in surface Siglec-8 could be the result of the protein being internalized or shed from the cell surface. While Siglec-8 internalization remains actively under investigation, new tests were needed to look for shedding. This led to the development of a novel assay to detect and quantify soluble Siglec-8 (sSiglec-8) levels. Here we report the validation of this assay and its use to screen sera from normal donors, those with allergies, and subjects with a variety of hypereosinophilic disorders. The objectives of this study were two-fold: (1) to determine whether we could use sSiglec-8 as a biomarker to differentiate among patients with various disorders, especially those associated with eosinophilia; and (2) to use this information to aid in the development of a more extensive validation study. The ultimate goal of this work is the development of an assay with clinical and/or prognostic utility.
2. Materials and methods
2.1. Reagents
Monoclonal Siglec-8 antibody 2C4 (IgG1 mouse anti-human Siglec-8) was generated using a Siglec-8-human IgG1 Fc fusion protein (Siglec-8-Fc) as previously described (Kikly et al., 2000). This dimeric Siglec-8-Fc fusion protein is the same reagent previously used to identify putative Siglec-8 glycan ligands (Bochner et al., 2005; Guo et al., 2011).
2.2. Biotinylation of Siglec-8-Fc
Siglec-8-Fc was dialyzed against PBS and biotinylated using 1 mg of biotin X-NHydroxysuccinimide ester (biotin-X-HSE, EMD Chemicals, Gibbstown, NJ), dissolved in N, N Dimethylformamide (100 μl), per mg of Siglec-8-Fc. The reaction mixture was stirred at room temperature for 1 h and then overnight at 4°C. After dialyzing again to remove free biotin, bovine serum albumin was added (10 mg per mg of Siglec-8-Fc-biotin), filtration sterilized and stored frozen at -20°C until used.
2.3. Eosinophil purification and generation of serum samples from various donors
Eosinophils from normal and allergic donors were purified from peripheral blood after density-gradient centrifugation using Percoll (Pharmacia, Uppsala, Sweden) for separation of mononuclear cells, followed by erythrocyte hypotonic lysis and immunomagnetic negative selection with CD16 negative selection employing Dynabeads™ from Invitrogen (Carlsbad, CA) (Matsumoto et al., 1997) or negative selection with Miltenyi microbeads (Auburn, CA). Eosinophil purity and viability were consistently higher than 98%, with neutrophils being the only contaminating cells.
Serum samples were obtained from normal donors (n=5) and those with mild allergies (n=5). Subjects from both groups had normal eosinophil counts < 300/mm3. Serum also was collected at the time of diagnosis, prior to initiation of therapy, and stored frozen at -80°C, from patients with benign eosinophilia (defined as subjects with eosinophil counts > 1,500/mm3 for ≥ 5 years without any symptoms or evidence of end organ involvement in the absence of theraphy) (n=3) eosinophilic gastrointestinal disease (EGID, n=2), the lymphocytic variant of HES (LHES, n=3), the myeloproliferative variant of HES associated with a deletion mutation on chromosome 4 involving FIP1L1-PDGFR (Cools et al., 2003), now best referred to according to the World Health Organization classification of PDGFRA-associated myeloproliferative neoplasm (Vardiman and Hyjek, 2011) (PDGFRA-associated MPN, n=2), other patients meeting criteria for HES but not classifiable as either LHES or PDGFRA-associated MPN (HES, n=7), and those diagnosed with helminthic parasitic diseases (PARA, n=3). The two parasitized subjects with high eosinophil counts (3272 and 1929/mm3) had Loa Loa infections, while the third subject (eosinophil count 710/mm3) had strongyloides. All sera were obtained from pre-treatment on the day of the eosinophil count provided.
