Abstract
Fatty liver disease is associated with obesity and type 2 diabetes, and hepatic lipid accumulation may contribute to insulin resistance by a variety of mechanisms. Here we show that mice with liver-specific deletion of histone deacetylase 3 (Hdac3) display severe hepatosteatosis and, notably increased insulin sensitivity without changes in insulin signaling or body weight. Hdac3 deletion reroutes metabolic precursors towards lipid synthesis and storage within lipid droplets (LDs). Reduced hepatic glucose production in Hdac3-depleted liver is a result of the metabolic rerouting rather than due to inherently defective gluconeogenesis. The lipid-sequestering LDs-coating protein Perilipin 2 is markedly induced upon Hdac3 deletion and contributes to the development of both steatosis and improved tolerance to glucose. These findings suggest that the sequestration of hepatic lipids ameliorates insulin resistance, and establish Hdac3 as a pivotal epigenomic modifier that integrate signals from the circadian clock in regulation of hepatic intermediary metabolism.
INTRODUCTION
Hepatic steatosis, the defining clinical hallmark of non-alcoholic fatty liver disease (NAFLD), is one of the most prevalent metabolic disorders throughout the world1,2. NAFLD is highly correlated with insulin resistance in many epidemiological studies, leading to speculation of a cause and effect relationship3,4. Insulin resistance in muscle and adipose tissue may increase carbohydrate and free fatty acid (FFA) influx into the liver, and thus promote hepatic de novo lipogenesis and triglyceride synthesis. Meanwhile, hyperinsulinemia, resulting from compensation for insulin resistance by the pancreas, also reinforces hepatic lipogenesis, and therefore contributes additionally to development of hepatosteatosis2,5. Moreover, intrahepatic accumulation of triglyceride may generate various deleterious lipid intermediates, including diacylglycerols (DAG) and ceramide, which could activate several cytosolic protein kinases either directly or indirectly through inflammation, resulting in modification and disruption of insulin signaling, and hence cause lipotoxicity and insulin resistance4,6.
These theories can have significant impact on the therapy of both NAFLD and diabetes. However, the use of insulin sensitizers to treat NAFLD in clinical trials have generated mixed outcomes3,7. Indeed, recent research has revealed that the inter-relationship between hepatosteatosis and hepatic insulin resistance is more complex1,8. Although the role of insulin resistance in the pathogenesis of NAFLD is supported by the observation that most hepatic lipid is derived from adipose-secreted FFA and de novo lipogenesis rather than directly from diet in humans9 and that insulin resistance precedes hepatosteatosis in several animal overnutrition models of NAFLD10, intact insulin signaling through insulin receptor, phosphoinositide 3-kinases and Akt (protein kinase B) is required to drive lipogenesis and hepatosteatosis as demonstrated by several knockout mouse models11–14. In addition, hepatic insulin hypersensitivity in the Pten (phosphatase and tensin homolog) liver-specific knockout mouse is sufficient to cause hepatosteatosis15. As for the role of hepatosteatosis in insulin resistance, although DAG and other lipid intermediates have been demonstrated to activate protein kinase C-ε (Prkce) and other cytosolic stress signals or inflammation that lead to disruption of insulin signaling, exceptions do exist and elevated total hepatic content of these lipid intermediates do not always cause hepatic insulin resistance, suggesting that detailed lipid species composition and their spatial distribution matters1,8,16–18.
While genetic factors contribute to the development of metabolic syndromes, environmental factors including diet, exercise, and rotating work shifts are critically important19,20. The environment regulates the expression of genes involved in carbohydrate and lipid metabolism by altering the epigenome, including changes in histone acetylation patterns in chromatin21. Histone acetylation is governed by opposing functions of two classes of enzymes: histone acetyltransferases (HATs) and histone deacetylases (HDACs). Given the availability of many small molecules that activate or inhibit HDAC activities and their therapeutic potential in treating a variety of diseases including metabolic disorders22, it is of great interest and importance to understand the biology of individual HDACs in metabolic regulation.
We recently showed that Hdac3 colocalizes with the Nuclear Receptor Corepressor (Ncor1) in chromatin near to genes involved in lipid metabolism in mouse liver, in a circadian pattern with the maximum occupancy during the day and the minimum at night23. The rhythm is orchestrated by Nr1d1, a nuclear receptor and a key component of the molecular circadian clock machinery23,24. These findings, along with others, demonstrate that Hdac3 is critical for the maintenance of hepatic lipid homeostasis23–25. However, it remains elusive how hepatic Hdac3 regulates carbohydrate metabolism. Here we demonstrate that the severe hepatic steatosis due to loss of Hdac3 in mouse liver engenders a notably insulin hypersensitive state. Systematic characterization of the physiological function of hepatic Hdac3 in energy metabolism establishes Hdac3 as a pivotal regulator of hepatic glucose production through an unexpected mechanism involving rerouting of metabolites.
RESULTS
Hdac3-depleted fatty liver is distinct from diet-induced
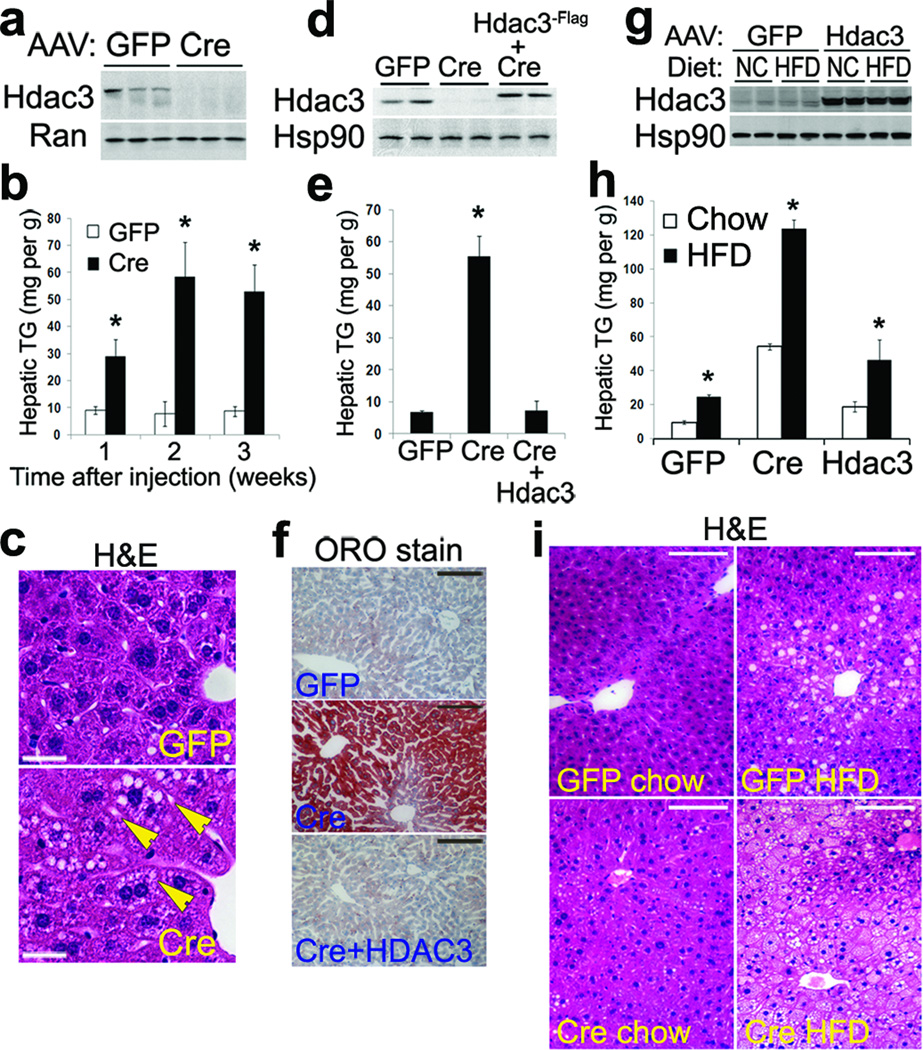
To delete Hdac3 specifically in liver, we injected Hdac3fl/fl C57BL/6 mice26 with adeno-associated virus (AAV) expressing either Cre recombinase or control green fluorescence protein (GFP) driven by the thyroxine-binding globulin (Tbg) promoter which specifically targets hepatocytes and not Kupffer cells or endothelial cells27, as shown by immunohistochemistry on liver from mice injected with AAV-Tbg-GFP (Supplementary Fig. 1a). This allows direct assessment of hepatic Hdac3 function in adult mice without potential interference of embryonic and neonatal development. This acute deletion approach also makes it possible to distinguish direct effects of Hdac3 deletion from secondary or compensatory effects. Depletion of hepatic Hdac3 protein was complete by 1-week after injection (Fig. 1a). Hepatic triglyceride content was significantly elevated 2–3 fold at 1-week after injection and was further increased 5–10 fold by 2–3 weeks after injection (Fig. 1b). By contrast, mice lacking Hdac3 in macrophages26 did not develop fatty liver (Supplementary Fig. 1b). Accumulation of very small lipid droplets within hepatocytes was evident after H&E staining (Fig. 1c). Exogenous Flag-tagged Hdac3 protein, when introduced by AAV at levels similar to endogenous Hdac3 (Fig. 1d), was able to completely rescue hepatosteatosis, as assessed by triglyceride measurement and Oil Red O (ORO) staining (Fig. 1e, f). This demonstrates that the metabolic phenotype was indeed due to the lack of Hdac3 in hepatocytes.
