Abstract
The human capacities for overcoming prepotent actions and flexibly switching between tasks represent cornerstones of cognitive control. Functional neuroimaging has implicated a diverse set of brain regions contributing to each of these cognitive control processes. However, the extent to which attentional switching and response conflict draw on shared or distinct neural mechanisms remains unclear. The current study examined the neural correlates of response conflict and attentional switching using event-related functional magnetic resonance imaging (fMRI) and a fully randomized 2×2 design. We manipulated an arrow-word version of the Stroop task to measure conflict and switching in the context of a single task decision, in response to a common set of stimuli. Under these common conditions, both behavioral and imaging data showed significant main effects of conflict and switching but no interaction. However, conjunction analyses identified frontal regions involved in both switching and response conflict, including the dorsal anterior cingulate cortex (dACC) and left inferior frontal junction. In addition, connectivity analyses demonstrated task-dependent functional connectivity patterns between dACC and inferior temporal cortex for attentional switching and between dACC and posterior parietal cortex for response conflict. These results suggest that the brain makes use of shared frontal regions, but can dynamically modulate the connectivity patterns of some of those regions, to deal with attentional switching and response conflict.
Keywords: Cognitive control, Attentional switching, Response conflict, Prefrontal cortex, fMRI, functional connectivity
1. INTRODUCTION
Cognitive control refers to the ability to integrate our thoughts and actions with internal goals in order to perform challenging tasks (Botvinick et al., 2001; Miller and Cohen, 2001). Two of the most commonly used paradigms to gauge cognitive control are switching and response conflict tasks. For example, in a typical response conflict task, subjects are required to respond to an arrow pointing left or right with a left or right button press. The incongruent condition (e.g., when a left button press is required for a right-pointing arrow) results in an increase in reaction time (RT) compared to the congruent condition (e.g., when a right button press is required for a right-pointing arrow), which is referred to as the conflict or interference effect. In a typical switching paradigm, subjects are asked to switch between two tasks based on relevant stimulus dimensions (e.g., making a decision about a shape or its color). The switch condition results in increased RT compared to the non-switch condition, which is referred to as the switch cost.
These two cognitive control tasks share many cognitive requirements such as the need to represent and update task-related information, shift attention, and deal with potential conflict (MacLeod, 1991; Monsell, 2003). However, the nature of conflicting representations is different in switching and response conflict conditions. In switching tasks, competition typically exists between stimulus dimensions themselves (e.g., between a shape and color), whereas in response conflict tasks competition stems from the incongruency between stimuli and their associated responses (e.g., a left button press is required for a right-pointing arrow). Thus, there may be both shared and distinct neural correlates of switching and response conflict tasks.
Many previous neuroimaging studies have separately explored the neural correlates of attentional switching (Crone et al., 2006; Dove et al., 2000a; Kim et al., 2011a; Sohn et al., 2000) or response conflict (Botvinick et al., 1999; Kerns et al., 2004; MacDonald et al., 2000). However, only a few studies have directly compared attentional switching and response conflict in a common group of subjects using a 2×2 factorial design with four conditions: nonswitch-congruent (NsCon), switch-congruent (SwCon), nonswitch-incongruent (NsInc), and switch-incongruent (SwInc) conditions (Barber and Carter, 2005; Sylvester et al., 2003). One of these studies reported a large switching by conflict behavioral interaction and an imaging interaction in regions such as left dorsolateral prefrontal cortex (DLPFC), medial PFC including dorsal anterior cingulate cortex (dACC), and bilateral parietal cortex (Sylvester et al., 2003). The other study focused on main effects and did not explore behavioral or imaging interactions (Barber and Carter, 2005).
In these previous studies, switching and conflict were instantiated at different levels. Switching was instantiated at the stimulus level (e.g., switching between relevant visual/perceptual features of the stimuli), and response conflict was instantiated at the level of opposing response rules. For example, in the congruent condition of Sylvester et al. (2003), subjects added to a running count when an arrow was facing its prepotent direction (count left for a left-pointing arrow), and in the incongruent condition subjects added to a running count when an arrow was opposite to its prepotent direction (count right for a left-pointing arrow). Different cues were used to indicate the compatible and incompatible rules, which were presented in different scanning blocks. Similarly, in the congruent condition of Barber and Carter (2005), subjects were required to respond with a right button press to the word “right”, and in the incongruent condition subjects were required to respond with a left button press to the word “right”.
In these kinds of designs, the SwInc condition is different from other conditions in that it is the only one in which two separate levels (stimulus and response rules) must be handled by the brain. That is, in the SwInc condition, the brain must deal not simply with the simultaneous requirement to switch and inhibit conflict, but it must also deal with the fact that these processes need to be instantiated at different levels (i.e., one must discriminate/switch between stimulus features and deal with an opposing response rule). Consequently, one would expect a large behavioral interaction associated with the increased difficulty of the SwInc condition compared to all other conditions. Thus, neural correlates of the SwInc condition of such designs may reflect some combination of a switching by conflict interaction, and increased difficulty of associated with the need to deal with different levels in the SwInc condition.
