Abstract
Fetal alcohol spectrum disorders are often associated with structural and functional hippocampal abnormalities, leading to long-lasting learning and memory deficits. The mechanisms underlying these abnormalities are not fully understood. Here, we investigated whether ethanol exposure during the 3rd trimester-equivalent period alters spontaneous network activity that is involved in neuronal circuit development in the CA3 hippocampal region. This activity is driven by GABAA receptors, which can have excitatory actions in developing neurons as a consequence of greater expression of the Cl− importer, NKCC1, with respect to expression of the Cl− exporter, KCC2, resulting in high [Cl−]i. Rat pups were exposed to ethanol vapor from postnatal day (P) 2 to 16 (4 hr/day). Weight gain was significantly reduced in pups exposed to ethanol compared to control at P15 and 16. Brain slices were prepared immediately after the end of the 4-hr exposure on P4–16 and experiments were also performed under ethanol-free conditions at the end of the exposure paradigm (P17–22). Ethanol exposure did not significantly affect expression of KCC2 or NKCC1, nor did it affect network activity in the CA3 hippocampal region. Ethanol exposure significantly decreased the frequency (at P9–11) and increased the amplitude (at P5–8 and P17–21) of GABAA receptor-mediated miniature postsynaptic currents. These data suggest that repeated in vivo exposure to ethanol during the 3rd trimester-equivalent period alters GABAergic transmission in the CA3 hippocampal region, an effect that could lead to abnormal circuit maturation and perhaps contribute to the pathophysiology of fetal alcohol spectrum disorders.
Keywords: Fetal alcohol syndrome, development, transporter, GABA, synaptic
Introduction
Drinking during pregnancy is prevalent around the world, leading to a constellation of alterations in offspring collectively known as fetal alcohol spectrum disorders (FASD). Neurobehavioral deficits are common in FASD patients, including learning and memory alterations that are thought to be a consequence of damage to the hippocampal formation (reviewed in Berman and Hannigan, 2000). Developmental ethanol exposure has been shown to produce structural and functional alterations in the CA3 hippocampal region (Farr et al., 1988; Sakata-Haga et al., 2003; Swartzwelder et al., 1988; Tanaka et al., 1991; West et al., 1981). For instance, aberrant intra- and infra-pyramidal mossy fibers were observed in a rodent model of 3rd trimester ethanol exposure (Livy et al., 2003; Maier and West, 2001; West and Hamre, 1985). The precise mechanisms responsible for these and other hippocampal abnormalities associated with developmental ethanol exposure remain to be elucidated.
Alterations in the spontaneous electrical activity that is essential for the development of neuronal circuits (Moody and Bosma, 2005; Spitzer, 2004) could play a role in the pathophysiology of FASD. Network driven-patterns of recurrent neuronal activity have been reported in several regions of the developing brain, including the hippocampus (reviewed in Ben-Ari, 2002). These events have been particularly well characterized in the CA3 hippocampal region where they are known as giant depolarizing potentials (GDPs) (Ben-Ari et al., 1989). Under current-clamp electrophysiological conditions, GDPs are observed as long-lasting, repetitive, large depolarizations (or inward currents in voltage-clamp) that are commonly observed during the first two weeks of life in rodents. Intrinsic bursting activity of CA3 pyramidal neurons, mediated by persistent Na+ and slow Ca2+ activated K+ currents, plays a central role in GDP generation (Sipila et al., 2005; Sipila et al., 2006). Pyramidal neuron bursting activity is facilitated by the depolarizing actions of type-A γ-aminobutyric acid (GABAA) and ionotropic glutamate receptors (Bolea et al., 1999; Sipila et al., 2005), and perhaps also by hyperpolarization activated currents (Bender et al., 2005; but see Sipila et al., 2006). During development, GABAA receptors can exert excitatory effects that are a consequence of elevated [Cl−]i in immature neurons, which predominantly express the Cl− importer, Na+-K+-Cl− co-transporter-1 (NKCC1) (Reviewed in Blaesse et al., 2009). Higher [Cl−]i shifts the Cl− equilibrium potential (ECl−) to a less negative membrane potential and, upon GABAA receptor activation, an outward Cl− current depolarizes the membrane potential. During maturation, neurons increasingly express the neuron-specific Cl− exporter, K+-Cl− co-transporter-2 (KCC2), leading to a reduction in [Cl−]i, a leftward shift in ECl−, and a switch in the function of GABAA receptors to inhibitory (Blaesse et al., 2009; Williams et al., 1999). The transient nature of the excitatory actions of GABAA receptors is thought to be predominantly responsible for the transient expression of GDPs. GDPs are associated with large [Ca2+]i elevations that regulate key developmental processes, such as release of neurotrophic factors and changes in gene expression, that ultimately control stabilization or elimination of synapses (Mohajerani and Cherubini, 2006).
We previously demonstrated that acute ethanol exposure increases GDP frequency in CA3 pyramidal neurons from neonatal rats (Galindo et al., 2005). We also showed that repeated ethanol vapor exposure of neonatal rats does not alter basal GDP frequency, nor does it lead to the development of tolerance to the acute ethanol-induced increase of GDP frequency (Galindo and Valenzuela, 2006). The purpose of this study was to further characterize the effects of repeated ethanol exposure during the 3rd trimester-equivalent period on GABAergic transmission in the CA3 hippocampal region. Specifically, we assessed whether ethanol exposure affects: 1) the expression of KCC2 and NKCC1, 2) the gradual disappearance of GDPs as a function of development, and 3) the developmental changes in GABAA receptor-mediated miniature postsynaptic currents (GABAA-mPSCs).