Figure 1. Liver-specific deletion Hdac3 in adult mice causes quick onset of hepatosteatosis that is distinct from high-fat diet-induced fatty liver.
(a) Western blot of total liver lysates at 1-week after AAV injection. (b) Hepatic triglyceride (TG) measurement, n = 3. (c) H&E staining of liver at 2-weeks after AAV injection. Arrows indicate small lipid droplets. Scale bar, 25 µm. (d) Western blot of liver lysates from the rescue experiment. (e) Hepatic TG measurement, n = 3. (f) Oil Red O (ORO) staining of liver at 2-weeks after AAV injection. Scale bar, 100 µm. (g) Western blot of total liver lysates. Mice were injected with AAV-GFP or AAV-Hdac3 followed by feeding on normal chow (NC) or high fat diet (HFD) for 6 weeks. Mice were then injected with AAV-Cre and maintained on their previous diet for another 2 weeks before tissue harvest. (h) Hepatic TG measurement in liver from mice described in (g), n = 3–4. (i) H&E staining of liver from mice described in (g). Scale bar, 100 µm. Error bars indicate s.e.m. * P < 0.05 between samples and the GFP controls;
To determine if Hdac3 plays a role in high fat diet (HFD)-induced fatty liver10, deletion and overexpression of Hdac3 in mouse liver was superimposed on a HFD feeding regimen. HFD feeding for 8 weeks did not alter endogenous or overexpressed exogenous Hdac3 protein levels (Fig. 1g), and had a synergistic effect with Hdac3 depletion in terms of promoting hepatic steatosis (Fig. 1h). In addition, Hdac3 overexpression failed to ameliorate HFD-induced fatty liver (Fig. 1h), suggesting that HFD feeding and hepatic Hdac3 depletion likely result in hepatic steatosis through independent mechanisms. Consistent with this, the morphologic changes in the liver differed drastically in the two situations. While Hdac3 depletion caused formation of tiny lipid droplets (LDs) that were hardly discernable under low magnification, HFD feeding resulted in accumulation of much bigger LDs that were more restricted to central vein areas (Fig. 1i). Depletion of Hdac3 in the setting of the HFD led to a striking reduction in LDs size (Cre on HFD versus GFP on HFD), even though total lipid content was higher in the Cre group than the GFP group (Fig. 1h, i). Taken together, liver-specific deletion of Hdac3 in adult mice causes hepatosteatosis that is quite distinct from HFD-induced fatty liver.
Glucose output and insulin sensitivity in Hdac3-null liver
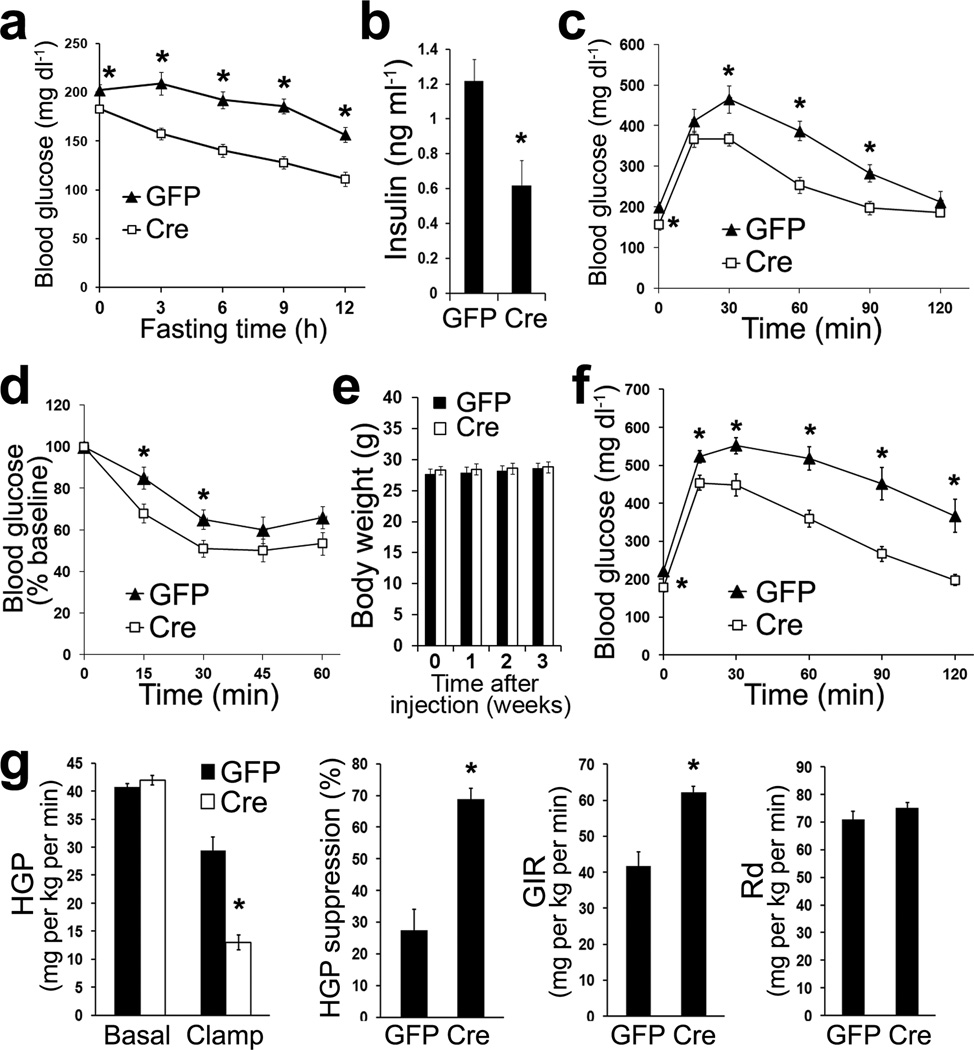
Despite the marked accumulation of triglyceride in liver, we were surprised to find that mice lacking hepatic Hdac3 had lower fasting blood glucose concentrations at 2-weeks after injection (Fig. 2a). This suggests that Hdac3 normally promotes hepatic glucose production during fasting. Mice without hepatic Hdac3 also displayed lower basal blood insulin concentrations (Fig. 2b), and were more tolerant to glucose and insulin (Fig. 2c, d), suggesting insulin hypersensitivity. Although most of the studies were performed in males, we observed similar phenotype in female mice (Supplementary Fig. 2a, b). These results are in line with a previous study reporting that mice with in utero deletion of hepatic Hdac3 using albumin-Cre (Alb:Hdac3fl/fl mice) develop hypoglycemia and hypoinsulinema, although the study was confounded by body weight differences and did not detect significant differences on whole-body glucose tolerance25. In the present study, depletion of hepatic Hdac3 in adult mice did not alter body weight up to 4-weeks after AAV-Cre injection compared to AAV-GFP-injected or non-injected controls (Fig. 2e and data not shown).