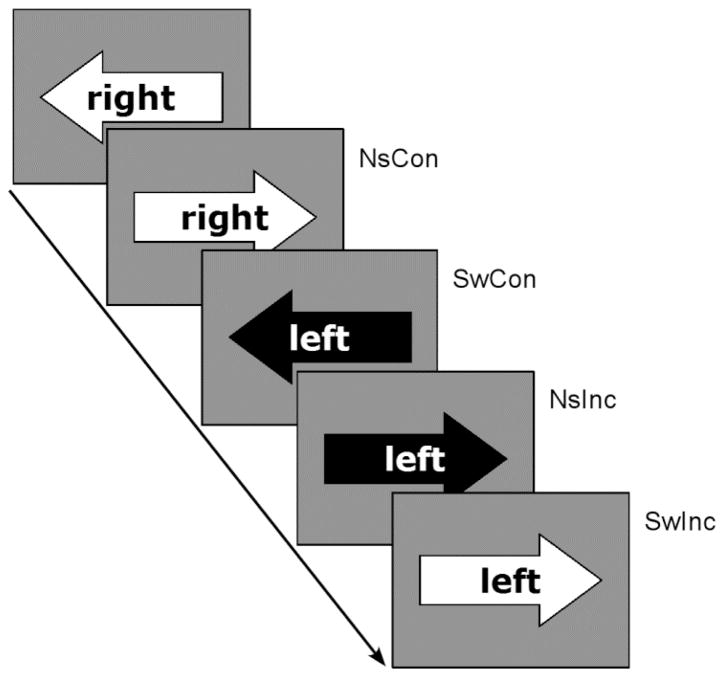
One way to focus more specifically on shared and distinct neural correlates of switching and response conflict would be to use a design in which these conditions are instantiated at the same level, such as the stimulus level. Toward that end, we designed an arrow-word version of the Stroop task in which task stimuli consisted of a word (“left” or “right”) embedded within a left-pointing or right-pointing arrow. The task required subjects to press the hand-held button (i.e., left or right) corresponding to the task appropriate cue (the color black) in each condition (Figure 1). In this kind of design, switching and response conflict are instantiated at a common stimulus level: subjects must switch between an arrow and a word, and must deal with conflict arising from the incongruency between the arrow and the word (i.e., the word left embedded within a right-pointing arrow).
Figure 1.
Illustration of the experimental design. Subjects are asked to respond to the direction of the arrow or word. The color black served as the task cue. For example, the correct response for the first trial is right (even though the arrow points left) because the word “right” is presented in black. Note: NsCon, nonswitch-congruent; NsInc, nonswitch-incongruent; SwCon, switch-congruent; SwInc, switch-incongruent.
The use of this kind of 2×2 event-related design, in which switching and response conflict are instantiated at the same stimulus level, is likely to eliminate potential interaction effects associated with the requirement to deal with multiple levels (e.g., stimuli and rules) in the SwInc condition. Using this kind of design, interaction effects would be more reflective of cognitive processes required by the brain in order to handle switching and conflict simultaneously (compared to dealing with these processes separately) and would identify regions contributing to these cognitive processes. In contrast, the lack of behavioral and imaging interaction effects (in the context of main effects) could be interpreted as suggestive that the brain can apply a shared set of cognitive processes to handle switching and conflict without encountering a large cost when these tasks are both instantiated at a common (stimulus) level.
In the present study, we also sought to identify brain regions that contribute significantly to both switching and conflict, and to provide insight about how such regions may contribute to information processing across these different cognitive domains. There is evidence that certain prefrontal cortex (PFC) regions can contribute to information processing across different cognitive domains through interaction with distinct posterior regions (e.g., Duncan, 2001; Gold and Buckner, 2002; Miller, 2000). Thus, based upon this literature, in the present study we were interested in (1) identifying PFC that may contribute to both switching and response conflict, and (2) determining if these shared PFC regions interact with distinct posterior regions during these switching and conflict tasks (see Figure 1 for task stimuli). Toward that end, we first used conjunction analyses to identify PFC regions that make a significant contribution to both switching and response conflict. We then performed functional connectivity analyses to determine if these shared PFC regions collaborate with distinct posterior regions during these different domains of cognitive control.
2. RESULTS
2.1. Behavioral data
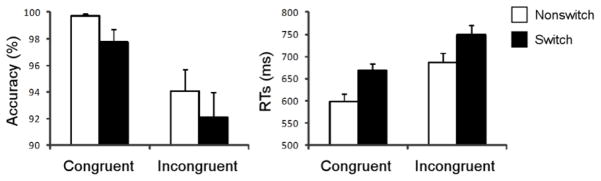
Mean accuracy and RTs are presented in Figure 2. Accuracy was generally high, with mean accuracies of all conditions ≥ 92 %. Accuracy and RT data were analyzed using two-way repeated measures ANOVAs. For accuracy, the main effect of switching was significant (F(1,15)=5.28, p=0.036), reflecting behavioral performance that was more accurate for nonswitch trials than switch trials. The main effect of conflict was also significant (F(1,15)=14.94, p=0.002), indicating the fact that accuracy was higher for congruent trials than incongruent trials. In contrast, there was no switching by conflict interaction (F(1,15)=0.00, p>0.999).
Figure 2.
Behavioral Results. Mean accuracy (left) and mean RTs (right) are presented for each task condition. Error bars represent the standard errors of the means.
Results from the RT analyses of correct trials generally paralleled the accuracy results. The two main effects of switch (F(1,15)=64.53, p<0.001) and conflict (F(1,15)=50.29, p<0.001) were significant. The switch cost was 66 ms and the conflict effect was 84 ms. The mean RTs for each condition were: 599 ms (SD=65) in the NsCon condition, 686 ms (SD=86) in the NsInc condition, 668 ms (SD=63) in the SwCon condition, and 749 ms (SD=83) in the SwInc condition. However, there was no switching by conflict interaction (F(1,15)=0.47, p=0.502).
