Abstract
It is not known whether behaviors unrelated to feeding are affected by hypothalamic regulators of hunger. We found that impairment of Agouti-related protein (AgRP) circuitry by either Sirt1 knockdown in AgRP-expressing neurons or early postnatal ablation of these neurons increased exploratory behavior and enhanced responses to cocaine. In AgRP circuit–impaired mice, ventral tegmental dopamine neurons exhibited enhanced spike timing–dependent long-term potentiation, altered amplitude of miniature postsynaptic currents and elevated dopamine in basal forebrain. Thus, AgRP neurons determine the set point of the reward circuitry and associated behaviors.
Hypothalamic neurons that co-produce neuropeptide Y (NPY), AgRP and GABA1 are fundamental for eliciting feeding behavior2–4, as their selective ablation in adult mice leads to cessation of feeding and, ultimately, death2,4. We sought to test whether these neurons also coordinate other complex behaviors.
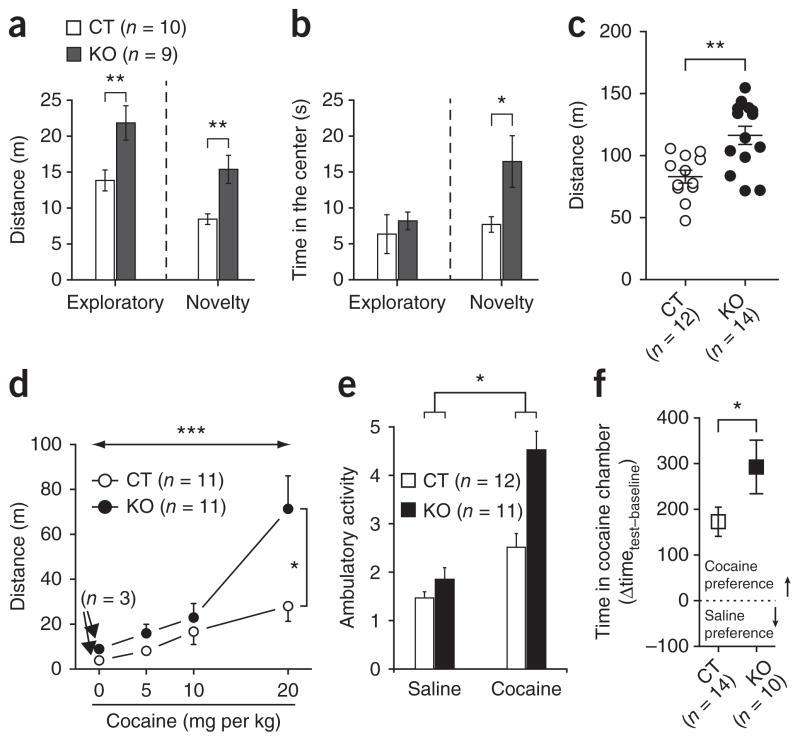
We analyzed behavioral responses of mice in which AgRP neuronal excitability was selectively altered by cell-specific deletion of Sirt1 in neurons expressing AgRP (AgRP-Sirt1 mice)5. We first analyzed the behavior of AgRP-Sirt1 mice and control littermates in an open-field test6,7. AgRP-Sirt1 mice exhibited increased activity compared with controls during both exploratory (F1,17 = 8.566, P = 0.009) and novelty stages (U = 11.00, P = 0.004; Fig. 1a), which is consistent with previous findings that brain infusion of AgRP reduces locomotor activity8. During the exploratory phase, no differences were observed in the time spent in the center of the open field (F1,17 = 2.435, P = 0.13; Fig. 1b). In the novelty stage, AgRP-Sirt1 mice explored the novel object significantly more than did controls (U = 18.00, P = 0.02; Fig. 1b). We tested another cohort of mice in a different open field for 60 min (in comparison with 5 min in the previous test), and we observed similar results (t24 = 3.604, P = 0.001; Fig. 1c). Motor coordination, skills and anxiety did not differ between genotypes (Supplementary Fig. 1). Differences in leptin levels, adiposity and body weight did not account for this phenotype (Supplementary Table 1 and Supplementary Figs. 2 and 3).