Figure 2. Hdac3-depleted liver has impaired glucose production and is insulin hypersensitive.
(a) Fasting blood glucose concentrations on chow at 2-weeks after AAV injection. Fasting started at 7 a.m., n = 7–8. (b) Basal blood insulin concentrations on chow at 5 p.m. at 2-weeks after injection, n = 7–8. (c) Glucose tolerance test on chow at 2-weeks after injection, n = 7–8. (d) Insulin tolerance test on chow at 3-weeks after injection, n = 7–8. (e) Body weight on chow, n = 7–8. (f) Glucose tolerance test at 2-weeks after injection after feeding HFD for 4-weeks, n = 8. (g) Hyperinsulinemic euglycemic clamp at 3-weeks after injection after feeding HFD for 4 weeks, n = 5. HGP: hepatic glucose production; GIR: glucose infusion rate; Rd: rate for disposal of glucose. Error bars indicate s.e.m. * P < 0.05 between GFP and Cre groups.
The improvement in glucose tolerance (Cre versus GFP) was even more prominent when mice were exposed to HFD (Fig. 2f), again without differences in body weight gain (Supplementary Fig. 2c), despite the severe hepatosteatosis in Hdac3-depleted liver under the same condition (Supplementary Fig. 2d). Hyperinsulinemic-euglycemic clamp studies using 3H-glucose as a tracer revealed that insulin-mediated suppression of hepatic glucose production (HGP) was significantly potentiated in the absence of hepatic Hdac3, resulting in a higher glucose infusion rate (GIR) in the clamp state (Fig. 2g, and supplementary Fig. 2e, f). The apparent lack of difference in basal HGP is likely due to the lower basal blood insulin levels in mice without hepatic Hdac3 compared to GFP controls. Thus, the loss of hepatic Hdac3 results in insulin hypersensitivity and deficient glucose production despite hepatic lipid accumulation.
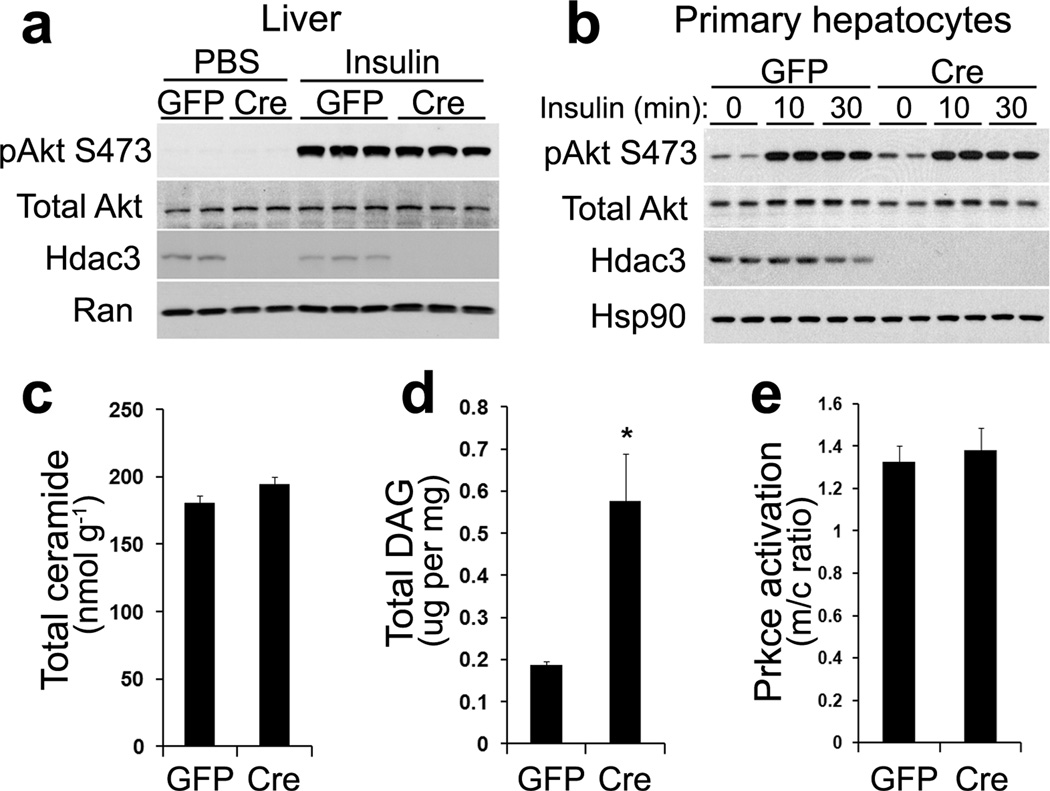
The prominent dissociation of hepatic steatosis and insulin resistance in Hdac3-depleted liver prompted us to further examine the insulin signaling pathway. We found that insulin-dependent phosphorylation of Akt was not altered upon Hdac3-deletion in either liver or isolated primary hepatocytes in response to different dosages of insulin (Fig. 3a, b and Supplementary Fig. 3a, b). Expression of pro-inflammatory genes and macrophage infiltration also remained unaltered (Supplementary Fig. 3c, d). Total hepatic ceramide content was not changed (Fig. 3c), although several ceramide species showed modest increases (Supplementary Fig. 3e). Hepatic DAG levels were indeed prominently elevated (Fig. 3d and Supplementary Fig. 3f), but did not lead to Prkce activation in Hdac3-depleted liver compared to GFP controls (Fig. 3e), as was noted in HFD-induced fatty liver compared to chow-fed controls (Supplementary Fig. 3g). This suggests that accumulated lipid species were somehow sequestered from activating cytosolic kinases such as Prkce and restrained from inducing inflammatory responses, which may underlie the dissociation of hepatosteatosis and insulin resistance28.
Figure 3. Molecular insulin signaling events remain undisturbed despite lipid accumulation in Hdac3-depleted liver.
(a) Western blot analysis of liver at 2-weeks after AAV injection. Mice were fasted for 18 h. Insulin was injected (2 mU g−1) followed by tissue harvest after 20 min. (b) Western blot analysis of primary hepatocytes isolated from AAV-injected mice after overnight fasting. Cells were challenged with 10 nM insulin for the indicated time and harvested. (c) Hepatic ceramide measured by LC/MS-MS, n = 5. (d) Hepatic DAG measured by gas chromatography, n = 5. (e) Prkce (PKCε) activation assay in liver, n = 6. m/c: membrane versus cytosolic Prkce protein amount. Error bars indicate s.e.m. * P < 0.05.