Materials and Methods
Ethanol vapor chamber exposure paradigm
Animal procedures were approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center and conformed to National Institutes of Health guidelines. Timed-pregnant Sprague-Dawley rats were obtained from Harlan Laboratories Inc. (Indianapolis, IN) and allowed to acclimate for one week before giving birth. Neonatal rat pups and dams were exposed to 3–4 g/dL of ethanol vapor (Fig 1A), as previously described (Galindo and Valenzuela, 2006; Puglia and Valenzuela, 2009, 2010). Briefly, paired air/ethanol litters were culled to an equal number of pups (8–12), prior to postnatal day (P) 4. Dams and rat pups were exposed daily for 4 hr from P2 until P16 (Fig 1A). Exposures were started at 07:00 hr (lights on at 06:00 hr and lights off at 18:00 hr). The only mortality witnessed was 1 pup in 1 ethanol litter.
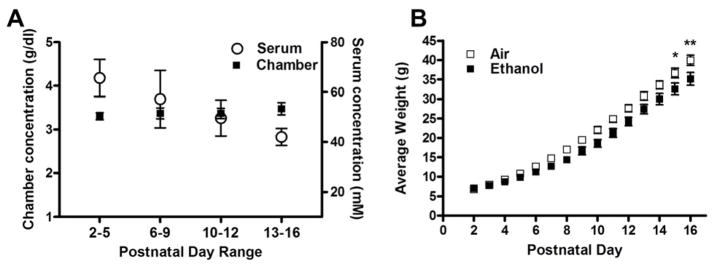
Figure 1. Chronic inhalation of ethanol vapor affected pup weight gain.
A) Ethanol vapor levels between 3–4 g/dL were recorded between P4–16, producing the indicated serum ethanol concentrations. (B) Pup weight gain was significantly affected by ethanol vapor exposure at P15 and 16 (*p<0.05, **p<0.01, respectively). Experiments were performed on 10 different litters per treatment group.
Brain Slice Preparation
Unless otherwise indicated, chemicals were purchased from Sigma (St. Louis, MO) or Tocris Cookson (Ellisville, MO). Male and female rat pups (P4–16) were euthanized 0.5–1.5 hr after the end of the 4 hr ethanol vapor exposure. In some cases, rats were euthanized after the end of the ethanol exposure paradigm at P17–22. Rats were euthanized by decapitation under deep anesthesia with ketamine (250 mg/kg). Serum was obtained by collecting trunk blood samples, allowing them to coagulate and centrifuging at 2.3 × g for 15 min at 4° C. Serum ethanol concentrations (SECs) were determined using an alcohol dehydrogenase-based assay, as previously described (Puglia and Valenzuela, 2009). Coronal brain slices (300 μm) were prepared using a vibratome, as previously described (Mameli et al., 2005). Slices were allowed to recover for a period of 40 min at 33–34°C followed by 30 min at room temperature in artificial cerebrospinal fluid (ACSF) containing the following: 124 mM NaCl, 7 mM KCl, 1.3 mM NaH2PO4, 26 mM NaHCO3, 10 mM glucose, 1 mM MgSO4, 2 mM CaCl2, and 400 μM of ascorbic acid (equilibrated with 95% O2 plus 5% CO2).
Western Immunoblotting
Brain slices were prepared as described above. Within 30 min after the recovery period, the CA3 hippocampal region was micro-dissected from coronal brain slices. Pooled CA3 regions from 1–2 rats were sonicated in homogenizing buffer containing 25 mM HEPES, 500 mM NaCl, 2 mM EDTA, 1 mM dithiothreitol, 0.1% Tween-20, 1 mM phenylmethanesulfonyl fluoride, 20 mM NaF, 1% v/v phosphatase cocktail (cat #P2850, Sigma), 5 μM cyclosporine A, and 1 Complete Mini Protease tablet/10 mL (cat #11 836 153 001, Roche Diagnostics, Mannheim, Germany) and stored in aliquots at −80°C. Protein concentration was determined using an assay based on the Bradford Method (BioRad, Hercules, CA); bovine serum albumin was used as standard. Samples were mixed with a 5X SDS-PAGE sample buffer containing 250 mM Tris-HCl (pH 6.8), 10% sodium dodecyl sulfate, 30% glycerol, 5% β-mercaptoethanol, 0.02% bromophenol blue and boiled for 5 min. The final protein concentration was 1 mg/mL. Samples were loaded at a concentration of 10 μg per lane and separated in 4–15% Tris-HCl precast gels (BioRad) at 110 V for 60–70 min. Control experiments demonstrated that this protein concentration was within the linear dynamic range for the western blot and Coomassie Blue assays (not shown). Proteins were electroblotted onto polyvinylidene fluoride (0.45 μm pore size) at 100 V for 70 min. Non-specific binding was prevented by incubating membranes for 2 hr with 5% non-fat dry milk in tris buffered saline (TBS) containing 0.1% Tween-20 (TBST). In most cases, membranes were initially incubated overnight at 4°C with monoclonal antibody anti-NKCC 1 (T4 antibody; 1:300; Developmental Studies Hybridoma Bank, Iowa City, IA). Membranes were stripped with Restore Western Blot Stripping Buffer for 15 min (Thermoscientific, Rockford, IL), re-blocked for 1 hr with 5% milk in TBST, and incubated with polyclonal anti-KCC2 antibody (cat #07–432. 1:1000; Chemicon, Temecula, CA). In some cases, membranes were probed once with either anti-NKCC1 or anti-KCC2 antibodies. Following each primary antibody incubation, membranes were incubated with anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibodies (1:1000 in TBS; Thermoscientific). Enhanced chemiluminescence was used for band detection (Thermoscientific). X-ray films were scanned and bands quantified using Counter One computer program (BioRad). Membranes were then incubated for 1 min in 0.025% (w/v) Coomassie Blue R-250, 40% methanol, and 7% acetic acid and washed overnight with a de-staining solution containing 50% methanol and 10% acetic acid. Membranes were re-scanned and re-quantified; a 50 kDa band from the Commassie stain was used to control for differences in lane loading. Pixel intensities for both KCC2 and NKCC1 were then normalized to the pixel intensity of the 50 kDa Coomassie band within each respective lane.