2.4. Quantification of sSiglec-8 using a competitive binding ELISA
Due to the lack of two antibodies that bind different determinants on Siglec-8 to set up a two-site immunoenzymetric assay, a competitive binding ELISA was developed to quantify sSiglec-8 levels in human serum. In this assay, 2C4 mAb was bound to plastic microtiter plates (optimal at 2 μg/ml in PBS, overnight at room temperature). The plate was then blocked with PBS-1% bovine serum albumin (1 h, room temperature, 0.3 ml/well). Following a buffer wash, 50 μl of the test sera or reference serum dilutions containing known amounts of Siglec-8-Fc were pipetted into their respective wells. Thirty minutes later, 50 μl of biotinylated Siglec-8-Fc (optimal at 30 ng/ml) was added. The plate was gently tapped to mix the contents in each well. If unlabeled Siglec-8-Fc was present, it would bind to the 2C4 mAb attached to the plate and thus proportionally block the binding of biotinylated Siglec-8-Fc. After 1 h incubation at room temperature, the primary binding reaction was stopped with a buffer wash (PBS-0.05% Tween20) and streptavidin-horseradish peroxidase (HRP, Sigma-Aldrich, St. Louis, MO) (1 μg/ml) was added to all wells to detect bound biotinylated Siglec-8-Fc. Following a final 1 h incubation at room temperature, the plate was washed with PBS-0.05% Tween20 and the quantity of bound biotinylated Siglec-8-Fc-avidin-HRP complexes was detected with the addition of ABTS substrate containing H2O2 (Sigma-Aldrich, St. Louis, MO). Optical density (OD405nm) was measured using a microtiter plate reader (Dynatech MR4000) and plotted as a function of the optical density obtained with the standards containing known quantities of unlabeled Siglec-8-Fc. All analyses were done in triplicate.
3. Results and discussion
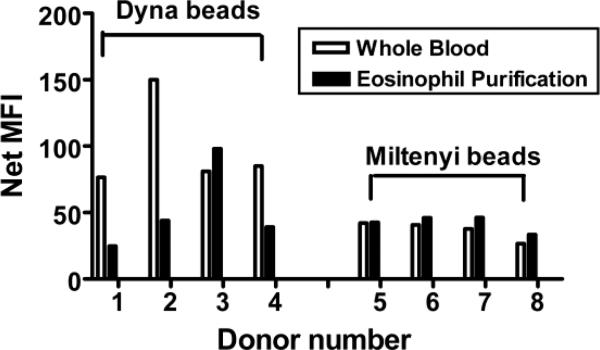
Figure 1 shows that purification of eosinophils using the DynaBeads system often results in a loss of Siglec-8 surface expression compared to baseline on cells in whole blood. This does not appear to occur using the Miltenyi microbeads negative selection system. These data suggest that under certain circumstances, levels of Siglec-8 decline with cell handling. While many siglecs are internalized after ligation (Tateno et al., 2007), no ligands were likely present under our experimental conditions. Thus, we hypothesized that Siglec-8 could be actively shed from the eosinophils in vivo and to test this hypothesis, an assay to measure sSiglec-8 was developed.
Figure 1.
Effect of eosinophil purification on cell surface levels of Siglec-8. Open bars represent data in whole blood (n=8) while black bars represent data after eosinophil purification (n=8). MFI, mean fluorescence intensity.
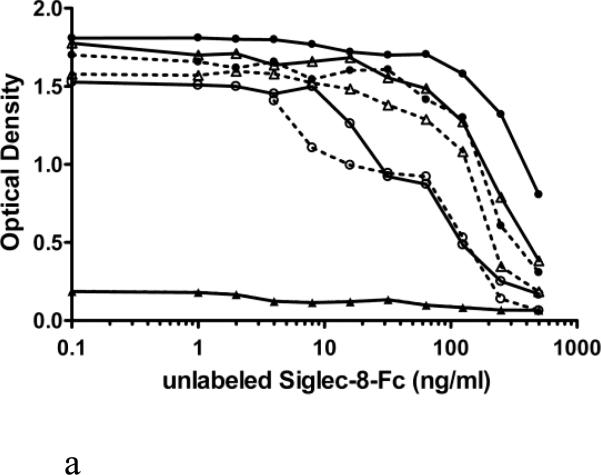
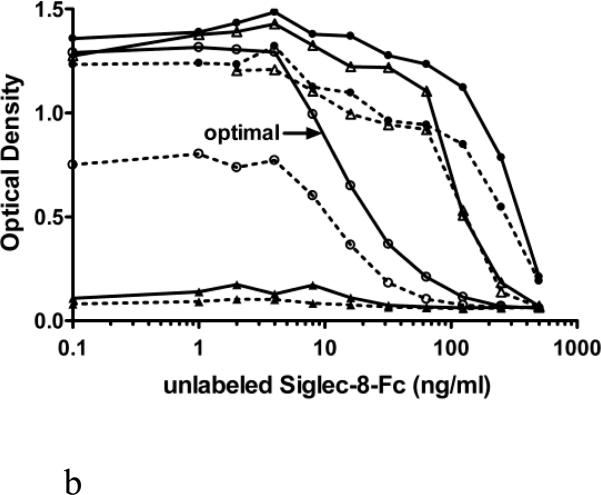
In Figure 2, two modes of reagent addition and selection of reagent concentrations are compared: either simultaneous addition of unlabeled (1-500 ng/ml) and biotin labeled (20 versus 40 ng/ml) Siglec-8-Fc for 1.5 h into a plate coated with mAb 2C4 at 1.25, 2.5, 5 and 10 μg/ml (panel A), or sequential addition of unlabeled (1-500 ng/ml, 30 min) Siglec-8-Fc followed by a 1 h incubation with biotin-labeled (20 versus 40 ng/ml) Siglec-8-Fc (without washing) into a plate coated with mAb 2C4 at 1.25, 2.5, 5 and 10 μg/ml. In comparison to simultaneous addition (Fig. 2A), the sequential addition of these reagents (Fig. 2B) resulted in an assay with the greatest analytical sensitivity. Therefore, in subsequent experiments, sequential addition of reagents was employed using 2.5 μg/ml for mAb 2C4 coating and 40 ng/ml for biotin-labeled Siglec-8-Fc. This resulted in an analytical sensitivity below 10 ng/ml (Figure 2B).