Hdac3 controls metabolic rerouting and lipid sequestration
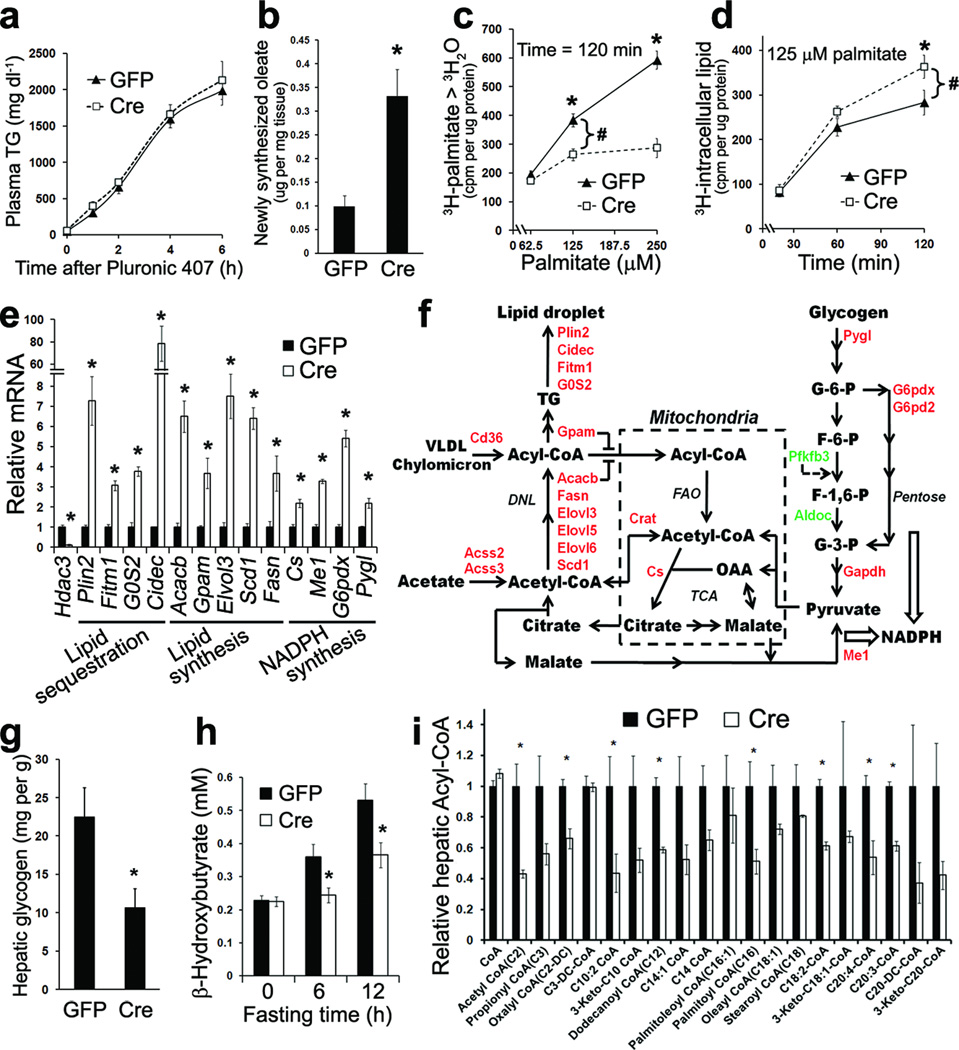
From a metabolic kinetic perspective, fatty liver is a result of imbalance among hepatic triglyceride secretion, de novo lipogenesis, fatty acid oxidation, and fatty acid uptake5. Hepatic triglyceride secretion rate, determined in vivo by measuring plasma triglyceride concentrations in a time course after blocking its turnover with the detergent Pluronic 407, were not affected by lack of Hdac3 (Fig. 4a). Hepatic de novo lipogenesis rate, measured in vivo by tracing newly synthesized oleate from deuterated water, was higher in Hdac3-depleted liver compared to GFP controls (Fig. 4b). Fatty acid oxidation and uptake were evaluated together in isolated primary hepatocytes by measuring production of 3H-labeled water from 3H-palmitate and acquisition of 3H-palmitate into the intracellular lipid pool, respectively. Hdac3-depleted hepatocytes showed lower fatty acid oxidation rate compared to GFP controls (Fig. 4c). More 3H-palmitate was found in the intracellular lipid pool in the absence of Hdac3, with the net gain in fatty acid acquisition roughly equal to the loss in fatty acid oxidation (Fig. 4d). Considering the unaltered triglyceride secretion, this suggests that the fatty acid uptake process per se was minimally affected by Hdac3 deletion.
Figure 4. Loss of Hdac3 reroutes precursors toward lipid synthesis and sequestration within lipid droplets.
(a) Hepatic triglyceride (TG) secretion rate evaluated by measuring time-dependent increase of plasma TG levels after blocking TG turnover, n = 6. (b) De novo lipogenesis rate measured in vivo by tracing hepatic 2H-oleate synthesized during a 4 h interval from 2H-H2O, n = 6–7. (c) Fatty acid oxidation rate measured in primary hepatocytes isolated from AAV-injected mice. After incubation of cells with the indicated amount of 3H-palmitate for 120 min, 3H-H2O was measured by scintillation counting. cpm, counts per minute. (d) Net fatty acid acquisition was measured in the same experiment as in (c). Cells were harvested after incubation with 125 uM 3H-palmitate for the indicated time, and intracellular lipid was extracted for radioactivity counting. # indicates that the gain in net fatty acid acquisition roughly equal to the loss in fatty acid oxidation. (e) RT-qPCR analysis of liver on chow at 2-weeks after injection, n = 5. (f) Biochemical pathways of genes that are upregulated (red) and downregulated (green) upon Hdac3 deletion. DNL, de novo lipogenesis; FAO, fatty acid oxidation; TCA, citric acid cycle. (g) Hepatic glycogen content, n = 5. (h) Fasting serum ketone body concentrations. Fasting started at 7 a.m. n = 7–8. (i) Hepatic acyl-CoA content measured by tandem mass spectrometry, n = 4. Error bars indicate s.e.m. * P < 0.05 between Cre and GFP groups.
In keeping with the metabolic tracing studies, comprehensive gene expression analysis showed that Hdac3 deletion in liver caused prominent upregulation of genes that promote lipid sequestration, lipid synthesis and NADPH synthesis (Fig. 4e, and Gene Expression Omnibus GSE25937). Upregulated expression of malic enzyme 1 (Me1) and citrate synthase (Cs) suggests an enhanced pyruvate/malate cycle that supplies NADPH and acetyl-CoA for lipid synthesis (Fig. 4e, f). Upregulated glycogen phosphorylase (Pygl) and glucose-6-phosphate dehydrogenase (G6pdx and G6pd2), along with downregulated 6-phosphofructo-2-kinase (Pfkfb3)29,30, predict that glycogen would be consumed to feed into the pentose phosphate pathway, again providing NADPH to be used for lipid synthesis (Fig. 4e, f). Indeed, hepatic glycogen content was significantly lower in Hdac3-depleted liver compared to the GFP control (Fig. 4g and Supplementary Fig. 4a). Mice without hepatic Hdac3 also displayed modestly higher FFA concentrations in serum (Supplementary Fig. 4b), potentially because of enhanced fat mobilization in adipose tissues due to lower blood insulin concentrations.