2.2. Imaging data
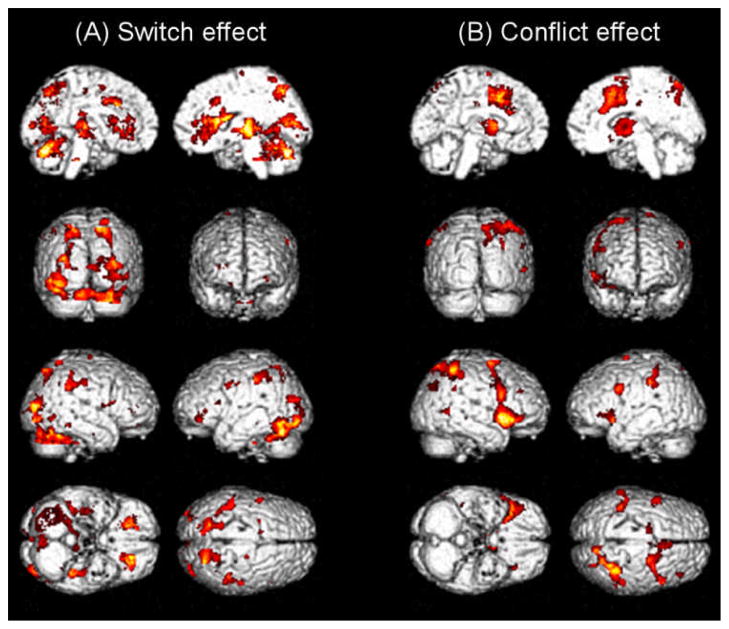
The imaging main effects of switching and conflict are presented in Figure 3, and cluster details are listed in Tables 1 and 2. The main effect of switching (switch > nonswitch) activated broad regions of frontal, parietal, and temporal cortices (Figure 3a and Table 1). Specifically, bilateral ventrolateral PFC regions (VLPFC; BA 47), left posterior lateral PFC regions including middle frontal gyrus (BA 9) and precentral gyrus (inferior frontal junction, IFJ; BA 6), and medial frontal regions including medial posterior portions of the superior frontal gyrus (BA 6) and the dorsal anterior region of the cingluate gyrus (dACC; BAs 24 and 32) were recruited. Activation in parietal regions was observed bilaterally in the precuneus (BA7), superior parietal lobule (BA 7), supramarginal gyrus (BA 40), and postcentral gyrus (BA 3). Temporal regions activated included the parahippocampal gyrus (BA 36) and inferior temporal and fusiform gyri (BAs 19 and 37). Finally, the insula and caudate were also activated by the main effect of switching.
Figure 3.
Imaging main effects. A distributed network of brain activations was observed for the switch effect (A) and the conflict effect (B).
Table 1.
Significant areas of activation for the main effect of switching.
| Region | Hem | Coordinates
|
BA | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Inferior Frontal Gyrus | L | −25 | 34 | −2 | 47 | 5.14 |
| R | 21 | 32 | −3 | 47 | 4.08 | |
| Middle Frontal Gyrus | L | −22 | −4 | 45 | 6 | 3.50 |
| Precentral Gyrus | L | −50 | −3 | 37 | 6 | 3.50 |
| Medial Frontal Gyrus | L | −13 | −5 | 54 | 6 | 3.55 |
| Anterior Cingulate | R | 4 | 29 | 20 | 24 | 3.56 |
| Cingulate Gyrus | L | −16 | 0 | 40 | 24/32 | 3.80 |
| Posterior Cingulate | L | −22 | −68 | 7 | 30 | 3.91 |
| Postcentral Gyrus | R | 26 | −24 | 40 | 3 | 3.86 |
| Supramarginal Gyrus | R | 41 | −42 | 32 | 40 | 3.65 |
| Precuneus | L | −20 | −67 | 41 | 7 | 3.97 |
| R | 19 | −68 | 45 | 7 | 3.94 | |
| Superior Parietal Lobule | R | 18 | −56 | 59 | 7 | 3.42 |
| Parahippocampal Gyrus | L | −38 | −34 | −9 | 36 | 4.43 |
| Inferior Temporal/Fusiform Gyri | R | 45 | −59 | −4 | 19/37 | 3.42 |
| Insula | L | −33 | 12 | 19 | 3.73 | |
| R | 34 | 2 | 19 | 3.77 | ||
| Caudate Body | L | −22 | 12 | 18 | 3.60 | |
| Putamen | R | 25 | 0 | 19 | 4.55 | |
| Cerebellum | R | 18 | −66 | −31 | 4.39 | |
Hem, hemisphere; BA, Brodmann area; L, left; R, right
Table 2.
Significant areas of activation for the main effect of conflict.