Figure 1.
AgRP neurons determine the behavioral response to novelty and cocaine. (a,b) Exploratory activity (a) and time spent in the center in the open-field test (b) comparing AgRP-Sirt1 (KO) and littermates control mice (CT). (c) Exploratory activity in a different open-field test during 60 min. (d,e) AgRP-Sirt1 mice displayed increased locomotor response to acute cocaine (d) and in a cocaine-sensitization procedure (e) compared with control mice. (f) In the CPP test, AgRP-Sirt1 mice had increased response compared with control mice. *P < 0.05, **P < 0.01, ***P < 0.001. Data are presented as mean ± s.e.m.
To further examine the effect of AgRP neuronal excitability on nonfood-related behaviors, we tested the responses of AgRP-Sirt1 and control mice to cocaine. First, we analyzed locomotor responses to cocaine. AgRP-Sirt1 mice had an increased response to cocaine in a dose-dependent manner (dose, F3,64 = 10.85, P < 0.001; genotype, F1,64 = 5.304, P = 0.02; interaction, F3,64 = 2.793, P = 0.04; Fig. 1d) and had elevated cocaine sensitization response compared with controls (challenge, F1,21 = 58.155, P < 0.001; genotype, F1,21 = 3.373, P = 0.08; interaction, F1,21 = 5.386, P = 0.03; Fig. 1e and Supplementary Fig. 4). In a conditioned place-preference test (CPP), AgRP-Sirt1 mice showed increased preference for cocaine compared with control mice (t22 = 1.935, P = 0.03, one tail; Fig. 1f).
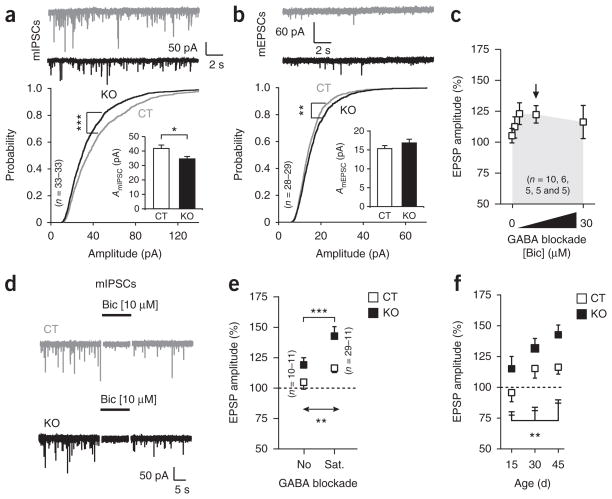
To explore the mechanism by which AgRP neurons affect reward circuits, we confirmed that AgRP fibers innervated the ventral tegmental area (VTA) in the midbrain9,10 (Supplementary Figs. 5 and 6). We then evaluated the synaptic input organization of the VTA dopamine cells and found a marked decrease in the average peak amplitude of miniature inhibitory postsynaptic currents (mIPSCs; t64 = 2.499, P = 0.01; Fig. 2a), but not of miniature excitatory postsynaptic currents (mEPSCs; Fig. 2b). However, we found an increased probability of lower amplitudes in the mIPSCs (k = 0.125, P < 0.001; Fig. 2a), with increases in the probability of events of higher amplitude for mEPSCs (k = 0.079, P < 0.01; Fig. 2b). Extracellular dopamine levels in the basal forebrain were significantly higher in AgRP-Sirt1 mice than in controls (control, 0.06 ± 0.01 nM; AgRP-Sirt1, 0.30 ± 0.05 nM; t6 = 4.353, P = 0.004). We found no differences in synaptic number (Supplementary Table 2) between control and AgRP-Sirt1 mice. The melanocortin receptor agonist MT-II and the melanocortin receptor antagonist SHU9119 did not affect mIPSCs in the VTA dopamine neurons (data not shown)11, suggesting that GABA is the main effector of the AgRP system in the alteration of VTA dopamine functions and that AgRP-triggered locomotor responses8 may be mediated by systems other than the midbrain dopamine neurons.