Expression of sterol regulatory element-binding proteins (Srebp) was not altered by Hdac3 deletion (Supplementary Fig. 4c). This, together with the finding that the genome-wide binding of Srebps does not extensively overlap with Hdac3 in mouse liver23,31,32, suggests that Srebps were unlikely to have contributed significantly to the hepatosteatosis phenotype in Hdac3-depleted liver. Expression of other transcriptional regulators of lipogenesis, including liver X receptors (Lxr) and carbohydrate response element-binding protein (Mlxipl), remained unchanged upon Hdac3 deletion (Supplementary Fig. 4c), consistent with the conclusion that Hdac3 directly represses transcription of lipogenic genes to which it is recruited by Nr1d123. Expression of genes involved in fatty acid oxidation (FAO) was unchanged or even upregulated upon Hdac3 deletion (Supplementary Fig. 4d), despite reduced FAO flux (Fig. 4c) and lower fasting serum ketone concentrations compared to GFP controls (Fig. 4h). These observations, along with the lack of accumulation of acyl-CoAs in liver (Fig. 4i) or acylcarnitines in plasma (Supplementary Fig. 4e), suggest that the FAO pathway is not inherently defective, but simply dominated by the enhanced lipid synthesis pathway in Hdac3-depleted liver. This could be accomplished through inhibition of acyl-CoAs transport into mitochondria by glycerol-3-phosphate acyltransferase 1 (Gpam) and acetyl-CoA carboxylase 2 (Acacb)5,33(Fig. 4f).
The finding that acyl-CoA species were reduced in Hdac3-depleted liver despite accumulation of triglyceride (Fig. 4i) suggests that these intermediates were shunted towards glycerolipid formation and subsequent sequestration in lipid droplets. Indeed, perilipin 2 (Plin2), cell death-inducing DFFA-like effector c (Cidec), fat storage-inducing transmembrane protein 1 (Fitm1), and G0/G1 switch gene 2 (G0S2), which encode proteins involved in lipolysis inhibition and lipid sequestration16,34–36, were among the most prominently upregulated genes in Hdac3-depleted liver (Fig. 4e). Their protein products were similarly upregulated and associated with lipid droplets (Supplementary Fig. 5a, b) in a highly specific manner, as other lipid droplet proteins such as Plin3, Cideb and Cidea was not significantly changed (Supplementary Fig. 5b, c). Thus, enhanced lipid sequestration causes reduced availability of fatty acids to the FAO machinery in mitochondria, resulting in reduced FAO activity without accumulation of acyl-CoAs. In sharp contrast, HFD-induced fatty liver is associated with elevated hepatic acyl-CoA species37–39 compared to chow-fed controls. When compared to Hdac3 deletion at the same degree of hepatosteatosis, HFD feeding caused less upregulated expression of genes involved in lipid storage and sequestration (Supplementary Fig. 6a, b), highlighting the importance of lipid sequestration in combating lipotoxicity. Taken together, loss of Hdac3 de-represses metabolic genes and reroutes metabolites towards lipid synthesis and sequestration, which underlies hepatic steatosis.
Metabolic rerouting reduces hepatic glucose production
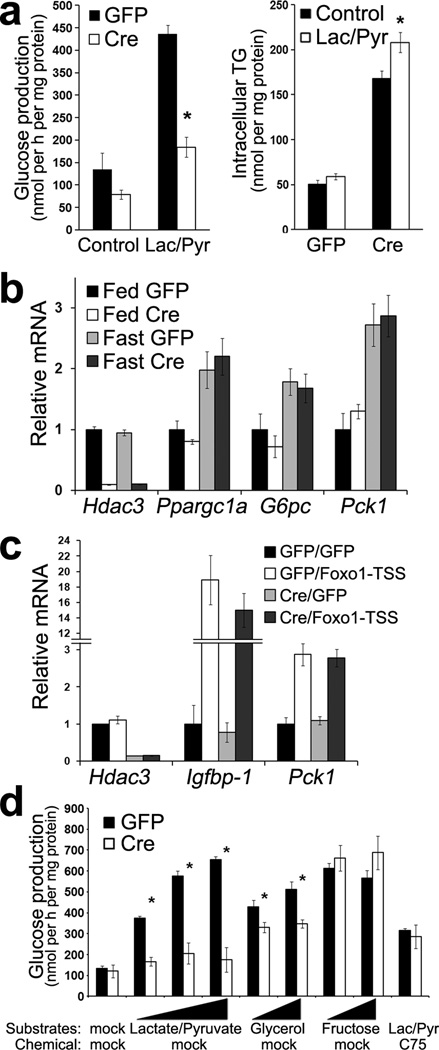
We next addressed the mechanism of how Hdac3 regulates glucose metabolism. In primary hepatocytes isolated from fasted mice without hepatic Hdac3, glucose production from precursors lactate and pyruvate was significantly reduced compared to GFP controls (Fig. 5a). Meanwhile, the already high levels of intracellular triglyceride in Hdac3-depleted hepatocytes were further increased upon exposure to lactate and pyruvate (Fig. 5a), suggesting that the gluconeogenic precursors were rerouted into intracellular lipid pools instead of being used for glucose production. Indeed, the reduction in glucose output calculated in carbon atoms was nearly 3 µmol per mg protein during the 2h incubation, and the increased triglyceride in carbons was about 2 µmol per mg protein (based on the mass of tripalmitin). Considering that only two out of the three carbons in lactate and pyruvate actually contribute to lipogenesis, these data strongly support the hypothesis that the reduced gluconeogenesis is explained by the enhanced lipogenesis in the absence of Hdac3.
Figure 5. Reduced hepatic glucose production in Hdac3-depleted liver is a result of the metabolic rerouting, rather than due to inherent defects of gluconeogenesis.
(a) Glucose production in primary hepatocytes isolated from AAV-injected mice after overnight fasting. Cells were incubated with Krebs buffer supplemented with or without 10 mM lactate (Lac) and 1 mM pyruvate (Pyr) for 2 h. The generated glucose was determined. Cells were harvested and intracellular triglyceride was extracted and measured. (b) RT-qPCR analysis of liver from mice at 2-weeks since AAV injection after either fed or fasted for 12 h from 7 p.m. to 7 a.m. n = 3. (c) RT-qPCR analysis of liver. At 9 d after AAV injection, mice were injected with adenovirus expressing either GFP or Foxo1-TSS (T24A, S256A, S319A). After another 5 d, mice were fasted for 12 h (7 p.m. −7 a.m.) and then refed for 6 h followed by tissue harvest. n = 4. (d) Glucose production in primary hepatocytes. Lac/Pyr were added together at 2 mM /0.2 mM, 10 mM /1 mM, 30 mM /3 mM; glycerol and fructose was added individually at 10 mM and 25 mM. Lipid synthesis inhibitor C75 was used at 5 ug ml−1. n = 3. Error bars indicate s.e.m. * P < 0.05 between Cre and GFP groups.
Recently Hdac3 was suggested to be directly involved in forkhead protein Foxo1-mediated transcriptional control of hepatic gluconeogenesis40. However, we found that deletion of Hdac3 in liver did not alter expression of key gluconeogenic genes including peroxisome proliferator-activated receptor gamma coactivator 1α (Ppargc1a), glucose 6-phosphatase (G6pc) and phosphoenolpyruvate carboxykinase (Pck1) under either fed or fasting conditions (Fig. 5b). To address whether Hdac3 is required specifically for Foxo1-mediated transcription, mice with or without hepatic Hdac3 were injected with adenovirus expressing constitutively active Foxo1. Gene expression analysis in liver showed that loss of Hdac3 did not interfere with Foxo1-depedent transcription of its target genes Igfbp-1 and Pck1 (Fig. 5c). These data suggest that gluconeogenesis is not inherently defective in Hdac3-depleted liver.