| Region | Hem | Coordinate
|
BA | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Inferior Frontal Gyrus/Insula | R | 49 | 14 | 2 | 45 | 5.16 |
| L | −32 | 26 | 6 | 45 | 4.00 | |
| Middle Frontal Gyrus | R | 42 | 9 | 32 | 9 | 4.84 |
| R | 39 | 29 | 36 | 9 | 3.82 | |
| Superior Frontal Gyrus | R | 8 | 9 | 48 | 6 | 4.86 |
| L | −15 | −12 | 70 | 6 | 4.28 | |
| Precentral Gyrus | L | −44 | −1 | 34 | 6 | 4.25 |
| Cingulate Gyrus | R | 6 | 11 | 41 | 24/32 | 4.79 |
| Superior Parietal Lobule /Precuneus | R | 28 | −53 | 43 | 7 | 4.82 |
| Inferior Parietal Lobule | L | −41 | −43 | 46 | 40 | 3.89 |
| L | −31 | −52 | 38 | 40 | 3.67 | |
| Supramarginal Gyrus | L | −57 | −42 | 35 | 40 | 3.89 |
| R | 48 | −48 | 29 | 40 | 3.56 | |
| Precuneus | R | 26 | −69 | 32 | 19 | 3.89 |
| Middle Temporal Gyrus | R | 52 | −53 | 6 | 21/37 | 3.94 |
| Claustrum | R | 34 | 10 | 2 | 4.94 | |
| L | −32 | 12 | 1 | 4.45 | ||
| Caudate | L | −8 | 1 | 10 | 4.54 | |
| R | 8 | 3 | 10 | 3.94 | ||
| Lentiform Nucleus | L | −20 | 1 | 13 | 4.06 | |
| R | 12 | 2 | 3 | 3.77 | ||
| Thalamus | R | 12 | −8 | 6 | 3.88 | |
Hem, hemisphere; BA, Brodmann area; L, left; R, right
The main effect of conflict activated a distributed network of fronto-cingulo-parietal regions (Figure 3b and Table 2). These regions included broad portions of frontal areas including bilateral VLPFC (BAs 45 and 47), right middle frontal gyrus (BA 9), bilateral superior frontal gyrus (BA 6), left precentral gyrus (IFJ; BA 6), and dACC (BAs 24 and 32). Parietal regions activated by conflict were the superior and inferior parietal lobules (BAs 7 and 40), precuneus (BAs 7 and 19), and supramarginal gyrus (BA 40). Other areas including middle temporal gyrus (BA21/37), caudate regions and thalamus were also activated by the main effect of conflict.
In contrast to the robust imaging main effects observed, no significant activations were observed in the analysis of the interaction effect between switch and conflict, even at a liberal uncorrected threshold (p<0.001) and no cluster threshold. Similarly, lowering the uncorrected threshold to p < 0.005, with no cluster threshold, resulted in the activation of only a few scattered voxels. Thus, any potential interaction effects, if present at all, were very weak.
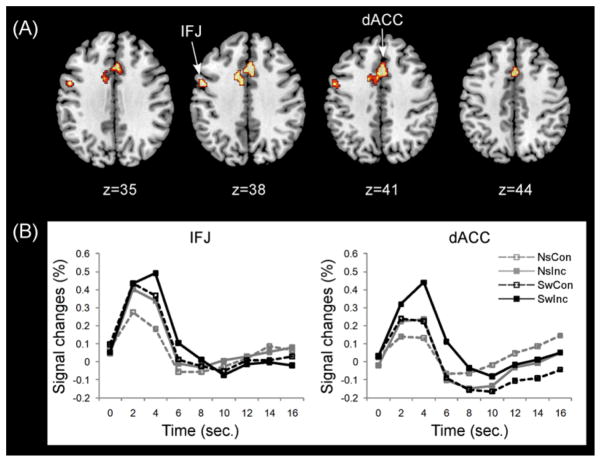
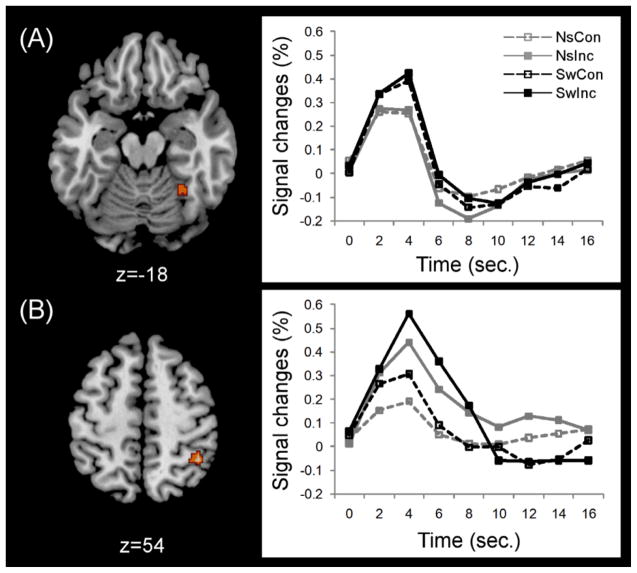
A conjunction analysis was then performed to identify common areas of activation across switch and conflict (Figure 4 and Table 3). The results showed prominent activation of precentral gyrus (IFJ; BA6) and dACC (BAs 24 and 32) within PFC.
Figure 4.
Common PFC activations across switching and conflict. (A) Significant PFC activations were observed in the left inferior frontal junction (IFJ) and dorsal anterior cingulate cortex (dACC). (B) BOLD responses in the left IFJ and dACC corresponding to each task condition. Note: NsCon, nonswitch-congruent; NsInc, nonswitch-incongruent; SwCon, switch-congruent; SwInc, switch-incongruent.
Table 3.
Common frontal areas of activation for both switching and conflict
| Region | Hem | Coordinate
|
BA | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Precentral Gyrus | L | −50 | −3 | 37 | 6 | 3.50 |
| Cingulate Gyrus | L | −1 | 13 | 42 | 24/32 | 3.44 |
Hem, hemisphere; BA, Brodmann area; L, left; R, right
Results from the PPI analyses demonstrated task-preferential increases in functional connectivity between dACC and posterior regions, but not between IFJ and posterior regions. Specifically, as shown in Figure 5, the PPI analyses revealed increased functional connectivity between dACC and right fusiform gyrus (x=37, y=−45, z=−16; BA 37) during switching compared to conflict. In contrast, increased functional connectivity was observed between dACC and right inferior parietal lobule (x=37, y=−51, z=49; BA 40) during conflict compared to switching. Correlations were then run between behavioral switch/conflict effects and PPI connectivity betas for dACC-fusiform gyrus and dACC-IPL. The results demonstrated negative correlations between PPI betas for dACC-IPL connectivity and behavioral conflict effect (r=−.59, p<.05), and the between PPI betas for dACC-fusiform gyrus connectivity and behavioral switch cost (r=−.53, p<.05).