Figure 2.
AgRP neuronal excitability determines connectivity of VTA dopamine neurons. (a,b) Representative traces, probability plots and average peak amplitude (insets) of miniature postsynaptic currents in control and AgRP-Sirt1 mice. (c) Dose response of GABA blockade on LTP propagation in control. (d) Saturating doses of GABA blockade (10 μM bicuculline (Bic), arrow in c) eliminated mIPSCs in both control and AgRP-Sirt1 mice. (e) AgRP-Sirt1 mice had increased LTP either in the absence of GABA blockade (No) or in the presence of saturating doses of GABA blocker (Sat). (f) Quantification of LTP during development in control and AgRP-Sirt1 mice. *P < 0.05, **P < 0.01, ***P < 0.001. Data are presented as mean ± s.e.m.
Next, we analyzed long-term potentiation (LTP) induced by a spike-timing dependent (STD) protocol. Blockade of GABA signaling facilitated STD-LTP in control mice12 (Fig. 2c). The GABA blocker bicuculline abolished synaptic responses in control and AgRP-Sirt1 mice (Fig. 2d). However, even in the absence of GABA blockade, AgRP-Sirt1 mice displayed significant facilitation of LTP (dose, F1,57 = 9.071, P = 0.003; genotype, F1,57 = 12.22, P < 0.001; Fig. 2e). AgRP fibers innervated the VTA more robustly during the first postnatal week (Supplementary Fig. 7), suggesting an effect on VTA development, as indicated by differences in LTP propagation between control and AgRP-Sirt1 mice when STD-LTP was recorded at different ages (age, F2,44 = 5.091, P = 0.01; genotype, F1,44 = 10.04, P = 0.002; Fig. 2f and Supplementary Fig. 7).
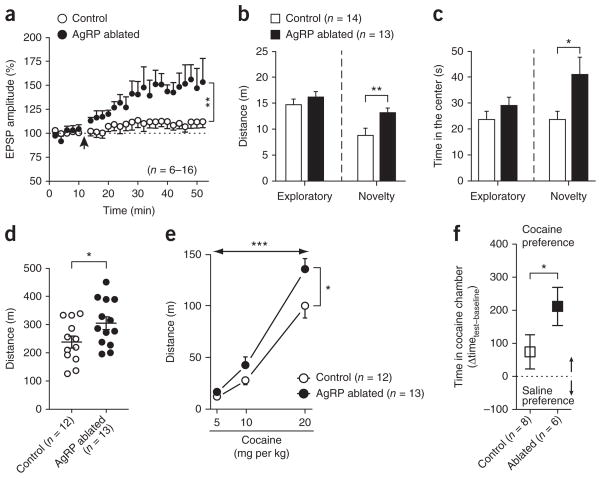
To further examine the effect of the AgRP neurons on VTA development, we used transgenic mice that express diphtheria toxin receptor under the control of the Agrp promoter2. There was significantly enhanced facilitation of LTP propagation in VTA dopamine cells from AgRP neuron–ablated mice compared with controls (t20 = 3.332, P = 0.001; Fig. 3a).
Figure 3.
AgRP neurons influence the development of VTA dopamine neurons and behavioral responses. (a) The STD-LTP protocol (arrow) was applied in the VTA dopamine cells from control and AgRP neuron–ablated mice. (b,c) Exploratory activity (b) and time in the center in the open field (c) comparing control and AgRP neuron–ablated mice. (d) Exploratory activity in a different open field over 120 min. (e) Locomotor responses of control and AgRP neuron–ablated mice to acute cocaine injections. (f) CPP test for cocaine in a different cohort of mice. *P < 0.05, **P < 0.01, ***P < 0.001. Data are presented as mean ± s.e.m.