Electrophysiological Recordings
Slices were maintained for up to 7 hr at room temperature in ACSF prior to electrophysiological experiments. During experiments, slices were transferred to a chamber (RC-27LD with slice support; Warner Instruments, Hamden, CT) perfused with ACSF at a rate of ~4.5 mL/min and maintained at 30–32°C. CA3 hippocampal pyramidal neurons were visualized using infrared-differential interference contrast microscopy and their electrophysiological activity assayed using the whole-cell patch-clamp configuration at a holding potential of −70 mV. Recordings were obtained with an Axopatch 200B amplifier connected to a Digidata 1440A, and data were acquired using pClamp10 (Molecular Devices, Sunnyvale, CA). When indicated, 3 mM of kynurenic acid (KYNA) and 0.5 μM of tetrodotoxin (TTX) were added to the ACSF. We used patch pipettes with resistances of 3 to 6 MΩ filled with an internal solution containing 140 mM CsCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM EGTA, 10 mM HEPES (pH 7.3), 4 mM Na2ATP, and 2 mM N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide (QX-314). Access resistance was less than 32 MΩ; if access resistance changed more than 25%, the recording was discarded. Data were analyzed using pClamp10 (Molecular Devices) or Mini Analysis Program (Synaptosoft, Decatur, GA). Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). A p ≤ 0.05 was considered to be statistically significant. Data are presented as mean ± SEM.
Results
Rat dams and pups were exposed to air (control) or ethanol via an inhalation paradigm to model exposure during the 3rd trimester-equivalent period of human pregnancy. Ethanol vapor levels were maintained between 3–4 g/dL, which yielded an overall SEC of 53 ±4.0 mM (0.24 ±0.018 g/dL; n = 33; Fig 1A). Maternal SECs are expected to be near 13 mM (Valenzuela et al., unpublished observation). This exposure paradigm significantly affected rat pup weight gain on P15 and 16 (p<0.05 and 0.01, respectively, by a repeated measures two-way ANOVA followed by Bonferroni’s posthoc test; Fig 1B).
Effect of ethanol on KCC2 and NKCC1 expression
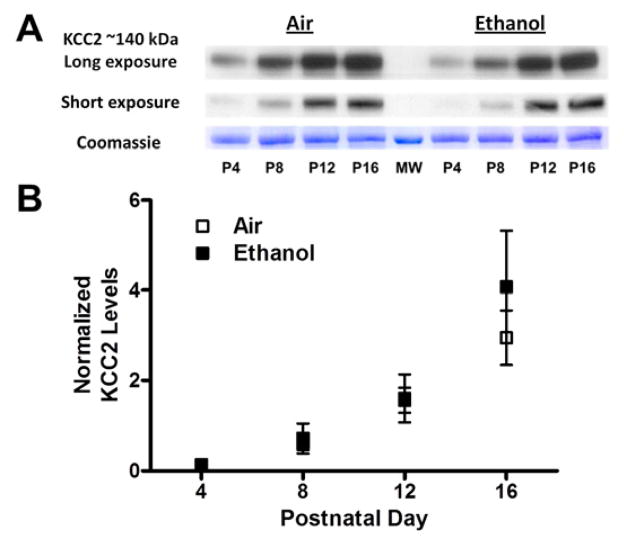
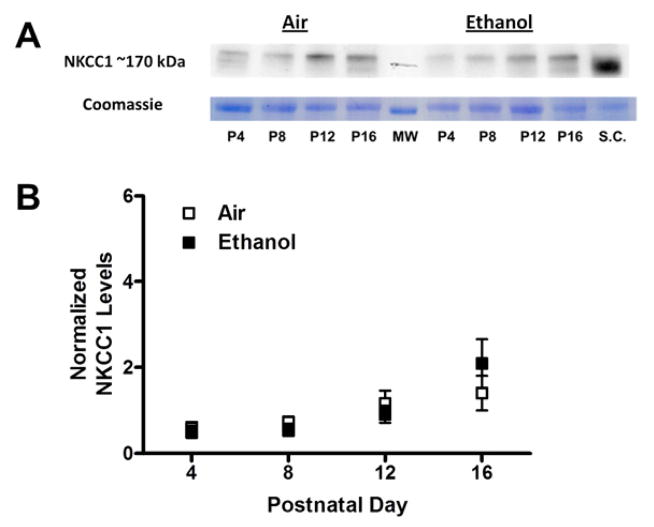
As discussed above, GDPs are, in part, driven by the excitatory actions of the Cl− permeable GABAA receptors as a consequence of high [Cl−]i resulting from the predominant expression of the Cl− importer NKCC1 and the low expression levels of the Cl− exporter KCC2 in immature neurons. We first measured the effect of repeated 3rd trimester-equivalent ethanol exposure on KCC2 expression in the CA3 region using western immunoblotting. Fig 2 shows that in both air and ethanol exposed pups, KCC2 expression gradually increased as a function of age; KCC2 expression was significantly higher at P12 and P16 vs. P4; P16 was also significantly greater than P8 (p<0.05 by repeated measures ANOVA followed by Bonferroni’s posthoc test). We also measured expression of the Cl− importer, NKCC1 (Fig 3). In air and ethanol exposed pups, the expression of NKCC1 was relatively constant between P4–P12 but was significantly higher at P16 than at P4 (p<0.05 by repeated measures ANOVA followed by Bonferroni’s posthoc test). The age-dependent increase in KCC2 levels was greater than that of NKCC1 levels (Figs 2–3). Ethanol did not significantly affect expression of KCC2 or NKCC1 at any of the developmental time points tested (Figs 2–3).