Figure 2.
Comparison of simultaneous (Panel A) versus sequential addition (Panel B) of unlabeled and biotinylated Siglec-8-Fc for optimization of the sSiglec-8 ELISA. Compared were four concentrations of 2C4 mAb (1.25 μg/ml, close triangle; 2.5 μg/ml, open circle; 5 μg/ml, open triangle; and 10 μg/ml, close circle) and two different concentrations of Siglec-8-Fc-biotin (20 ng/ml, dashed lines; 40 ng/ml, solid lines) with addition of non-biotinylated Siglec-8-Fc (1-500 ng/ml). The designation of “optimal” in Panel B refers to the best assay conditions, namely sequential addition of unlabeled Siglec-8-Fc, followed by addition of 40 ng/ml Siglec-8-Fc biotin, using 2.5 μg/ml of mAb 2C4 as the capture reagent.
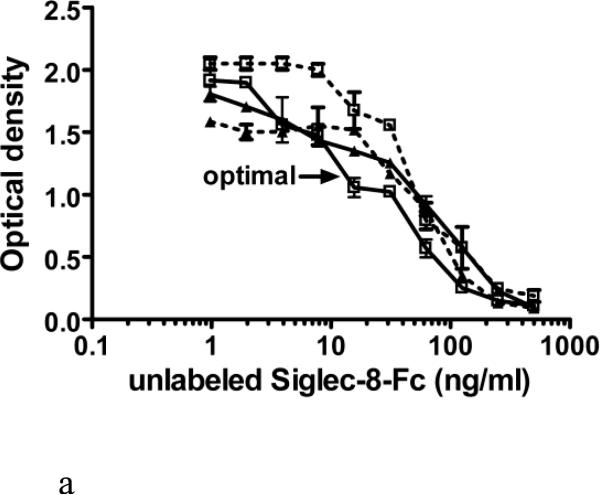
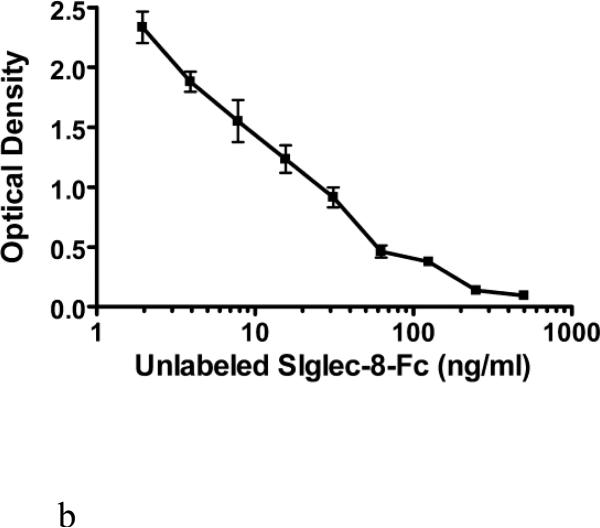
Figure 3 shows further efforts towards optimization of the calibration curve. In these experiments, the concentrations of unlabeled Siglec-8-Fc were varied, while comparing either 2 or 2.5 μg/ml of immobilized 2C4 mAb and either 30 or 40 ng/ml of biotinylated Siglec-8-Fc. These experiments showed that the addition of 30 ng/ml of biotin-Siglec-8-Fc into a plate coated with 2C4 at 2 μg/ml produced the most analytically sensitive ELISA (Figure 3A). These conditions were then replicated in three separate experiments, yielding the sSiglec-8 calibration curve shown in Figure 3B, with a potential range of detection from 2-500 ng/ml.