We then tested control and AgRP neuron–ablated mice in the open-field test. During the novelty stage, AgRP neuron–ablated mice had higher activity (t25 = 2.591, P = 0.007) and spent more time exploring the new object (t25 = 2.393, P = 0.012) than control mice (Fig. 3b,c). When AgRP neuron–ablated mice were allowed to explore a new environment for 2 h, a slight increase in activity became evident (t23 = 2.130, P = 0.04; Fig. 3d). AgRP neuron–ablated mice had normal home-cage activity and food intake (Supplementary Fig. 8). AgRP neuron–ablated mice had elevated responses to cocaine compared with control mice in an acute cocaine dose-response experiment (dose, F2,46 = 132.4, P < 0.001; group, F1,46 = 6.333, P = 0.01; interaction, F2,46 = 2.759, P = 0.07; Fig. 3e) and in the CPP test (t12 = 1.76, P = 0.05; Fig. 3f).
Our results highlight a previously unsuspected role for hypothalamic hunger-promoting neurons in setting midbrain dopamine neuronal activity and nonfood-related behaviors.
ONLINE METHODS
Animals
Female transgenic mice were used in these studies. Littermate controls and AgRP-Sirt1 mice were generated as described previously5. All animals were kept in temperature- and humidity-controlled rooms on a 12-h:12-h light: dark cycle, with lights on from 7:00 a.m. to 7:00 p.m. Mice were group housed (3–5 mice per cage) and food and water were provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Yale University. All behavioral and electrophysiology experiments were blinded.
The following transgenic mice were used: AgRP-Cre mice donated by A. Xu (University of California San Francisco), AgRPDTR mice donated by R. Palmiter (University of Washington), Dat-Cre mice donated by N.-G. Larssons (Karolinska Institute). AgRP-Cre mice (#012899), Sirt1loxP/loxP mice (#008041), R26-LSL-LacZ reporter mice (#002292), R26-LSL-tdTomato reporter mice (#007914) and NPY-hrGFP mice (#006417) were from Jackson Laboratories.
Ablation of the AgRP neurons
AgRPDTR or wild-type mice from the same litter received injection of diphtheria toxin at postnatal day 5. Diphtheria toxin was injected using previously described methods2.
Elevated plus-maze test
The apparatus consisted of four elevated arms (40 cm from the floor, 25 cm long and 5.2 cm wide) arranged at right angles (cross-like disposition). Two opposite arms were enclosed by 15-cm-high walls, and the other two were open (no walls). The number of entries in each arm and the time spent in each arm were recorded for 5 min. Mice were 3 and 6 months of age.
Open field
The apparatus consists of a Plexiglas open field (37 cm × 37 cm × 37 cm). AgRP-Sirt mice (3–6 months old) were first put in the open field for 5 min (exploratory stage). Immediately after, mice were returned to their home cages for 2 min. A new object (a cylinder of 5-cm radius and 10 cm high) was placed in the center of the arena. Mice were then returned to the open field for an additional 5 min (novelty stage). The same mice were injected with murine leptin 7 weeks later (5 mg per kg of body weight, intraperitoneal, 3–4 h before test, PreproTech, cat #450-31, lot #101076) and were re-tested in the open field using a different object as a novel stimulus. For the experiment using low and high body-weight mice, we purchased 40 C57B6/J female mice (8 weeks old) from Jackson Laboratory. At 10 weeks of age, the eight heaviest and eight lightest mice were selected for the open-field test. One mouse in the low body-weight group was not used because it had malocclusion. Similar procedures as described above were used to test these mice in the open-field test. For the experiment using AgRP neuron–ablated mice, female adult (45–55 d old) mice were used, and all tests were performed as described above.
Open-field acclimation and cocaine dose response
Female control (n = 14) and AgRP-Sirt1 (n = 14) mice (3–6 months old) were allowed to explore a novel environment (45 × 24 × 20 cm) for 60 min before receiving an injection of vehicle (phosphate-buffered saline (PBS), intraperitoneal). Sixty min after the PBS injection, 11 mice in each group received cocaine injections (5, 10 and 20 mg per kg, every 120 min, intraperitoneal), and three mice in each group (control and AgRP-Sirt1) received PBS injections. For the cocaine dose-response plot, the first 20 min of each block were considered for analyses. The software did not appropriately track two mice during the acclimation stage, which were subsequently excluded from the analysis of this stage. Similar procedures were performed for AgRP neuron–ablated mice, but no vehicle control mice were run during the studies.