Figure 2. Chronic ethanol exposure did not affect KCC2 levels in the CA3 hippocampal region.
(A) Sample western immunoblots for the air and ethanol groups at the indicated postnatal days (P). Signals obtained with short (3 s) and long (10 s) X-ray film exposures are shown for comparison. At the bottom is the Coomassie blue stained 50 kDa control band. Also shown is a 50 kDa molecular weight marker (MW). (B) Expression of KCC2 (normalized to that of the Coomassie 50 kDa band) was not significantly different between the treatment groups. Experiments were performed on 6 different litters per treatment group.
Figure 3. Chronic ethanol exposure did not affect NKCC1 levels in the CA3 hippocampal region.
(A) Sample western immunoblots for the air and ethanol groups at the indicated postnatal days (P). A sample from spinal cord (S.C.) from a P14 male rat, where NKCC1 levels are high, was loaded as a reference. At the bottom is the Coomassie blue stained 50 kDa control band. Also shown is a 50 kDa molecular weight marker (MW). (B) Expression of NKCC1 (normalized to that of the Coomassie 50 kDa band) was not significantly different between the treatment groups. Experiments were performed on 6 different litters per treatment group.
Effect of ethanol on GDPs and GABAA-mPSCs
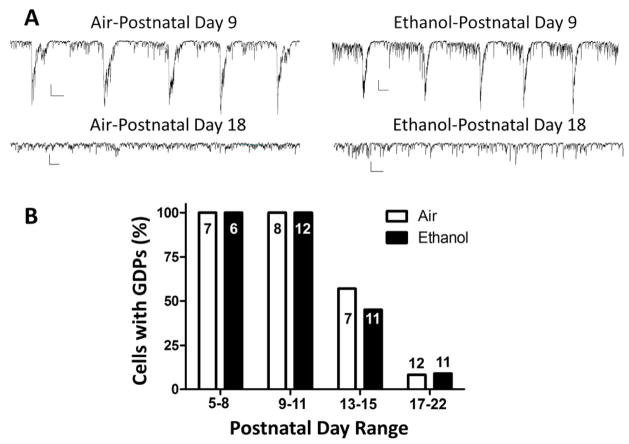
To characterize the functional effects of repeated ethanol exposure during the 3rd trimester-equivalent, we performed whole-cell patch-clamp recordings from CA3 pyramidal neurons. Under our recording conditions, GDPs were visualized as large spontaneous inward currents (Galindo et al., 2005) (Fig 4A). Under basal conditions, GDPs can be absent or occur at a low frequency in a significant number of CA3 pyramidal neurons (Mohajerani and Cherubini, 2006). We therefore used ACSF containing a 7 mM concentration of KCl to increase the frequency of events. Under this condition, 100% of CA3 pyramidal neurons tested at P5–11 exhibited GDPs (Fig 4B). The percent of neurons with GDPs decreased to about 50% at P13–15 and to less than 10% at P17–22 (Fig 4B). Ethanol exposure did not significantly affect the percent of cells with GDPs at any of the developmental time points (p>0.05 by Fisher’s exact test).
Figure 4. Ethanol exposure did not affect the age-dependent decrease of GDP occurrence in CA3 pyramidal neurons.
A) Sample traces illustrating GDPs recorded from P9 slices for the air or ethanol groups. Also shown are traces illustrating the lack of GDPs in slices from P18 rats. Scale bars: 200 pA and 0.5 s. B) Percent of cells with GDPs at the indicated postnatal day ranges for the air and ethanol groups. Numbers of recorded cells are given inside or above the bars. Experiments were performed on 6 different litters per treatment group.