Figure 3.
Further fine optimization of the sSiglec-8 ELISA (Panel A) and validation of the selected assay conditions in replicate experiments (Panel B). Panel A shows sequential addition (30 min) of unlabeled Siglec-8-Fc (1-500 ng/ml) followed by a 1 hour incubation of Siglec-8-Fc biotin (30 ng/ml, solid lines or 40 ng/ml, dashed lines) without washing into a plate coated with 2C4 mAb at either 2 μg/ml (squares) or 2.5 μg/ml (triangles). The designation of “optimal” in Panel A refers to the best assay conditions, namely sequential addition of unlabeled Siglec-8-Fc, followed by addition of 30 ng/ml Siglec-8-Fc biotin, using 2 μg/ml of mAb 2C4 as the capture reagent, the conditions used to generate the averaged data (n=3, mean ± SD) shown in Panel B.
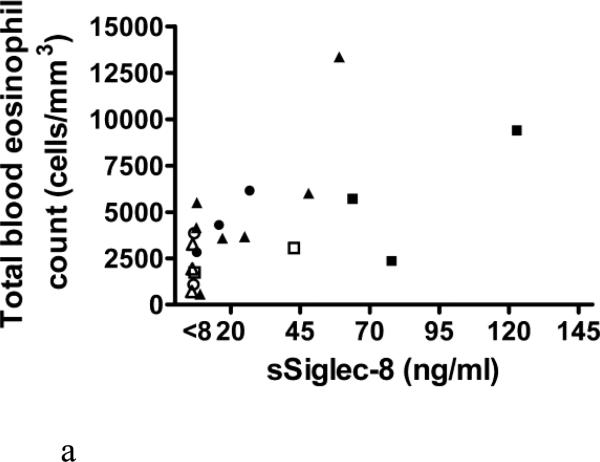
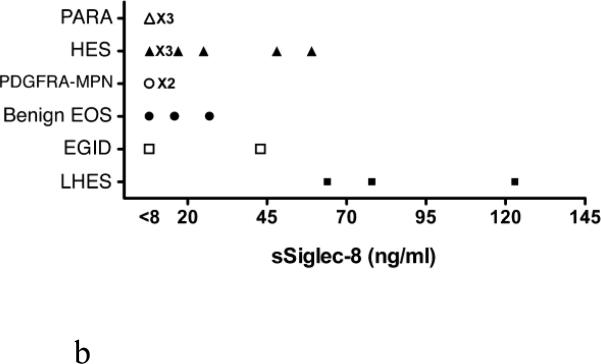
Levels of sSiglec-8 were then determined in ten serum samples from subjects with normal eosinophil counts (<300/mm3, five from atopics, five from non-atopics), and in all cases sSiglec-8 levels were undetectable (data not shown). Next, 17 serum samples from patients with various forms of eosinophilia were masked and assayed for sSiglec-8 levels. As shown in Figure 4A, sSiglec-8 was detectable in some but not all subjects with eosinophilia and weakly correlated with total blood eosinophil counts across all diagnoses. Although the numbers were small, more impressive correlations (those with R2 values displayed in the Figure 4A legend) were seen between total eosinophil counts and sSiglec-8 levels among subjects with HES, benign eosinophilia (albeit with low sSiglec-8 levels) and LHES. When these same sSiglec-8 levels were re-plotted by diagnosis (Figure 4B), it became more readily observable that subjects with PDGFRA-associated MPN and PARA (2 subjects were infected by Loa loa, 1 subject by strongyloides) had undetectable sSiglec-8 levels, while LHES sera contained the highest sSiglec-8 levels measured and values for HES and EGID varied.
Figure 4.
Analysis of serum samples for levels of sSiglec-8 using the conditions in Figure 3B. Sera from 17 newly diagnosed and untreated patients with various disorders including parasitic diseases (PARA, △), hypereosinophilic syndrome (HES, ▲), PDGFRA-associated MPN (PDGFRA-MPN, ○), benign eosinophilia (benign EOS, ●), eosinophilic gastrointestinal disorders (EGID, □) and lymphocytic HES (LHES, ■). Panel A. Correlations between total eosinophil counts and levels of sSiglec-8. Although the numbers are small, statistically significant correlations (those with R2 values) are seen for some disorders. Panel B: Replotting of data in Panel A by diagnosis to better show the disease-related levels of sSiglec-8.