To circumvent the competition for substrates from lipogenesis, we sought to determine if the reduced glucose production in Hdac3-depleted hepatocytes could be rescued with substrates that are more direct precursors for gluconeogenesis but less direct precursors for lipogenesis. Lactate and pyruvate were unable to rescue even at high concentrations (Fig. 5d), presumably due to enhanced pyruvate and malate cycle driven by continuous consumption of acetyl-CoA and NADPH in lipogenesis (Fig. 4f), and limited pyruvate carboxylase activities due to low acetyl-CoA levels (Fig. 4i). Glycerol significantly boosted glucose production in Hdac3-depleted hepatocytes but still could not completely rescue it (Fig. 5d), in line with the fact that glycerol is able to bypass the TCA cycle in gluconeogenesis but can also be directly used for fatty acid esterification. Fructose, a more direct glucose precursor when used at a concentration (10–25 mM) well above its Km for hexokinase (about 1–2 mM), completely rescued glucose production in Hdac3-depleted hepatocytes (Fig. 5d), excluding possibilities for any inherent defects in the last several steps of gluconeogenesis, including the one catalyzed by G6pc, in these cells. Exposure of hepatocytes to a fatty acid synthase inhibitor C75 also masked the difference in glucose production (Fig. 5d), supporting the notion that the metabolic rerouting towards lipid synthesis and sequestration contributes to reduced glucose production in Hdac3-depleted hepatocytes.
Lipid sequestration, steatosis, and insulin resistance
Our studies in primary hepatocytes provided in vitro evidence that hepatic glucose production is dominated by the lipid synthesis and sequestration pathway when Hdac3 is deleted. To test this hypothesis in vivo, we sought to specifically inhibit lipid synthesis and sequestration in liver. Several considerations prompted us to target Plin2: (i) the lipid droplet-coating protein has been known to play essential role in lipid sequestration and inhibition of basal lipolysis34; (ii) our gene expression analysis showed that Plin2 is among the most prominently upregulated genes in Hdac3-depleted liver; (iii) it is known that Plin2 is required for hepatosteatosis and upregulated lipogenic gene expression under pro-diabetic conditions41,42; (iv) anti-sense oligonucleotides (ASO) against Plin2 is available for liver-specific knockdown42.
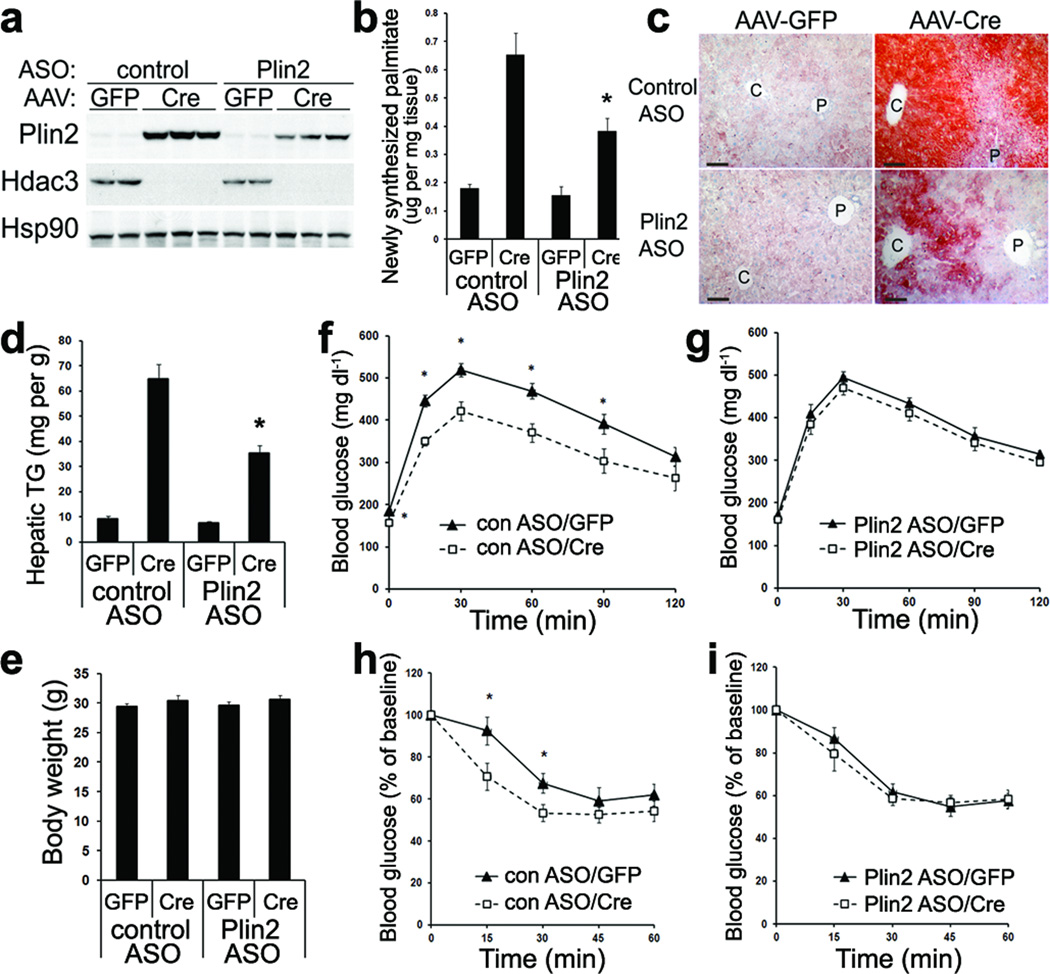
We found that treatment of mice with ASO against Plin2 blunted the prominent upregulation of Plin2 by Hdac3 deletion in liver (Fig. 6a). Consistent with previous findings42, knockdown of Plin2 suppressed the hepatic de novo lipogenesis flux that was enhanced by Hdac3 deletion (Fig. 6b), suggesting crosstalk between lipid storage and lipid synthesis. Plin2 knockdown clearly ameliorated the hepatosteatosis induced by Hdac3 deletion (Fig. 6c, d), and the remaining steatotic areas were primarily in the regions proximal to central veins (Fig. 6c). Thus, enhanced lipogenesis and lipid sequestration contributed significantly to the development of hepatosteatosis upon Hdac3 deletion. Of note, although Plin2 knockdown did not alter body weight (Fig. 6e), it masked the improved tolerance to glucose (Fig. 6f, g) as well as to insulin (Fig. 6h, i) in the hepatic Hdac3-depleted mice, strongly supporting that lipid sequestration is a key causal factor in dissociation of hepatosteatosis from insulin resistance. Consistent with this, hepatic overexpression of Plin2 (Supplementary Fig. 7a, b) caused hepatosteatosis (Supplementary Fig. 7c, d) associated with hypoglycemia (Supplementary Fig. 7e), hypoinsulinemia (Supplementary Fig. 7f), and improved whole-body insulin sensitivity (Supplementary Fig. 7g) without changes in body weight (Supplementary Fig. 7h). Collectively, these data demonstrate that enhanced lipid synthesis and sequestration contribute to altered carbohydrate metabolism and insulin hypersensitivity in mice lacking hepatic Hdac3.
Figure 6. Lipid sequestration is required for development of severe hepatosteatosis and improved glucose tolerance in Hdac3-depleted liver.
(a) Western blot analysis of total liver lysates from mice injected with either control anti-sense oligo (ASO) or ASO against Plin2 along with the indicated AAV vectors. (b) Hepatic de novo lipogenesis rate measured by tracing 2H-palmitate synthesized from 2H2O, n = 6. (c) ORO staining of liver. C: central vein; P: portal vein. Scale bar, 50 µm. (d) Hepatic TG measurement, n = 6. (e) Body weight measurement at 2-weeks after AAV injection, n = 7–8. (f, g) Glucose tolerance test at 2-weeks after AAV injection, n = 7–8. (h, i) Insulin tolerance test at 2-weeks after AAV injection, n = 7–8. Error bars indicate s.e.m. * P < 0.05 between Cre and GFP groups.