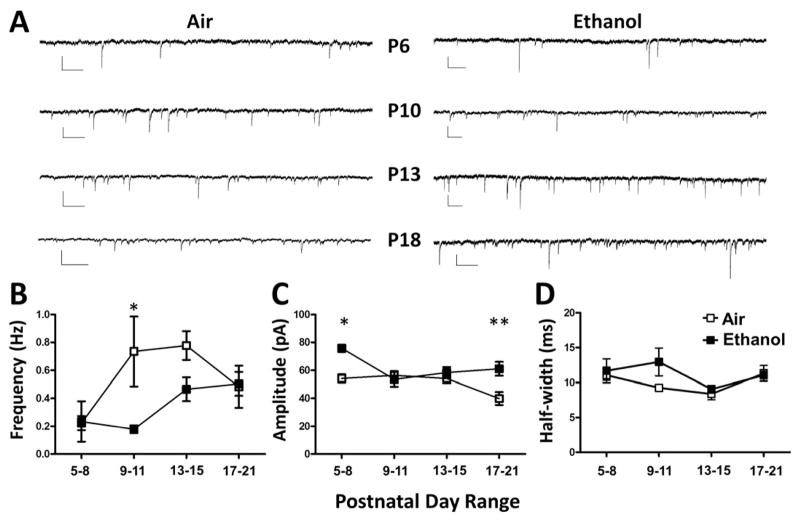
We next examined the effect of repeated vapor exposure on GABAergic synaptic transmission in CA3 pyramidal neurons. Activation of ionotropic glutamate receptors is required for GDP generation (Ben-Ari et al., 1989; Bolea et al., 1999; Sipila et al., 2005; Sipila et al., 2006); therefore, we used the antagonist of ionotropic glutamate receptors, KYNA, to block GDPs and isolate GABAA receptor-mediated synaptic activity. TTX was added to isolate GABAA-mPSCs. GABAA-mPSC frequency was significantly lower in the ethanol vs. air groups at P9–11 (Fig 5A–B). At P5–8 and P17–21, ethanol exposure significantly increased GABAA-mPSC amplitude (Fig 5A–C). GABAA-mPSC half-width was not significantly affected by ethanol exposure (Fig 5D).
Figure 5. Ethanol exposure altered GABAA-mPSC frequency and amplitude in developing CA3 pyramidal neurons.
A) Sample traces of GABAA-mPSCs recorded at the indicated postnatal day ranges. Scale bars: 50 pA and 0.5 s. B) Ethanol exposure significantly reduced GABAA-mPSC frequency at P9–11 (*p<0.05 by two-way ANOVA followed by Bonferroni’s posthoc test). C) Ethanol exposure significantly increased GABAA-mPSC amplitude at P5–8 and P17–21 (*p<0.05, **p<0.01 by two-way ANOVA followed by Bonferroni’s posthoc test). D) Ethanol exposure did not significantly affect GABAA-mPSC half-width. Control: P5–8 (n=4); P9–11 (n=5); P13–15 (n=7); and P17–21 (n=6). Ethanol: P5–8 (n=3); P9–11 (n=4); P13–15 (n=7); and P17–21 (n=6). Experiments were performed on 3 different litters per treatment group.
Discussion
We found that exposure to ethanol during the 3rd trimester-equivalent period produces the following effects in the CA3 region of the rat hippocampus: 1) it does not affect expression of KCC2 and NKCC1, 2) it does not impair the gradual disappearance of GDPs during the first 2–3 weeks of life, and 3) it delays the developmental increase in GABAA-mPSC frequency and increases the amplitude of these events at P5–8 and P17–21. These findings suggest that 3rd trimester-equivalent ethanol exposure affects GABAergic transmission, but not network activity or Cl− co-transporter expression in this hippocampal region.
In agreement with the literature (Ben-Ari et al., 1989; Khazipov et al., 2004), we observed that GDPs gradually decrease with age in CA3 pyramidal neurons, becoming very infrequent in neurons from pups older than P17, a period that coincides with the switch in GABAA receptor function from excitatory to inhibitory (Khazipov et al., 2004; Romo-Parra et al., 2008). Increased expression and oligomerization of KCC2, leading to decreased [Cl−]i and a negative shift in ECl−, has been linked to the switch in GABAA receptor function to predominantly inhibitory (Reviewed in Blaesse et al., 2009). The mechanism responsible for the increased expression and function of KCC2 is unclear, with reports implicating: GABA release (Ganguly et al., 2001; Leitch et al., 2005 but see Ludwig et al., 2003; Titz et al., 2003), BDNF/trkB/ERK1/2-induced expression of early growth response 4 (Egr4) (Aguado et al., 2003; Carmona et al., 2006; Ludwig et al., 2011; Shulga et al., 2008; Wake et al., 2007), and de-repression of KCC2 transcription in a RE1-silencing transcription factor-dependent manner (Yeo et al., 2009). We found that ethanol did not affect the ontogenetic decrease in GDPs that occurs in parallel with the increase in KCC2 expression, suggesting that the above-mentioned mechanisms responsible for KCC2 up-regulation during the 3rd trimester-equivalent are insensitive to ethanol. Thus, the ethanol exposure-induced inhibition of BDNF release, which our lab recently characterized in CA3 pyramidal neurons from neonatal rats, does not appear to have an impact on KCC2 expression (Zucca and Valenzuela, 2010). A possible explanation is that dendritic BDNF release from CA3 pyramidal neurons predominantly acts in a paracrine fashion, retrogradely regulating afferent axonal terminals, rather than acting in an autocrine fashion to modulate gene expression in these neurons (Gubellini et al., 2005; Mohajerani and Cherubini, 2006).