There are several shortcomings of the data in its current form. First, the exact source of the measured sSiglec-8 in these serum samples cannot be determined (e.g., they could come from mast cells or basophils). Second, the mechanism of sSiglec-8 generation is unknown and will require additional studies, including those that explore whether cell surface shedding is occurring. Certainly the present findings require further verification in larger cohorts of patients both prospectively and before and after medical intervention. Studies would also need to be done in which disease activity and severity are monitored along with total blood eosinophil counts and sSiglec-8 levels in order to ascertain whether the latter measurements would be useful either as a disease-specific biomarker or as a clinical test that influences medical management, perhaps by reflecting disease control. Nevertheless, these results suggest that sSiglec-8 levels correlate with simultaneously obtained peripheral blood total eosinophil counts in some disorders, such as HES and LHES. It is especially tempting in future studies to explore the diagnostic utility of sSiglec-8 levels >60 ng/ml in the diagnosis of LHES.
4. Conclusions
Over the years, a variety of serum, cell surface, tissue-based and other markers of eosinophil activation and involvement in disease have been studied. So far, none have been clearly found to be superior to simply measuring blood eosinophil counts as a way to diagnose allergic or eosinophilic diseases, or to follow disease activity and response to treatment. Here we report the development of a sensitive competitive ELISA to detect sSiglec-8 in human serum samples obtained from patients with various forms of eosinophilia. This analysis has allowed us to identify sSiglec-8 as a potential biomarker for certain hypereosinophilic diseases such as HES and LHES. Whether these measurements would be useful in distinguishing LHES from other forms of eosinophilia, or for predicting disease exacerbations, remains to be determined. Further research is required to validate the utility of all eosinophil-related biomarkers before their use in clinical practice can be recommended with confidence.
Acknowledgements
We thank Sherry Hudson for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman SJ, Bochner BS. Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol Allergy Clin North Am. 2007;27:357–75. doi: 10.1016/j.iac.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigelman W, Chodosh S, Pizzuto D, Cupples LA. Sputum and blood eosinophils during corticosteroid treatment of acute exacerbations of asthma. Am. J. Med. 1983;75:929–36. doi: 10.1016/0002-9343(83)90871-9. [DOI] [PubMed] [Google Scholar]
- Bochner BS. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy. 2009;39:317–24. doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–12. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Gleich GJ. What targeting eosinophils has taught us about their role in diseases. J Allergy Clin Immunol. 2010;126:16–25. doi: 10.1016/j.jaci.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolos-Sy AM, Bochner BS, Togias A, Schleimer RP, Beck LA. Surface expression of CCR3 and CD49d on eosinophils is not increased in allergic asthma and allergic rhinitis. J. Allergy Clin. Immunol. 2000;105:S259. [Google Scholar]
- Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, Coyle AJ, Koike M, Spitalny GL, Kiener PA, Geba GP, Molfino NA. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–1244. doi: 10.1016/j.jaci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, Wilkins HJ, Henkel T, Nair P. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–32. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- Caulfield JP, Hein A, Rothenberg ME, Owen WF, Soberman RJ, Stevens RL, Austen KF. A morphometric study of normodense and hypodense human eosinophils that are derived in vivo and in vitro. Am. J. Pathol. 1990;137:27–41. [PMC free article] [PubMed] [Google Scholar]
- Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, Kutok J, Clark J, Galinsky I, Griffin JD, Cross NC, Tefferi A, Malone J, Alam R, Schrier SL, Schmid J, Rose M, Vandenberghe P, Verhoef G, Boogaerts M, Wlodarska I, Kantarjian H, Marynen P, Coutre SE, Stone R, Gilliland DG. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–14. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- Davenpeck KL, Brummet ME, Sterbinsky SA, Mayer RJ, Bochner BS. Activation of human leukocytes reduces surface P-selectin glycoprotein ligand-1 (PSGl-1, CD162) and adhesion to P-selectin in vitro. J. Immunol. 2000;165:2764–2772. doi: 10.4049/jimmunol.165.5.2764. [DOI] [PubMed] [Google Scholar]
- Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–8. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa M, Bochner BS, Schleimer RP. Eosinophil-endothelial interactions and transendothelial migration. In: Bochner BS, editor. Adhesion molecules in allergic diseases. Marcel Dekker, Inc.; New York: 1997. pp. 173–186. [Google Scholar]
- Ebisawa M, Schleimer RP, Bickel C, Bochner BS. Phenotyping of purified human peripheral blood eosinophils using the blind panel mAb. In: Schlossman S, Boumsell L, Gilks W, Harlan J, Kishimoto T, Morimoto C, Ritz J, Shaw S, Silverstein R, Springer T, Tedder T, Todd R, editors. Leukocyte Typing V: White Cell Differentiation Antigens. Vol. 1. Oxford University Press; New York: 1995. pp. 1036–1038. [Google Scholar]
- Fernvik E, Lundahl J, Magnusson CG, Hallden G. The effect of in vitro activation and platelet interaction on the CD9 distribution and adhesion properties of human eosinophils. Inflamm Res. 1999;48:28–35. doi: 10.1007/s000110050383. [DOI] [PubMed] [Google Scholar]
- Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003a;112:1029–36. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003b;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, Steel J, Crocker PR. Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J. Biol. Chem. 2000;275:861–6. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- Georas SN, Liu MC, Newman W, Beall WD, Stealey BA, Bochner BS. Altered adhesion molecule expression and endothelial activation accompany the recruitment of human granulocytes to the lung following segmental antigen challenge. Am. J. Respir. Cell Mol. Biol. 1992;7:261–269. doi: 10.1165/ajrcmb/7.3.261. [DOI] [PubMed] [Google Scholar]
- Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, Holtappels G, Tavernier J, van Cauwenberge P, Bachert C. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–41. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, De Ruyck N, Blomme K, Sousa AR, Marshall RP, Bachert C. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–95. e1–8. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- Gibson PG, Dolovich J, Girgis-Gabardo A, Morris MM, Anderson M, Hargreave FE, Denburg JA. The inflammatory response in asthma exacerbation - changes in circulating eosinophils, basophils and their progenitors. Clin. Exp. Allergy. 1990;20:661–668. doi: 10.1111/j.1365-2222.1990.tb02705.x. [DOI] [PubMed] [Google Scholar]
- Guo JP, Brummet ME, Myers AC, Na HJ, Rowland E, Schnaar RL, Zheng T, Zhu Z, Bochner BS. Characterization of expression of glycan ligands for Siglec-F in normal mouse lungs. Am J Respir Cell Mol Biol. 2011;44:238–43. doi: 10.1165/rcmb.2010-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijnen D, De Bruin-Weller M, Oosting B, Lebre C, De Jong E, Bruijnzeel-Koomen C, Knol E. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell- attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol. 2004;113:334–40. doi: 10.1016/j.jaci.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- Jahnsen FL, Brandtzaeg P, Halstensen TS. Monoclonal antibody EG2 does not provide reliable immunohistochemical discrimination between resting and activated eosinophils. J. Immunol. Methods. 1994;175:23–36. doi: 10.1016/0022-1759(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Johansson MW, Barthel SR, Swenson CA, Evans MD, Jarjour NN, Mosher DF, Busse WW. Eosinophil beta 1 integrin activation state correlates with asthma activity in a blind study of inhaled corticosteroid withdrawal. J Allergy Clin Immunol. 2006;117:1502–4. doi: 10.1016/j.jaci.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Johansson MW, Han ST, Gunderson KA, Busse WW, Jarjour NN, Mosher DF. Platelet Activation, P-selectin, and Eosinophil beta1-integrin Activation in Asthma. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201109-1712OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol. 2008;180:7622–35. doi: 10.4049/jimmunol.180.11.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius P, Luttmann W, Knoechel B, Kroegel C, Matthys H, Virchow JC., Jr. CD69 surface expression on human lung eosinophils after segmental allergen provocation. Eur Respir J. 1999;13:1253–9. doi: 10.1183/09031936.99.13612609. [DOI] [PubMed] [Google Scholar]
- Kahn JE, Grandpeix-Guyodo C, Marroun I, Catherinot E, Mellot F, Roufosse F, Blétry O. Sustained response to mepolizumab in refractory Churg-Strauss syndrome. J. Allergy Clin. Immunol. 2010 doi: 10.1016/j.jaci.2009.10.014. in press. [DOI] [PubMed] [Google Scholar]