DISCUSSION
Liver is the central nexus of energy metabolism and governs fate of substrates in response to nutritional and hormonal signals. Under normal physiological conditions, both hepatic glucose production and lipid synthesis display circadian rhythm20. Our findings establish that rhythmic rerouting of metabolic intermediates between the two synthetic pathways is directly regulated by the molecular circadian clock through the epigenomic modifier Hdac3 (Supplementary Fig. 8). During the day, when the nocturnal mouse is inactive and feeding less, Hdac3 is recruited to the genome and represses expression of genes involved in lipid synthesis and sequestration, and allows metabolic precursors to be directed towards gluconeogenesis in order to maintain normoglycemia. During the active and feeding night period, the virtual absence of Hdac3 at these genomic regions de-represses lipogenesis, and reroutes metabolic intermediates towards lipid synthesis for energy storage at the expense of glucose production. This model is supported by the following findings: i) depletion of Hdac3 in liver results in upregulation of genes in lipid synthesis and sequestration, but does not alter expression of key gluconeogenic genes; ii) the chromatin occupancy of Hdac3 is predominantly on genes in lipid metabolism and has a circadian pattern with the maximum occupancy during fasting and minimum occupancy during feeding23; iii) hepatic glycogen is reduced upon Hdac3 deletion, contrary to what would be expected if there were a primary defect in G6pc activity and inability of generating glucose from glycogen40,43; iv) glucose synthesis from canonical precursors pyruvate and lactate is reduced in Hdac3-depleted hepatocytes, concurrent with increased lipid synthesis from the same precursors; v) the low glucose production in Hdac3-depleted hepatocytes can be rescued by fructose, a six carbon precursors of glucose, or by inhibition of lipid synthesis, suggesting that gluconeogenesis is inherently intact, but dominated by the routing of three carbon metabolites toward lipid synthesis; vi) the low acetyl-CoA content is accompanied with enhanced lipogenesis flux in Hdac3-depleted liver, suggesting that acetyl-CoA is shunted toward lipogenesis, which may limit pyruvate carboxylase activity and thus reduce gluconeogenesis; vii) the improved glucose and insulin tolerance in Hdac3 liver-specific knockout mice is attenuated by suppression of the metabolic rerouting towards lipid synthesis and storage through knockdown of lipid sequestering LDs-coating protein Plin2; and viii) overexpression of Plin2 in liver in wild-type mice results in hepatosteatosis and improved insulin sensitivity, mimicking the metabolic phenotype in Hdac3 liver-specific knockout mice.
The present study highlights the integrated nature of metabolism. It shows that a given metabolic process can be modulated by rerouting of metabolites, without altering enzymes immediately involved in that process, as exemplified by the indirect regulation of gluconeogenesis by Hdac3. Notably, a parallel and independent metabolic rerouting may be coordinated by hepatic FoxOs, which are active in driving gluconeogenic genes expression during fasting and are inhibited by Akt-mediated phosphorylation and p300-mediated acetylation during feeding44. Constitutively active Foxo1 increases glucose production, and seems to reduce hepatic lipid output as evidenced by hypolipidemia45,46. Conversely, depletion of hepatic FoxOs decreases glucose production, and tends to increase lipid synthesis as evidenced by hepatosteatosis or hyperlipidemia47,48. In both situations, the effects of FoxOs on gluconeogenesis are through well-characterized direct transcriptional activation, while the effects of FoxOs on lipid metabolism are less well-defined and somewhat controversial. Of note, acetylation of FoxOs decreases their transactivation activity44, and it has been shown that recombinant Hdac3 protein can deacetylate Foxo1 in vitro40. However, our findings that deletion of Hdac3 in liver does not alter Foxo1-dependent transcription, and does not cause accumulation of glycogen as predicted by the in vitro model40 clearly indicate that the potential deacetylation of Foxo1 is not essential for effects of Hdac3 on glucose metabolism in vivo. We speculate that FoxOs-mediated transcriptional activation and Hdac3-mediated transcriptional repression may function collectively, but independently, to divert metabolic intermediates in between glucose production and lipid synthesis during the fasting/feeding and light/dark cycles. Although Hdac3 clearly functions as an epigenomic modifier in liver23, it may also have non-histone protein targets as well as deacetylase-independent functions.
In contrast to the circadian shifting between hepatic lipogenesis and gluconeogenesis under normal physiological conditions, pathological overnutrition provides excess metabolic intermediates that oversaturate and constantly activate both pathways. This leads to co-existence of hepatosteatosis and insulin resistance. However, the concurrent insulin hypersensitivity and severe hepatosteatosis in mice without hepatic Hdac3 clearly demonstrates that hepatosteatosis and insulin resistance per se do not necessarily have a cause and effect relationship. Among the most prominently upregulated genes in Hdac3-depleted liver are those encoding lipid droplets-associated proteins, including Plin2, Cidec, Fitm1 and G0S2, that negatively regulate lipolysis and subsequent release of lipids in a cooperative manner8,16,34,49,50,35,36. Low acyl-CoA content is accompanied with reduced FAO flux in Hdac3-depleted liver, suggesting that acyl-CoAs are shunted toward glycerolipid synthesis and subsequent sequestration from mitochondrial oxidation machinery. Prkce activity and inflammation is not enhanced despite elevated total DAG content and other lipid species in Hdac3-depleted liver, suggesting that lipid is sequestered from activating cytosolic kinases. Dampening the upregulation of Plin2 masked the insulin hypersensitivity in mice without hepatic Hdac3, while hepatic overexpression of Plin2 in wild type mice results in steatosis and improves whole-body insulin sensitivity, stressing the essential role of lipid sequestration in preventing lipotoxicity and lipid-induced hepatic insulin resistance. Consistent with our finding, the insulin sensitizing effect of Plin2 was observed previously in cultured hepatocytes where depletion of Plin2 and other perilipins decreased cellular responses to insulin51. In addition, dissociation of hepatosteatosis and insulin resistance was observed in mice or humans that are deficient in either lipolysis or hepatic triglyceride secretion8,18,52–54. All these findings can be unified under the hypothesis that hepatic insulin resistance is determined by whether lipid droplets sequester lipid content well enough to prevent cytosolic accumulation of deleterious lipid species.
Although hepatic lipogenesis is upregulated and required for the development of steatosis in both chow-fed mice lacking hepatic Hdac3 and in wild type mice on HFD, a key difference lies in the balance between lipid sequestration capacity and the content of metabolic intermediates. In overnutrition mouse models (HFD or ob/ob), excess delivery of metabolic intermediates to the liver is the primary event, while increased hepatic lipid synthesis and storage is an adaptive response. But the lipid sequestration capacity is insufficient relative to the overwhelming lipid content influx, leading to accumulation of harmful lipid intermediates, such as DAG and ceramide that promote insulin resistance. In this situation, ablation of Plin2 reduces lipid synthesis and thus reduces generation of harmful lipid species, leading to improved insulin sensitivity42,55. In the Hdac3-deficient model, the primary event of upregulated lipogenesis diverts metabolic precursors away from glucose production. In addition, reduced fatty acid oxidation and acetyl-CoA levels may contribute to decrease pyruvate carboxylase activity and thus limit the flux of pyruvate to glucose production. Collectively, these changes result in reduced glucose output and insulin hypersensitivity. Knockdown of Plin2 on this background reduces lipid synthesis and the metabolic rerouting, and therefore ameliorates insulin hypersensitivity. This highlights the concept that hepatic lipid accumulation have diverse pathogenesis and require specifically targeted therapies, which is critically important in light of the failure of large therapeutic trials (such as ACCORD) that lump all diabetes together56.
Finally, our data establish Hdac3 as a pivotal epigenomic modifier that directly receives signal input from the molecular circadian clock and promotes hepatic glucose production via rerouting of gluconeogenic metabolites away from lipid synthesis rather than direct transcriptional regulation of gluconeogenic genes. This novel mechanism highlights the dissociation of hepatic steatosis from insulin resistance due to lipid sequestration, which may explain similar phenotypes that have been observed in other mouse models8,17,18,53,57.