NKCC1 expression has also been shown to promote GDP generation in the CA3 hippocampal region. Blockade of this transporter with bumetanide abolished the depolarizing actions of GABA and GDP activity (Sipila et al., 2006; Sipila et al., 2009). In neonatal rats, global expression levels of NKCC1 are typically higher than those of KCC2, contributing to the generation of high [Cl−]i in neurons (Dzhala et al., 2005). In agreement with previous hippocampal studies (Bray and Mynlieff, 2009; Yan et al., 2001), we found that NKCC1 protein expression increases by P16, although to a lesser extent than KCC2. Repeated ethanol exposure during the neonatal period did not affect NKCC1 expression. It should be noted that NKCC1 has been shown to redistribute from soma to dendrite during the 2nd and 3rd postnatal weeks, leading to GABAA receptor-mediated depolarizing responses in dendrites of CA3 pyramidal neurons (Marty et al., 2002; Romo-Parra et al., 2008). It is possible that neonatal ethanol exposure affects this NKCC1 dendritic relocation process, leading to alterations in dendritic development. In addition, while KCC2 is neuron specific, NKCC1 is also associated with glial cells (Munoz et al., 2007). Therefore, whether ethanol selectively affects NKCC1 expressed in neurons vs. glial cells should be investigated.
Consistent with the lack of an effect of repeated neonatal ethanol exposure on KCC2 and NKCC1 expression, we found that ethanol exposure did not affect the normal disappearance of GDPs in CA3 pyramidal neurons (Ben-Ari et al., 1989; Khazipov et al., 2004). These results confirm our previous finding that single or repeated exposures to ethanol vapor during P4–6 do not significantly affect basal GDP frequency (Galindo and Valenzuela, 2006). Short-term (7 min or 3–4 hr) exposure of hippocampal slices to ethanol did increase GDP frequency and this effect persisted after ethanol vapor exposure (Galindo and Valenzuela, 2006; Galindo et al., 2005). The findings of these studies, taken together with the results presented here, suggest that GDP frequency is only affected while ethanol is present and sensitivity to this effect of ethanol is retained after repeated ethanol exposure. However, basal GDP frequency is not permanently altered nor is the normal developmental time course of GDP disappearance.
Although ethanol did not affect network activity or Cl− transporter expression in the CA3 hippocampal region, it altered action potential-independent GABAergic transmission in these cells. In slices from control rats, we found that GABAA-mPSC frequency increased with age in CA3 pyramidal neurons, in general agreement with the literature (Gubellini et al., 2001; Isaeva et al., 2006). Ethanol significantly delayed the increase in GABAA-mPSC frequency, which could be a consequence of a transient decrease in the probability of GABA release and/or the number of active GABAergic synapses. A potential link between the decrease in GABAA-mPSC frequency and the ethanol-induced impairment of retrograde release of BDNF from CA3 pyramidal neurons should be investigated (Zucca and Valenzuela, 2010). Our findings are consistent with those of previous studies demonstrating that developmental up-regulation of GABAA receptors in both in vivo and in vitro models of 3rd trimester-equivalent ethanol exposure is blunted in medial septum/diagonal band and cerebellar neurons (DuBois et al., 2004; DuBois et al., 2006; Hsiao et al., 2001; Hsiao et al., 1999). Regarding GABAA-mPSC amplitude and half-width, these parameters did not significantly change as a function of age, suggesting that quantal content and/or receptor subunit composition remains relatively stable between P5 and P22. Ethanol exposure produced a significant increase in GABAA-mPSC amplitude at P5–8 and P17–21, indicating that it age-dependently increases receptor function, receptor density and/or GABA vesicular content. The data could also be consistent with the possibility that ethanol exposure potentiates GABAA-mPSC amplitude at P5–8, with tolerance developing at P9–15 and reappearance of the effect after the end of ethanol exposure (i.e., >P16). The link between the changes in GABAA-mPSC amplitude and the decrease in BDNF retrograde release that we previously reported is an important topic for future studies (Zucca and Valenzuela, 2010).
A limitation that must be kept in mind is that the GABAA-mPSC recordings were performed with slices prepared soon after the end of the 4 hr ethanol exposure paradigm at the developmental time points indicated in Fig 5. Future studies should examine whether changes in GABAA-mPSC frequency can also be detected in slices prepared at later time points after the end of ethanol vapor exposure (e.g., the day after). These studies will provide a more complete picture of the relationship between the timing of ethanol exposure and the duration of alterations in GABAergic transmission. These studies are currently ongoing in our laboratory.
In conclusion, the studies presented here suggest that 3rd trimester-equivalent ethanol exposure transiently alters spontaneous GABAergic synaptic transmission in the hippocampal CA3 region. Based on the literature (Ganguly et al., 2001; Leitch et al., 2005), this effect would have been expected to decrease expression of the Cl− co-transporters and delay GDP disappearance. Our finding that ethanol exposure does not affect these processes lends support to studies suggesting that spontaneous GABAergic transmission itself is not required for the increase in KCC2 expression and GDP disappearance (Ludwig et al., 2003; Titz et al., 2003). Ongoing studies in our laboratory are investigating whether the ethanol-induced decreases in spontaneous GABAergic activity lead to abnormalities in neuronal circuitry development in the CA3 and perhaps other regions of the CNS.