Figure 5.
Task-preferential increases in functional connectivity between dACC and posterior regions. (A) Increased functional connectivity between dACC and right fusiform gyrus during switching compared to conflict. (B) Increased functional connectivity between dACC and right inferior parietal lobule during conflict compared to switching. Middle panels plot BOLD timecourses for each condition. Right panels show correlations between PPI betas for right fusiform gyrus and behavioral switch costs and between PPI betas for right inferior parietal lobule and conflict effects. Note: NsCon, nonswitch-congruent; NsInc, nonswitch-incongruent; SwCon, switch-congruent; SwInc, switch-incongruent.
3. DISCUSSION
The current study explored the neural correlates of attentional switching and response conflict instantiated at a common stimulus level. Subjects were required to shift attention between an arrow and a word, and deal with conflict arising from the incongruency between the arrow and the word. Under these common conditions, both behavioral and imaging data yielded main effects for switching and response conflict. In addition, conjunction analyses identified regions involved in both switching and response conflict, and PPI analyses demonstrated task-dependent functional connectivity patterns between dorsal anterior cingulate cortex and different posterior regions. These results suggest that the brain makes use of shared frontal regions, but can dynamically modulate the connectivity patterns of some of those regions, to deal with attentional switching and response conflict.
3.1. Neural correlates of attentional switching
Our data demonstrated that the main effect of switching (switch > nonswitch) was associated with activation of bilateral anterior-VLPFC (~BA47/11; pars orbitalis/orbital gyri), left posterior lateral frontal regions such as the inferior frontal junction (IFJ), the dorsal anterior region of the cingluate gyrus (dACC), bilateral posterior parietal cortex (PPC), and inferior temporal cortex (ITC). This network of regions is consistent with previous attentional switching studies (Brass and von Cramon, 2004; Dove et al., 2000b; Dreher and Berman, 2002; Kim et al., 2011a; Kim et al., 2012).
Among the regions associated with switching, the prominent activation of anterior-VLPFC during switching may relate to conceptual retrieval processes. Switch trials required the retrieval of a previously utilized conceptual rule (e.g., retrieve the meaning of the word “left”) compared to nonswitch trials, and anterior-VLPFC has been linked with conceptual rule retrieval (Bunge et al., 2003; Crone et al., 2006). In particular, the left anterior-VLPFC has been linked with semantic retrieval, which is a form of conceptual retrieval (Badre et al., 2005; Gold and Buckner, 2002; Gold et al., 2006). In addition, right anterior-VLPFC has been linked with retrieval of non-verbal conceptual representations (Bunge et al., 2004). Our results suggest that bilateral recruitment of anterior-VLPFC may be required in order to switch between rules associated with linguistic (e.g., the word “left”) and shape (e.g., left-pointing arrow) information.
3.2. Neural correlates of response conflict
The main effect of conflict (incongruent > congruent) was associated with a fronto-cingulo-parietal network including DLPFC (BA 9), bilateral mid-VLPFC (~BA 45; pars triangularis), dACC (BA 24/32), and PPC (BA 40). Among these areas, DLPFC has been shown to contribute to regulatory processes in response to conflict (Botvinick et al., 1999; Botvinick et al., 2001; Kerns et al., 2004; MacDonald et al., 2000). The activation of right and left mid-VLPFC regions we observed may relate to the resolution of response conflict and semantic conflict, respectively. Right mid-VLPFC has been implicated in inhibitory response control in tasks such as the Go/No-Go and stop-signal tasks (Garavan et al., 1999; Garavan et al., 2002; Konishi et al., 1999; Rubia et al., 2003) and its activation during incongruent trials thus likely reflects inhibition of the competing (but incorrect) response. In contrast, left mid-VLPFC has been associated with greater demands on selection of relevant semantic representations from among competitors (Badre et al., 2005; Gold et al., 2006; Thompson-Schill et al., 1997) and its activation during incongruent trials is thus likely to reflect inhibitory control of interfering semantic representation (e.g., the meaning of the word “left” has to be suppressed when it is embedded within a black, right-pointing arrow). However, future research will be required to directly compare the roles of left and right mid-VLPFC regions in conflict processing.
3.3. Common cognitive control mechanisms involved in attentional switching and response conflict
In contrast to the robust main effects observed, the use of a single task and common stimulus set resulted in no behavioral or imaging interactions between switching and response conflict. However, it is important to note that additive behavioral effects were observed: the behavioral RT in the SwInc condition was longer than those in the SwCon or NsInc conditions. This suggests that attentional switching and response conflict associated with a common task and stimulus set may be handled by common brain regions in a serial manner. Results from our conjunction analysis served to identify two of those common brain regions.
In particular, prominent activation common to switching and response conflict was observed in the dACC and left IFJ, suggesting that these regions may support cognitive processes common to these tasks. If this were the case, then two expectations should follow. The first expectation is that there should be previous evidence that these commonly recruited frontal regions have been activated in previous studies of switching or response conflict tasks. There is evidence for this expectation, as activation of dACC and left IFJ has frequently been reported in previous studies which have separately explored switching or conflict (Dove et al., 2000b; Kim et al., 2010; Kim et al., 2011a; Kim et al., 2011b; MacDonald et al., 2000; Sohn et al., 2000).