- Kay AB, Klion AD. Anti-interleukin-5 therapy for asthma and hypereosinophilic syndrome. Immunol Allergy Clin North Am. 2004;24:645–66. doi: 10.1016/j.iac.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Kayaba H, Yamada Y, Cui CH, Saito N, Honda K, Kobayashi Y, Urayama O, Chihara J. Expression of VLA-4 on eosinophils decreases in patients with eosinophilia. Int. Arch. Allergy Immunol. 2001;125:33–37. doi: 10.1159/000053850. [DOI] [PubMed] [Google Scholar]
- Kikly KK, Bochner BS, Freeman S, Tan KB, Gallagher KT, D'Alessio K, Holmes SD, Abrahamson J, Hopson CB, Fischer EI, Erickson-Miller CL, Tachimoto H, Schleimer RP, White JR. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells and basophils. J. Allergy Clin. Immunol. 2000;105:1093–1100. doi: 10.1067/mai.2000.107127. [DOI] [PubMed] [Google Scholar]
- Kim S, Marigowda G, Oren E, Israel E, Wechsler M. Mepolizumab as a steroid-sparing treatment in patients with Churg-Strauss syndrome. J. Allergy Clin. Immunol. 2010;125:1336–43. doi: 10.1016/j.jaci.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Kita H, Adolphson CR, Gleich GJ. Biology of eosinophils. In: Adkinson JNF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Allergy Principles and Practice. 6th Edition Mosby; Philadelphia: 2003. pp. 305–332. [Google Scholar]
- Koh GC, Shek LP, Goh DY, Van Bever H, Koh DS. Eosinophil cationic protein: is it useful in asthma? A systematic review. Respir Med. 2007;101:696–705. doi: 10.1016/j.rmed.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Konikoff MR, Blanchard C, Kirby C, Buckmeier BK, Cohen MB, Heubi JE, Putnam PE, Rothenberg ME. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1328–36. doi: 10.1016/j.cgh.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Luijk B, Lindemans CA, Kanters D, van der Heijde R, Bertics P, Lammers JW, Bates ME, Koenderman L. Gradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challenge. J Allergy Clin Immunol. 2005;115:997–1003. doi: 10.1016/j.jaci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Appiah-Pippim J, Schleimer RP, Bickel CA, Beck LA, Bochner BS. CD44 and CD69 represent different types of cell surface activation markers for human eosinophils. Am. J. Respir. Cell Mol. Biol. 1998;18:860–866. doi: 10.1165/ajrcmb.18.6.3159. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Sterbinsky SA, Bickel CA, Zhou DW, Kovach NL, Bochner BS. Regulation of a4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1 (VCAM-1). J. Allergy Clin. Immunol. 1997;99:648–656. doi: 10.1016/s0091-6749(97)70027-7. [DOI] [PubMed] [Google Scholar]
- Mawhorter SD, Stephany DA, Ottesen EA, Nutman TB. Identification of surface molecules associated with physiologic activation of eosinophils - application of whole-blood flow cytometry to eosinophils. J. Immunol. 1996;156:4851–4858. [PubMed] [Google Scholar]
- Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, Kay AB, Denburg J. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111:714–9. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Loegering DA, Kita H, Kephart GM, Gleich GJ. Reactivity of monoclonal antibodies EG1 and EG2 with eosinophils and their granule proteins. J. Leukoc. Biol. 1999;66:447–54. doi: 10.1002/jlb.66.3.447. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Morii T, Ako H, Hamada K, Saito S, Narita N. In vivo expression of CD69 on lung eosinophils in eosinophilic pneumonia - CD69 as a possible activation marker for eosinophils. J Allerg Clin Immunol. 1992;90:169–174. doi: 10.1016/0091-6749(92)90068-d. [DOI] [PubMed] [Google Scholar]
- Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, Leiferman KM, Nutman TB, Pfab F, Ring J, Rothenberg ME, Roufosse F, Sajous MH, Sheikh J, Simon D, Simon HU, Stein ML, Wardlaw A, Weller PF, Klion AD. Hypereosinophilic syndrome: A multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319–1325. doi: 10.1016/j.jaci.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps S, Flood-Page P, Menzies-Gow A, Ong YE, Kay AB. Intravenous anti-IL-5 monoclonal antibody reduces eosinophils and tenascin deposition in allergen-challenged human atopic skin. J Invest Dermatol. 2004;122:1406–12. doi: 10.1111/j.0022-202X.2004.22619.x. [DOI] [PubMed] [Google Scholar]