Cocaine sensitization
Female control (n = 12) and AgRP-Sirt1 (n = 11) mice (between 10–14 weeks old) were used in this experiment. On day 0, mice were acclimated in clear rat cages for 60 min. Locomotor activity was assessed by measuring the number of beam breaks. On each of the next 3 d (days 1, 2 and 3), mice received an injection of saline just before being placed in the recording chambers, and activity was recorded for 20 min. On days 4–8, mice received an injection of cocaine (10 mg per kg, intraperitoneal). After 4 d of treatment withdrawal, mice were challenged again with saline (day 13) and cocaine (day 14) to test for contextual and drug sensitization, respectively. Data during the sensitization protocol (saline acclimation and cocaine injections) were normalized by the individual’s activity on day 0.
Rotarod
A total of 12 control and 12 AgRP-Sirt1 female mice (9–11 weeks old) were used in this test. The apparatus consists of a rotating bar located 20 cm above the floor. On days 1–5, all mice were trained on the rotarod (two trials per day, initial speed = 4 rpm, maximum speed = 60 rpm, time to reach maximum speed = 300 s). The cut-off time on the rotarod was 600 s. The latency to fall from the rotating bar was recorded. The average latency of both trials in each day was used for analyzes.
Cocaine place preference
A total of 34 female adult mice were used as previously described13 (3–4 months old). A total of 14 mice were analyzed in the control group and 10 mice in the AgRP-Sirt1 group. In addition, a third group of mice was used as a control group for the conditioning chambers. We used similar methods to study CPP in female AgRP neuron–ablated mice. The AgRP neuron–ablated (n = 6) and littermate control mice (n = 8) were 50–55 d old at the time of CPP test.
Home cage monitoring
AgRP neuron–ablated mice and their littermate controls were left to acclimate in TSE LabMaster home cages for at least 5 d before recording food intake, water intake and activity.
AgRP reporter mice
We bred AgRP-Cre mice (Jax 012899) to R26-LSL- tdTomato mice to study the projections of the AgRP neurons to the VTA. We further crossed these mice (AgRP-Cre:R26-LSL-tdTomato) to NPY-hrGFP mice to confirm that the AgRP neurons in the arcuate nucleus in the hypothalamus expressing tdTomato were also NPY neurons. Adult mice (about 3 months old) were used for these studies.
Immunohistochemistry for tyrosine hydroxylase and AgRP
Mice were perfused with 4% paraformaldehyde (vol/vol), 0.1% gluteraldehyde (vol/vol), 15% picric acid (vol/vol), in 0.1 M phosphate buffer, pH = 7.4. Vibratome sections were cut (50 μm) and incubated with primary antibodies (guinea pig antibody to AgRP, 1:3,000 donated by K. Grove; mouse anti–tyrosine hydroxylase antisera, 1:5,000) and developed with Nickel-DAB and DAB, consecutively. Alternatively, slices were incubated with secondary antibodies coupled to Alexa488 to visualize AgRP–tyrosine hydroxylase cell juxtaposition (tyrosine hydroxylase–positive cells in which AgRP fibers and/or buttons were found in close proximity, apparently contacting each other) in a confocal microscope.
Microdialysis
Female adult mice were used in this study (3–5 months old). CMA-7 microdialysis guide cannulas, with dummy cannulas within (CMA, #P000137), were inserted into the following coordinates in relation to bregma: +1.3 mm anterior-posterior, −0.6 mm lateral and −3.6 mm dorsal-ventral. CMA/7 microdialysis probes with 1-mm membrane length (CMA, #P000082) were attached through FEP tubing to a PHD-Ultra syringe pump (Harvard Apparatus). Artificial cerebrospinal fluid was then continuously pumped through the probes at 1.2 μl min−1 for 1.5 h before samples were collected. Eight samples were collected at 15-min intervals for dopamine analyzes using high-performance liquid chromotagraphy (Eicom). All mice were perfused and brains were sectioned to check for probe and cannula location and orientation.
Tyrosine hydroxylase staining, quantitative synaptology and mitochondria counting
Electron microscopy analysis of the dopamine neurons in the VTA was performed as previously described14.