A second and more specific expectation is that there should be previous evidence that frontal regions commonly activated in our conjunction analysis contribute to core cognitive processes considered essential to switching and response conflict. There is evidence for this expectation. Two cognitive processes thought to be involved in both switching and conflict are monitoring competition and updating task-related representations (MacLeod, 1991; Monsell, 2003). There appears to be good correspondence between these processes and the known functions of the dACC and left IFJ, respectively.
First, dACC has a well-established role in detection of conflict between responses, as described in the conflict monitoring theory (Botvinick et al., 1999; Botvinick et al., 2001; MacDonald et al., 2000). In addition to detection of response conflict, emerging evidence suggests that dACC is also involved in monitoring competition between task-related representations and performance outcomes (Kim et al., 2010; Kim et al., 2011b; Ridderinkhof et al., 2004; Rushworth et al., 2002; Rushworth et al., 2004). The present finding of dACC activation for both switching and response conflict provides support for the emerging view that dACC captures the occurrence of competition across multiple cognitive domains. Specifically, our results suggest that dACC is involved in monitoring competition at the level of both stimulus dimensions (switching) and S-R mappings (response conflict).
Second, emerging evidence suggests that the left IFJ, a posterior lateral region at the junction of the precentral and inferior frontal sulci (~BA 44/6/9), contributes to core cognitive processes used in multiple cognitive control tasks. For example, Derrfuss and colleagues have found that the left IFJ is associated with updating representations across the Stroop task and attentional switching paradigms (Derrfuss et al., 2004; Derrfuss et al., 2005). In addition, IFJ is activated across multiple forms of attentional switching (Derrfuss et al., 2004; Kim et al., 2011a; Kim et al., 2012). In the present study, switch trials required subjects to update a previously relevant stimulus dimension (i.e., word or arrow). In addition, the incongruent trials required subjects to update the relevant stimulus-response (S-R) mappings (e.g., the word “left” embedded within a black, right-pointing arrow must be mapped onto a right button-press). Our results thus provide further evidence that IFJ contributes to updating task-related representations in a domain-general manner.
3.4. Functional connectivity of dACC with posterior regions
As previously discussed, results from the conjunction analysis and previous work suggest that dACC is involved in monitoring conflict from multiple sources such as response conflict and attentional switching. This raises the following question: what determines whether the nature of dACC conflict monitoring is directed toward switching or response conflict? Results from the connectivity analysis suggest that the direction of dACC monitoring resources toward switching or response conflict are based in part on the nature of underlying conflict engendered by these conditions and dACC’s potential for flexible interaction with distinct posterior regions. Specifically, the PPI analysis demonstrated task-dependent functional connectivity between dACC and right ITC (fusiform gyrus) for switching and between dACC and right PPC (inferior parietal lobule) for response conflict. Furthermore, these functional connectivity patterns were inversely related to behavioral switch costs in a task preferential manner.
Switching conditions invoke task-related conflict associated with the occurrence of a new task (e.g., read the word) and the requirement to inhibit the previous task (e.g., register the arrow’s direction). Our switching condition required subjects to shift attention between two kinds of visual stimuli (from word to arrow or vice versa). Compared to nonswitch trials, switch trials required increased visual selective attention associated with the competing visual ‘what’ representations of the word and arrow (i.e. conflict at the ‘what’ level of visual representation). Under this form of task-conflict, dACC showed preferential connectivity with ITC. While novel, this task-dependent connectivity pattern is consistent with the known functioning of ITC. The ITC is a part of the well-known ventral stream (the ‘what’ pathway) which processes visual features of objects (Ungerleider and Haxby, 1994). A promising interpretation of functional connectivity between dACC and ITC is that dACC is involved in detecting task-related visual ‘what’ competition represented in ITC.
In contrast to the task conflict associated with switch conditions, incongruent trials invoke response conflict because the word and the arrow indicate opposite (conflicting) responses. Under this form of conflict, dACC showed preferential connectivity with right PPC. Right PPC has an established role in representing stimulus-response mappings (Garavan et al., 1999; Hazeltine et al., 2003; Hester et al., 2007). Thus, a promising interpretation of the connectivity pattern between dACC and right PPC is that dACC is involved in detecting competition between relevant and irrelevant S-R mappings represented in PPC. This interpretation is consistent with results from previous studies which have linked dACC with conflict monitoring and right PPC in representing S-R mappings (Garavan et al., 1999; Hazeltine et al., 2003; Hester et al., 2007).
One likely factor contributing to PPC’s role in representing S-R mappings may be the inherent spatial nature of this form of conflict. PPC has a well-established role in visuospatial information processing (Ungerleider and Haxby, 1994). Consistent with our right-lateralized finding, right PPC is known to represent the majority of the spatial attention field, with its left-sided homologue playing a more minor role (Driver and Mattingley, 1998; Driver and Vuilleumier, 2001). In addition, because PPC uses visuospatial representations to help guide motor responses, it is considered to be a key part of the dorsal visual stream’s ‘how’ pathway (Wise et al., 1997). Thus, in the present study, dACC may be involved in detecting competition between incongruent S-R mappings held in PPC. At the broadest level, our connectivity results, combined with the inherent visuomotor nature of S-R incongruency, suggest that dACC and PPC may represent a critical pathway for detecting ‘how’ conflict (i.e. how to respond to a stimulus in a particular context).