Acknowledgments
This work was supported by NIH grant RO1-AA15614. We thank Drs. Partridge, Diaz, Morton, and Zamudio-Bulcock for critically reading the manuscript and Aya Wadleigh for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RA, Galindo R, Mameli M, Gonzalez-Vega R, Valenzuela CF, Baram TZ. Synchronized network activity in developing rat hippocampus involves regional hyperpolarization-activated cyclic nucleotide-gated (HCN) channel function. Eur J Neurosci. 2005;22:2669–2674. doi: 10.1111/j.1460-9568.2005.04407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bolea S, Avignone E, Berretta N, Sanchez-Andres JV, Cherubini E. Glutamate controls the induction of GABA-mediated giant depolarizing potentials through AMPA receptors in neonatal rat hippocampal slices. J Neurophysiol. 1999;81:2095–2102. doi: 10.1152/jn.1999.81.5.2095. [DOI] [PubMed] [Google Scholar]
- Bray JG, Mynlieff M. Influx of calcium through L-type calcium channels in early postnatal regulation of chloride transporters in the rat hippocampus. Dev Neurobiol. 2009;69:885–896. doi: 10.1002/dneu.20749. erratum 897–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona MA, Pozas E, Martinez A, Espinosa-Parrilla JF, Soriano E, Aguado F. Age-dependent spontaneous hyperexcitability and impairment of GABAergic function in the hippocampus of mice lacking trkB. Cereb Cortex. 2006;16:47–63. doi: 10.1093/cercor/bhi083. [DOI] [PubMed] [Google Scholar]
- DuBois DW, Parrish AR, Trzeciakowski JP, Frye GD. Binge ethanol exposure delays development of GABAergic miniature postsynaptic currents in septal neurons. Brain Res Dev Brain Res. 2004;152:199–212. doi: 10.1016/j.devbrainres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- DuBois DW, Trzeciakowski JP, Parrish AR, Frye GD. GABAergic miniature postsynaptic currents in septal neurons show differential allosteric sensitivity after binge-like ethanol exposure. Brain Res. 2006;1089:101–115. doi: 10.1016/j.brainres.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Farr KL, Montano CY, Paxton LL, Savage DD. Prenatal ethanol exposure decreases hippocampal 3H-vinylidene kainic acid binding in 45-day-old rats. Neurotoxicol Teratol. 1988;10:563–568. doi: 10.1016/0892-0362(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Galindo R, Valenzuela CF. Immature hippocampal neuronal networks do not develop tolerance to the excitatory actions of ethanol. Alcohol. 2006;40:111–118. doi: 10.1016/j.alcohol.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo R, Zamudio PA, Valenzuela CF. Alcohol is a potent stimulant of immature neuronal networks: implications for fetal alcohol spectrum disorder. J Neurochem. 2005;94:1500–1511. doi: 10.1111/j.1471-4159.2005.03294.x. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Ben-Ari Y, Gaiarsa JL. Activity- and age-dependent GABAergic synaptic plasticity in the developing rat hippocampus. Eur J Neurosci. 2001;14:1937–1946. doi: 10.1046/j.0953-816x.2001.01823.x. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Ben-Ari Y, Gaiarsa JL. Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. J Neurosci. 2005;25:5796–5802. doi: 10.1523/JNEUROSCI.0824-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao SH, Acevedo JL, DuBois DW, Smith KR, West JR, Frye GD. Early postnatal ethanol intubation blunts GABA(A) receptor up-regulation and modifies 3alpha-hydroxy-5alpha-pregnan-20-one sensitivity in rat MS/DB neurons. Brain Res Dev Brain Res. 2001;130:25–40. doi: 10.1016/s0165-3806(01)00194-8. [DOI] [PubMed] [Google Scholar]
- Hsiao SH, West JR, Mahoney JC, Frye GD. Postnatal ethanol exposure blunts upregulation of GABAA receptor currents in Purkinje neurons. Brain Res. 1999;832:124–135. doi: 10.1016/s0006-8993(99)01480-8. [DOI] [PubMed] [Google Scholar]
- Isaeva E, Isaev D, Khazipov R, Holmes GL. Selective impairment of GABAergic synaptic transmission in the flurothyl model of neonatal seizures. Eur J Neurosci. 2006;23:1559–1566. doi: 10.1111/j.1460-9568.2006.04693.x. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Leitch E, Coaker J, Young C, Mehta V, Sernagor E. GABA type-A activity controls its own developmental polarity switch in the maturing retina. J Neurosci. 2005;25:4801–4805. doi: 10.1523/JNEUROSCI.0172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Li H, Saarma M, Kaila K, Rivera C. Developmental up-regulation of KCC2 in the absence of GABAergic and glutamatergic transmission. Eur J Neurosci. 2003;18:3199–3206. doi: 10.1111/j.1460-9568.2003.03069.x. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Uvarov P, Soni S, Thomas-Crusells J, Airaksinen MS, Rivera C. Early growth response 4 mediates BDNF induction of potassium chloride cotransporter 2 transcription. J Neurosci. 2011;31:644–649. doi: 10.1523/JNEUROSCI.2006-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Carta M, Partridge LD, Valenzuela CF. Neurosteroid-induced plasticity of immature synapses via retrograde modulation of presynaptic NMDA receptors. J Neurosci. 2005;25:2285–2294. doi: 10.1523/JNEUROSCI.3877-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, Wehrle R, Alvarez-Leefmans FJ, Gasnier B, Sotelo C. Postnatal maturation of Na+, K+, 2Cl− cotransporter expression and inhibitory synaptogenesis in the rat hippocampus: an immunocytochemical analysis. Eur J Neurosci. 2002;15:233–245. doi: 10.1046/j.0953-816x.2001.01854.x. [DOI] [PubMed] [Google Scholar]