Body composition
An EchoMRI-100 machine was used to measure body composition.
Mouse leptin assay
Mice were fasted overnight and serum was collected from eye bleed. Leptin levels were measured using a kit (Millipore, cat #EZML-82K).
Electrophysiology
Horizontal brain slices containing the VTA were cut from female control and AgRP-Sirt1 mice, and were freshly prepared for electrophysiology recordings as described previously12,14. For LTP measurements, mice were 13–16 d old for the 15-d-old group, 27–31 d old for the 30-d-old group and 44–55 d old for the 45-d-old group. mIPSCs and mEPSCs were recorded in ~45-d-old mice. For the recordings in the AgRP neuron–ablated mice, all mice were 29–31 d old, and dopamine cells were identified by the red fluorescence in Dat-Cre: R26-LSL-tdTomato:AgRPDTR mice. For the LTP studies, one cell was recorded per mouse.
Statistical analysis
For all tests, P ≤ 0.05 was considered to be statistically significant. Data distribution was first tested for homogeneity of variances using Levene’s test. If homogeneity was assumed, then one- or two-way ANOVA was used. For two-way ANOVA with repeated measures, sphericity was tested using the Mauchly’s test of sphericity, and the degrees of freedom were adjusted using the epsilon calculated by the Greenhouse-Geisser procedure when necessary. To test for LTP response, we used a paired t test (one tail) between pre- and post-stimulation values. When homogeneity was not assumed, Mann-Whitney U test or Friedman’s test was used. PASW Statistics 18.0 for Mac software was used for all analysis.
Supplementary Material
Acknowledgments
We thank L. Kus from the GENSTAT Project, which provided important information on the AgRP-EGFP mouse (MT25), A. Xu for providing breeding pairs of AgRP-Cre mice (University of California San Francisco), R. Palmiter for providing breeding pairs of the AgRP-DTR mice (University of Washington), and the personnel of the Horvath laboratory for support. This work was supported by a US National Institutes of Health Director’s Pioneer Award to T.L.H. I.A. was supported by National Institute on Deafness and Other Communication Disorders (US National Institutes of Health) grants. D.O.S. was supported by grants from Financiadora de Estudos e Projetos/Ministério de Ciência e Tecnologia (FINEP/MCT, Brazil) and Instituto Nacional de Ciência e Tecnologia em Excitotoxicidade e Neuroproteção (Brazil). M.O.D. was partially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil).
Footnotes
Note: Supplementary information is available in the online version of the paper.
AUTHOR CONTRIBUTIONS
M.O.D. designed, executed, collected and analyzed all of the data (except for the electrophysiology experiments). Z.-W.L. designed, executed and analyzed the electrophysiology experiments. J.B. help to design and execute the experiments that involved dopamine measurements and immunohistochemistry, and aided with animal maintenance and genotyping. Y.M. helped with the CPP experiment. M.P. helped to design and discussed the experiments and results of the cocaine experiments. J.G.F. and L.A.T. helped with the microdialysis experiment. I.A. designed, discussed and helped analyze the dopamine measurement experiment. D.O.S. helped to design and discussed the behavioral experiments. X.-B.G. helped to design and analyze the electrophysiology experiments. T.L.H. designed and analyzed all of the experiments. M.O.D. and T.L.H. wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 2.Luquet S, Perez F, Hnasko T, Palmiter R. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 3.Gropp E, et al. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 4.Wu Q, Boyle M, Palmiter R. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrich MO, et al. J Neurosci. 2010;30:11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. J Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazlauckas V, et al. Behav Brain Res. 2005;162:272–278. doi: 10.1016/j.bbr.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Tang-Christensen M, et al. Endocrinology. 2004;145:4645–4652. doi: 10.1210/en.2004-0529. [DOI] [PubMed] [Google Scholar]
- 9.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. Proc Natl Acad Sci USA. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Q, Howell MP, Palmiter RD. J Neurosci. 2008;28:9218–9226. doi: 10.1523/JNEUROSCI.2449-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu QS, Pu L, Poo MM. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abizaid A, et al. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.