Taken together, our results demonstrate that the brain makes use of shared frontal regions to deal with switching and response conflict when these tasks are instantiated at a common stimulus level. In particular, the same left IFJ and dACC regions were significantly activated during switching and response conflict, suggesting that these regions contribute to cognitive processes involved in both switching and response conflict, such as monitoring competition and updating task-related representations. However, our connectivity results showed that dACC interacted with right ITC during switching and with right PPC during response conflict. These results suggest that dACC’s ability to monitor conflict associated with different kinds of underlying representations is in part based on its ability for flexible interaction with distinct domain-preferential posterior regions.
4. EXPERIMENTAL PROCEDURE
4.1. Subjects
Sixteen healthy young adult subjects between the ages of 18 and 35 years (mean age= 23.6, SD = 2.9; 9 females) participated in this study. All subjects were right-handed native English speakers who reported no history of head injury, psychiatric or neurological problems. Subjects provided written informed consent in a manner approved by the University of Kentucky Institutional Review Board and were paid for participating.
4.2. Materials and procedure
An arrow-word version of the Stroop task was designed to simultaneously measure conflict and switch effects on a common task and common stimulus set. These two factors (switching and conflict) were fully crossed in a 2×2 within-subject design comprised of 4 conditions: nonswitch-congruent (NsCon), nonswitch-incongruent (NsInc), switch-congruent (SwCon), and switch-incongruent (SwInc). Subjects received training on the task prior to fMRI scanning. Task stimuli consisted of a word (“left” or “right”) embedded within a left-pointing or right-pointing arrow, presented in the middle of a gray screen (see Figure 1).
The task required subjects to press the hand-held button (i.e., left or right) corresponding to the task appropriate cue. Tasks were cued by the color black. In the word task, the target (i.e., the word “left” or “right”) was presented in black and the distracter (i.e., a left-pointing or right-pointing arrow) was presented in white. The color presentations were reversed in the arrow task, in which the target arrow was presented in black and the distracter word was presented in white.
In the switch trials (i.e., SwCon and SwInc), the target switched from word to arrow (or vice versa). In the nonswitch trials (i.e., NsCon and NsInc), the target dimension was the same as the previous trial. In the incongruent trials (i.e., NsInc and SwInc), the word and arrow were in conflict (e.g., the word, “left” embedded within a right-pointing arrow). In the congruent trials (i.e., NsCon and SwCon), the word and arrow were consistent (e.g., the word, “left” embedded within a left-pointing arrow; See Figure 1). An event-related design was employed in which a stimulus was presented for 1.1 seconds, with an inter-trial-interval ranging from 1.7 to 7.3 sec (average 3 sec). Across the experiment, there were a total of 42 trials in each of the NsInc, SwCon, and SwInc conditions. There were a total of 126 trials in the NsCon condition to maximize the opportunity for strong effects of conflict, switching and their potential interaction. The experiment was divided into three scanning runs, each lasting 370 sec. The experiment was programmed using E-Prime v1.2.
4.3. Imaging acquisition
Functional and anatomical images were acquired on a 3-T Siemens TIM scanner at the Magnetic Resonance Imaging and Spectroscopy Center at University of Kentucky. T2*-weighted functional images were collected using a gradient-echo (EPI) sequence (33 interleaved slices, repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 77°, field of view (FOV) = 224 mm2, matrix = 64×64, isotropic 3.5 mm voxels). A double-echo gradient-echo sequence (TE1 = 5.19 ms, TE2 = 7.65 ms) with slice position and spatial resolution matching those of the EPI acquisition was used to map the spatial inhomogeneity of the B0 field. A T1-weighted structural images for all subjects were collected using the magnetization-prepared rapid gradient-echo (MPRAGE) sequence (FOV = 224×256×192 mm, resolution=1 mm3, sagittal partitions).
4.4. fMRI Data analysis
Statistical Parametric Mapping (SPM 5; Wellcome Department of Cognitive Neurology, UCL, London, UK) was used in preprocessing and statistical analyses of fMRI data. The first five functional volumes were discarded prior to preprocessing. In the first preprocessing step, sinc interpolation was used to correct slice timing (Henson et al., 1999). The timing-corrected images were spatially realigned to the first volume in order to correct for head motion. These images were unwarped via B0 field maps to reduce magnetic field distortions. The images were then coregistered with structural images (MPRAGE) and were spatially normalized into the Montreal Neurological Institute (MNI) template, using both 12-parameter affine and non-linear transformations (2mm cubic voxels). Images were smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel. High-pass filtering (a 128 second cutoff) was applied to the images to remove low-frequency drifts.
Statistical analyses at the subject-level were conducted in the context of the general linear (GLM) model using a canonical hemodynamic response function (HRF) with temporal and dispersion derivatives. Only correct trials from each of the four individual experimental conditions (NsCon, NsInc, SwCon, and SwInc) were included in the GLM, with head movement parameters in six dimensions as covariates of no interest. Error trials were modeled separately as a condition of non-interest. For second-level group analyses, subject’s individual contrast images of each of the four experimental conditions compared to baseline were entered into a 2×2 repeated measures ANOVA. The first factor was switching (nonswitch and switch), and the second was conflict (congruent and incongruent). These main effects and their interaction were thresholded at p < 0.01 using the false discovery rate (FDR) for multiple comparisons correction (Genovese et al., 2002).
As described in the introduction, we next sought to identify frontal regions that may contribute to both switching and response conflict, and to determine if these regions may interact with distinct posterior regions during switching and conflict tasks. Thus, subsequent analyses were restricted to regions showing significant activation in the main effect analyses described above. Toward that end, Monte Carlo simulations using the AlphaSim program were used to determine the appropriate combination of the significance level and cluster threshold required to reach a corrected significance level of p < 0.05 for the specific dimensions of the search space tested in each comparison (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). The Monte Carlo simulations used 1000 iterations.