- Mohajerani MH, Cherubini E. Role of giant depolarizing potentials in shaping synaptic currents in the developing hippocampus. Crit Rev Neurobiol. 2006;18:13–23. doi: 10.1615/critrevneurobiol.v18.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Moody WJ, Bosma MM. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol Rev. 2005;85:883–941. doi: 10.1152/physrev.00017.2004. [DOI] [PubMed] [Google Scholar]
- Munoz A, Mendez P, DeFelipe J, Alvarez-Leefmans FJ. Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia. 2007;48:663–673. doi: 10.1111/j.1528-1167.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. AMPAR-mediated synaptic transmission in the CA1 hippocampal region of neonatal rats: unexpected resistance to repeated ethanol exposure. Alcohol. 2009;43:619–625. doi: 10.1016/j.alcohol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Repeated third trimester-equivalent ethanol exposure inhibits long-term potentiation in the hippocampal CA1 region of neonatal rats. Alcohol. 2010;44:283–290. doi: 10.1016/j.alcohol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo-Parra H, Trevino M, Heinemann U, Gutierrez R. GABA actions in hippocampal area CA3 during postnatal development: differential shift from depolarizing to hyperpolarizing in somatic and dendritic compartments. J Neurophysiol. 2008;99:1523–1534. doi: 10.1152/jn.01074.2007. [DOI] [PubMed] [Google Scholar]
- Sakata-Haga H, Sawada K, Ohta K, Cui C, Hisano S, Fukui Y. Adverse effects of maternal ethanol consumption on development of dorsal hippocampus in rat offspring. Acta Neuropathol. 2003;105:30–36. doi: 10.1007/s00401-002-0606-9. [DOI] [PubMed] [Google Scholar]
- Shulga A, Thomas-Crusells J, Sigl T, Blaesse A, Mestres P, Meyer M, Yan Q, Kaila K, Saarma M, Rivera C, et al. Posttraumatic GABA(A)-mediated [Ca2+]i increase is essential for the induction of brain-derived neurotrophic factor-dependent survival of mature central neurons. J Neurosci. 2008;28:6996–7005. doi: 10.1523/JNEUROSCI.5268-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci. 2005;25:5280–5289. doi: 10.1523/JNEUROSCI.0378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Voipio J, Kaila K. Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarizing potentials are driven by a persistent Na+ current and terminated by a slow Ca2+-activated K+ current. Eur J Neurosci. 2006;23:2330–2338. doi: 10.1111/j.1460-9568.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Yamada J, Afzalov R, Voipio J, Blaesse P, Kaila K. Compensatory enhancement of intrinsic spiking upon NKCC1 disruption in neonatal hippocampus. J Neurosci. 2009;29:6982–6988. doi: 10.1523/JNEUROSCI.0443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. Coincidence detection enhances appropriate wiring of the nervous system. Proc Natl Acad Sci USA. 2004;101:5311–5312. doi: 10.1073/pnas.0401270101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Farr KL, Wilson WA, Savage DD. Prenatal exposure to ethanol decreases physiological plasticity in the hippocampus of the adult rat. Alcohol. 1988;5:121–124. doi: 10.1016/0741-8329(88)90008-0. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Nasu F, Inomata K. Fetal alcohol effects: decreased synaptic formations in the field CA3 of fetal hippocampus. Int J Dev Neurosci. 1991;9:509–517. doi: 10.1016/0736-5748(91)90037-m. [DOI] [PubMed] [Google Scholar]
- Titz S, Hans M, Kelsch W, Lewen A, Swandulla D, Misgeld U. Hyperpolarizing inhibition develops without trophic support by GABA in cultured rat midbrain neurons. J Physiol. 2003;550:719–730. doi: 10.1113/jphysiol.2003.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Watanabe M, Moorhouse AJ, Kanematsu T, Horibe S, Matsukawa N, Asai K, Ojika K, Hirata M, Nabekura J. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J Neurosci. 2007;27:1642–1650. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Hamre KM. Effects of alcohol exposure during different periods of development: changes in hippocampal mossy fibers. Brain Res. 1985;349:280–284. doi: 10.1016/0165-3806(85)90155-5. [DOI] [PubMed] [Google Scholar]
- West JR, Hodges CA, Black AC., Jr Prenatal exposure to ethanol alters the organization of hippocampal mossy fibers in rats. Science. 1981;211:957–959. doi: 10.1126/science.7466371. [DOI] [PubMed] [Google Scholar]
- Williams JR, Sharp JW, Kumari VG, Wilson M, Payne JA. The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J Biol Chem. 1999;274:12656–12664. doi: 10.1074/jbc.274.18.12656. [DOI] [PubMed] [Google Scholar]
- Yan Y, Dempsey RJ, Sun D. Expression of Na(+)-K(+)-Cl(−) cotransporter in rat brain during development and its localization in mature astrocytes. Brain Res. 2001;911:43–55. doi: 10.1016/s0006-8993(01)02649-x. [DOI] [PubMed] [Google Scholar]
- Yeo M, Berglund K, Augustine G, Liedtke W. Novel repression of Kcc2 transcription by REST-RE-1 controls developmental switch in neuronal chloride. J Neurosci. 2009;29:14652–14662. doi: 10.1523/JNEUROSCI.2934-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca S, Valenzuela CF. Low concentrations of alcohol inhibit BDNF-dependent GABAergic plasticity via L-type Ca2+ channel inhibition in developing CA3 hippocampal pyramidal neurons. J Neurosci. 2010;30:6776–6781. doi: 10.1523/JNEUROSCI.5405-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]