- Pignatti P, Perfetti L, Galdi E, Pozzi V, Bossi A, Biale C, Moscato G. Increased CD69 expression on peripheral blood eosinophils after specific inhalation challenge. Allergy. 2002;57:411–6. doi: 10.1034/j.1398-9995.2002.23454.x. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, Schwartz LB, Rosenwasser LJ, Ring J, Griffin EF, Haig AE, Frewer PI, Parkin JM, Gleich GJ. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–28. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Owen WF, Jr., Silberstein DS, Woods J, Soberman RJ, Austen KF, Stevens RL. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J. Clin. Invest. 1988;81:1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Wardlaw A, Rothenberg ME. Organ-specific eosinophilic disorders of the skin, lung and gastrointestinal tract. J. Allergy Clin. Immunol. 2010;126:45–9. doi: 10.1016/j.jaci.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, 3rd, O'Gorman MA, Abonia JP, Young J, Henkel T, Wilkins HJ, Liacouras CA. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, Filipovich AH, Assa'ad AH, Rothenberg ME. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006;118:1312–9. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Stein ML, Villanueva JM, Buckmeier BK, Yamada Y, Filipovich AH, Assa'ad AH, Rothenberg ME. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol. 2008;121:1473–83. doi: 10.1016/j.jaci.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, Beglinger C, Smith DA, Patel J, Byrne M, Simon HU. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2009;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, Saito H, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–53. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- Tai PC, Spry CJ, Peterson C, Venge P, Olsson I. Monoclonal antibodies distinguish between storage and secreted forms of eosinophil cationic protein. Nature. 1984;309:182–184. doi: 10.1038/309182a0. [DOI] [PubMed] [Google Scholar]
- Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6'-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–35. doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, Paulson JC. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol. 2007;27:5699–710. doi: 10.1128/MCB.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardiman J, Hyjek E. World health organization classification, evaluation, and genetics of the myeloproliferative neoplasm variants. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2011;2011:250–6. doi: 10.1182/asheducation-2011.1.250. [DOI] [PubMed] [Google Scholar]
- Varki A, Angata T. Siglecs - the major sub-family of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007;81:1434–44. doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. Ann N Y Acad Sci. 2008;1143:61–82. doi: 10.1196/annals.1443.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedi B, Wieczorek D, Stünkel T, Breuer K, Kapp A. Staphylococcal exotoxins exert proinflammatory effects through inhibition of eosinophil apoptosis, increased surface antigen expression (CD11b, CD45, CD54, and CD69), and enhanced cytokine-activated oxidative burst, thereby triggering allergic inflammatory reactions. J. Allergy Clin. Immunol. 2002;109:477–484. doi: 10.1067/mai.2002.121702. [DOI] [PubMed] [Google Scholar]
- Werfel S, Yednock T, Matsumoto K, Sterbinsky SA, Schleimer RP, Bochner BS. Functional regulation of b1 integrins and human eosinophils by divalent cations and cytokines. Am. J. Respir. Cell Mol. Biol. 1996;14:45–52. doi: 10.1165/ajrcmb.14.1.8534485. [DOI] [PubMed] [Google Scholar]
- Wilson TM, Maric I, Shukla J, Brown M, Santos C, Simakova O, Khoury P, Fay MP, Kozhich A, Kolbeck R, Metcalfe DD, Klion AD. IL-5 receptor alpha levels in patients with marked eosinophilia or mastocytosis. J Allergy Clin Immunol. 2011;128:1086–92. e1–3. doi: 10.1016/j.jaci.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–7. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi H, Choi OH, Hubbard W, Lee H-S, Canning BJ, Lee HH, Ryu S-D, Bickel CA, Hudson SA, MacGlashan DW, Jr., Bochner BS. Inhibition of FcεRI-dependent mediator release and calcium flux from human mast cells by Siglec-8 engagement. J. Allergy Clin. Immunol. 2008;121:499–505. doi: 10.1016/j.jaci.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Yoon J, Terada A, Kita H. CD66b regulates adhesion and activation of human eosinophils. J Immunol. 2007;179:8454–62. doi: 10.4049/jimmunol.179.12.8454. [DOI] [PubMed] [Google Scholar]
- Zimmerman B, Lanner A, Enander I, Zimmerman RS, Peterson CG, Ahlstedt S. Total blood eosinophils, serum eosinophil cationic protein and eosinophil protein X in childhood asthma: relation to disease status and therapy. Clin. Exp. Allergy. 1993;23:564–70. doi: 10.1111/j.1365-2222.1993.tb00895.x. [DOI] [PubMed] [Google Scholar]