As a first step, a conjunction analysis was performed to identify common frontal regions involved in both conflict and switching. The conjunction analysis was to test against the conjunction null (Friston et al., 2005) to identify frontal regions that were commonly activated by both switching and conflict main effects. We first generated a mask using frontal regions activated by either the switch or conflict main effect and then performed the conjunction analysis to identify common frontal regions within this frontal mask that were significantly activated by both conflict and switching. A significance threshold of p < 0.005 and a minimum cluster size of 17 contiguously activated voxels (136 mm3) were employed for this analysis based on the Monte Carlo simulations to achieve a corrected significance level of p < 0.05.
As a second step, psychophysiological interaction analysis (PPI) analyses was conducted in order to determine if these commonly activated PFC regions may interact with different posterior regions during switching and conflict tasks. PPI analyses assess whether interactions between one brain region and another brain region covary as a function of task conditions (Friston et al., 1997; Gitelman et al., 2003). Specifically, the PPI analysis involves a regressor of the deconvolved time course data in a seed region (the physiological factor), a regressor of the task condition (the psychological factor), and a regressor of the cross product of physiological and psychological factors (the psychophysiological interaction term).
First, we extracted the deconvolved time course data in the two PFC regions of interest (ROIs) identified in the conjunction analysis (left IFJ and dACC, see Results) from individual subjects’ data using a 6 mm radius sphere centered at the peak activation coordinates in these two ROIs. The left IFJ and dACC time course data were multiplied with the psychological factors of interest (NsInc and SwCon) and convolved with the canonical HRF to create the psychophysiological interaction term. The NsInc and SwCon were selected as the psychological factors of interest because these two conditions were selectively associated with either conflict (NsInc) or switching (SwCon). The time course of the seed region (the physiological factor), the task condition (the psychological factor), and the interaction term were entered into a new GLM model, along with 6 head movement parameters as covariates of no interest.
These analyses were conducted separately for both the psychological factors of interest (NsInc and SwCon) and both seed regions (left IFJ and dACC). These PPI analyses were carried out for each subject and the resulting individual images of contrast estimates were then submitted into the second-level group analysis for each seed region. Paired-sample t-tests were carried out to contrast PPI patterns between NsInc and SwCon conditions. These analyses were restricted to posterior regions (occipital, temporal, and parietal cortices) activated by either the switch or conflict main effect. A significance threshold of p < 0.005 and a minimum cluster size of 12 contiguously activated voxels (96 mm3) were employed for this analysis based on the Monte Carlo simulations to achieve a corrected significance level of p < 0.05.
Acknowledgments
This study was supported by NIA Grant R01 AG033036 and NSF Grant BCS-0814302. We thank Sara Cilles for help in recruiting and scheduling subjects.
References
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–18. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Barber AD, Carter CS. Cognitive Control Involved in Overcoming Prepotent Response Tendencies and Switching Between Tasks. Cereb Cortex. 2005;15:899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Selection for cognitive control: a functional magnetic resonance imaging study on the selection of task-relevant information. J Neurosci. 2004;24:8847–52. doi: 10.1523/JNEUROSCI.2513-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis JD, Miller EK, Wagner AD. Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol. 2003;90:3419–28. doi: 10.1152/jn.00910.2002. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain Cogn. 2004;56:141–52. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–86. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–12. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Cognitive Brain Research. 2000a;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000b;9:103–9. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Berman KF. Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci U S A. 2002;99:14595–600. doi: 10.1073/pnas.222193299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Mattingley JB. Parietal neglect and visual awareness. Nat Neurosci. 1998;1:17–22. doi: 10.1038/217. [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79:39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–9. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. NeuroImage. 2005;25:661–7. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–6. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–9. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of Statistical Maps in Functional Neuroimaging Using the False Discovery Rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage. 2003;19:200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–12. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. J Neurosci. 2006;26:6523–32. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JD. Material-dependent and material-independent selection processes in the frontal and parietal lobes: an event-related fMRI investigation of response competition. Neuropsychologia. 2003;41:1208–17. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Henson RN, Buchel C, Josephs O, Friston KJ. The slice-timing problem in event-related fMRI. NeuroImage. 1999;9:125. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Hester R, D’Esposito M, Cole MW, Garavan H. Neural mechanisms for response selection: comparing selection of responses and items from working memory. NeuroImage. 2007;34:446–454. doi: 10.1016/j.neuroimage.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, III, Stenger VA, Carter CS. Anterior Cingulate Conflict Monitoring and Adjustments in Control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kim C, Chung C, Kim J. Multiple cognitive control mechanisms associated with the nature of conflict. Neurosci Lett. 2010;496:156–160. doi: 10.1016/j.neulet.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Kim C, Johnson NF, Cilles SE, Gold BT. Common and Distinct Mechanisms of Cognitive Flexibility in Prefrontal Cortex. The Journal of Neuroscience. 2011a;31:4771–4779. doi: 10.1523/JNEUROSCI.5923-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Kroger JK, Kim J. A functional dissociation of conflict processing within anterior cingulate cortex. Human Brain Mapping. 2011b;32:304–312. doi: 10.1002/hbm.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT. Domain general and domain preferential brain regions associated with different types of task switching: A Meta-Analysis. Human Brain Mapping. 2012;33:130–142. doi: 10.1002/hbm.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–91. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–8. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–92. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends in Cognitive Sciences. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sohn M-H, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C-YC, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Current Opinion in Neurobiology. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]