METHODS
Methods and any associated references are available in the online version of the paper.
Supplementary Material
ACKNOWLEDGEMENTS
We thank J. Millar and the Metabolic Tracer Resource at the Penn Institute for Diabetes, Obesity and Metabolism for lipogenesis flux assay. We thank the Mouse Metabolic Phenotyping Core and the Viral Vector Core at Penn Diabetes Research Center (P30 DK19525), and the Penn Digestives Disease Center Morphology Core (P30 DK050306) at the University of Pennsylvania. We thank the Vanderbilt (DK59637) and Yale (U24 DK059635) Mouse Metabolic Phenotyping Centers, and the Children's Hospital of Philadelphia Pathology Core. We thank B. Agarwal and S. Mullican for helpful discussion. This work was supported by R37 DK43806 (M.A.L.), P01 DK49210 (M.A.L., M.J.B., R.S.A.), R01 DK40936 (G.I.S.), R01 DK075017 (C.S.), the Department of Veteran Affairs Merit Review Program (T.G.U.), F32 DK079572 (R.A.M.), the JPB Foundation, and the Cox Institute for Medical Research.
Abbreviations
- AAV
adeno-associated virus
- LDs
lipid droplets
- HFD
high fat diet
- DAG
diacylglyceride
Footnotes
AUTHOR CONTRIBUTIONS
Z.S. and M.A.L. conceived of the hypothesis and designed the experiments. Z.S., R.A.M., R.T.P., J.C., R.D., H.W. and D.Z. performed the experiments. Z.S., R.A.M., R.D., T.G.U., G.I.S., C.S., M.J.B., R.S.A., M.J.B. and M.A.L. analyzed and interpreted the data. M.J.G. provided reagents. Z.S. and M.A.L. wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 4.Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J. Lipid Res. 2009;50(Suppl):S74–S79. doi: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonardo A, Bellentani S, Ratziu V, Loria P. Insulin resistance in nonalcoholic steatohepatitis: necessary but not sufficient - death of a dogma from analysis of therapeutic studies? Expert Rev Gastroenterol Hepatol. 2011;5:279–289. doi: 10.1586/egh.11.19. [DOI] [PubMed] [Google Scholar]
- 8.Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J. Gastroenterol. Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- 11.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu. Rev. Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi CM, et al. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Stiles B, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected] Proc. Natl. Acad. Sci. U.S.A. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg AS, et al. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzaka T, et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat. Med. 2007;13:1193–1202. doi: 10.1038/nm1662. [DOI] [PubMed] [Google Scholar]
- 18.Brown JM, et al. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuomilehto J, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 20.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol. Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 22.Lawless MW, Norris S, O’Byrne KJ, Gray SG. Targeting histone deacetylases for the treatment of immune, endocrine & metabolic disorders. Endocr Metab Immune Disord Drug Targets. 2009;9:84–107. doi: 10.2174/187153009787582441. [DOI] [PubMed] [Google Scholar]
- 23.Feng D, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z, Feng D, Everett LJ, Bugge A, Lazar MA. Circadian Epigenomic Remodeling and Hepatic Lipogenesis: Lessons from HDAC3. Cold Spring Harbor Symposia on Quantitative Biology. 2011 doi: 10.1101/sqb.2011.76.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutson SK, et al. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. EMBO J. 2008;27:1017–1028. doi: 10.1038/emboj.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullican SE, et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25:2480–2488. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell P, et al. Evaluation of adeno-associated viral vectors for liver-directed gene transfer in dogs. Hum. Gene Ther. 2011;22:985–997. doi: 10.1089/hum.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuel VT, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calvo MN, et al. PFKFB3 gene silencing decreases glycolysis, induces cell-cycle delay and inhibits anchorage-independent growth in HeLa cells. FEBS Lett. 2006;580:3308–3314. doi: 10.1016/j.febslet.2006.04.093. [DOI] [PubMed] [Google Scholar]
- 30.Bolaños JP, Almeida A, Moncada S. Glycolysis: a bioenergetic or a survival pathway? Trends Biochem. Sci. 2010;35:145–149. doi: 10.1016/j.tibs.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Seo Y-K, et al. Genome-wide analysis of SREBP-1 binding in mouse liver chromatin reveals a preference for promoter proximal binding to a new motif. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13765–13769. doi: 10.1073/pnas.0904246106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo Y-K, et al. Genome-wide localization of SREBP-2 in hepatic chromatin predicts a role in autophagy. Cell Metab. 2011;13:367–375. doi: 10.1016/j.cmet.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim. Biophys. Acta. 2009;1791:501–506. doi: 10.1016/j.bbalip.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Gong J, Sun Z, Li P. CIDE proteins and metabolic disorders. Curr. Opin. Lipidol. 2009;20:121–126. doi: 10.1097/MOL.0b013e328328d0bb. [DOI] [PubMed] [Google Scholar]
- 36.Puri V, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J. Biol. Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 37.Samuel VT, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 38.Hammond LE, et al. Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J. Biol. Chem. 2005;280:25629–25636. doi: 10.1074/jbc.M503181200. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson A, Thomassen MS, Christiansen E. Long-chain Acyl-CoA levels in liver from rats fed high-fat diets: is it of significance for an increased peroxisomal beta-oxidation? Lipids. 1984;19:187–194. doi: 10.1007/BF02534796. [DOI] [PubMed] [Google Scholar]
- 40.Mihaylova MM, et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang BH-J, et al. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol. Cell. Biol. 2006;26:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai Y, et al. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132:1947–1954. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 43.Mutel E, et al. Control of Blood Glucose in the Absence of Hepatic Glucose Production During Prolonged Fasting in Mice: Induction of Renal and Intestinal Gluconeogenesis by Glucagon. Diabetes. 2011 doi: 10.2337/db11-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogt PK, Jiang H, Aoki M. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle. 2005;4:908–913. doi: 10.4161/cc.4.7.1796. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 46.Banks AS, et al. Dissociation of the Glucose and Lipid Regulatory Functions of FoxO1 by Targeted Knockin of Acetylation-Defective Alleles in Mice. Cell Metab. 2011;14:587–597. doi: 10.1016/j.cmet.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haeusler RA, Han S, Accili D. Hepatic FoxO1 ablation exacerbates lipid abnormalities during hyperglycemia. J. Biol. Chem. 2010;285:26861–26868. doi: 10.1074/jbc.M110.134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tao R, et al. Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J. Biol. Chem. 2011;286:14681–14690. doi: 10.1074/jbc.M110.201061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadereit B, et al. Evolutionarily conserved gene family important for fat storage. Proc. Natl. Acad. Sci. U.S.A. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X, et al. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bell M, et al. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–2045. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kantartzis K, et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58:2616–2623. doi: 10.2337/db09-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minehira K, et al. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res. 2008;49:2038–2044. doi: 10.1194/jlr.M800248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schonfeld G, Yue P, Lin X, Chen Z. Fatty liver and insulin resistance: not always linked. Trans. Am. Clin. Climatol. Assoc. 2008;119:217–223. discussion 223–224. [PMC free article] [PubMed] [Google Scholar]
- 55.Chang BH-J, Li L, Saha P, Chan L. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J. Lipid Res. 2010;51:2132–2142. doi: 10.1194/jlr.M004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerstein HC, et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monetti M, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J. Lipid Res. 2009;50:1621–1629. doi: 10.1194/jlr.M800614-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumashiro N, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. U.S.A. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sztalryd C, et al. Functional compensation for adipose differentiation-related protein (ADFP) by Tip47 in an ADFP null embryonic cell line. J. Biol. Chem. 2006;281:34341–34348. doi: 10.1074/jbc.M602497200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.