Abstract
Objective
To identify the effect of controlled metabolic diet on reducing urinary calcium oxalate supersaturation in subjects with hyperoxaluric nephrolithiasis after potentially malabsorptive forms of bariatric surgery.
Materials and Methods
Subjects with a history of CaOx kidney stones and mild hyperoxaluria after bariatric surgery (n=9) collected baseline 24-hour urine samples while on a free choice diet. They were then placed on a controlled diet low in oxalate (70 – 80 mg/day), normal in calcium (1000 mg/day), and moderate in protein prior to 2 final 24-hour urine collections.
Results
Overall urinary CaOx supersaturation fell from 1.97 ± 0.49 delta Gibbs (DG) on the free choice diet to 1.13 ± 0.75 DG on the controlled diet (P<0.01). This happened in the absence of a significant change in urinary oxalate excretion, (0.69 ± 0.29 mmol/day) on the free choice diet compared to (0.66 ± 0.38 mmol/day) on the controlled diet. Urinary volume, citrate and pH all increased, although not significantly (p>0.05), contributing to the significant CaOx supersaturation change.
Conclusions
A controlled metabolic diet normal in calcium, moderate in protein and reduced in oxalate can positively impact urinary CaOx supersaturation after bariatric surgery. However, this diet did not appear to decrease urinary oxalate excretion. Therefore, restriction of dietary oxalate alone may not be enough to reduce urinary oxalate excretion to normal levels in this group of known enteric hyperoxaluric patients. Additional strategies may be necessary, such as use of oral calcium supplements as oxalate binders and a lower fat diet.
Keywords: Bariatric surgery, calcium oxalate, diet therapy, enteric hyperoxaluria, nephrolithiasis, urolithiasis
INTRODUCTION
As much as 20% of the United States population is currently classified as obese (body mass index (BMI) > 30 kg/m2), including 11.5 million who are morbidly obese (BMI >40 kg/m2) 1. Furthermore, approximately 5 million Americans are deemed to have medically-complicated obesity, since they have weight-related co-morbidities such as diabetes mellitus, hypertension, or sleep apnea.
Studies suggest that bariatric surgery is an effective treatment for sustainable weight loss among the morbidly obese 2. Two separate studies demonstrated that bariatric surgery also imparts long-term survival benefits compared with conventional methods of weight loss 3, 4. Bariatric surgical procedures include jejunoileal bypass (JIB) which is no longer employed, vertical banded gastroplasty (VBG), laparoscopic adjustable gastric band (LAGB), biliopancreatic diversion with duodenal switch (BPDDS) and Roux-en-Y Gastric bypass (RYGB) 5. Of these, RYGB is currently the most commonly performed bariatric surgery in the United States 1.
Nevertheless, it is also recognized that bariatric surgery carries risk for short- and long-term complications. For example, it has recently observed that certain patients develop enteric hyperoxaluria and kidney stone disease after modern bariatric surgical procedures, even though many were stone-free pre-operatively 6, 7. The optimal treatment for this complication remains undefined, although a low-fat, low oxalate diet is often recommended 6. Therefore, in the current study urinary chemistries were evaluated in a group of patients with kidney stone disease after bariatric surgical procedures after they were placed on a controlled diet normal in calcium, moderate in protein and reduced in oxalate that was formulated to reduce kidney stone risk.
MATERIALS AND METHODS
This study was approved by the Mayo Clinic Institutional Review Board and registered with the United States National Library of Medicine (NCT 00597041; www.clinicaltrials.gov).
Study Population
Nine bariatric surgery patients evaluated in the Mayo Nephrology Clinic for kidney stone disease after their surgeries were recruited between Jan. 2007 and May 2009. Nephrolithiasis was diagnosed by the presence of radio-opaque stones on X-ray together with a history consistent with passage of a stone, or of stone surgery or extracorporeal shock wave lithotripsy. All stones analyzed were ≥ 50% CaOx. If patients were taking oral calcium, citrate or vitamin D at the time of recruitment of the study, the dosage of these medications were not changed. Therefore, during the diet phase of the study 8 patients were taking calcium citrate timed with meals (1438 ± 840 mg/day), 6 were taking potassium citrate (60 ± 19 mg/day) and 4 were taking vitamin D (50,000 U/week). None were currently taking any fiber supplements or probiotics.
Study Design
All patients were required to collect two 24-h urines as a baseline, obtained while on their currently prescribed medications and a free-choice diet. A subset completed a food frequency questionnaire (FFQ) to capture diet history prior to the study. This electronic computer-administered format was adapted from the Women's Health Initiative FFQ (Viocare Technologies, Princeton, NJ, USA), queries intake over the last three months and employs the Minnesota NDS nutritional analysis database for nutrient analyses. The Viocare FFQ was validated and compares well with nutrient intake validated against 4-day food records and four 24-hr dietary recalls 8. On day 0 each began a controlled metabolic diet which was calculated using ProNutra nutritional analysis software (Viocare Technologies Version 3.1.0.13., Copyright© 2002) and individualized to take allergies and intolerance into account. The daily diet contained reduced oxalate (70–80 mg), adequate protein (15–19% of total calories), low fat (<25% of total calories), moderate sodium (120–150mq), and the recommended daily amount of calcium (1000 mg) and vitamin C (100–150mg). Subjects were asked to continue their usual fluid intake but this was not monitored daily. Together with supplements, total calcium intake was 2458 ± 840 mg/day. Calorie intake was calculated to avoid weight loss using Harris-Benedict calculations plus an activity factor of 20 – 50% depending on normal daily activities and / or exercise. Subjects were instructed to avoid fiber supplements, probiotics and dietary yeast supplements during the controlled diet phase. Average fiber content was 14.5 g per day (range: 10 – 18 g). All meals were prepared in the Mayo Clinic Clinical Research Unit. While on the diet, patients came to the unit daily for breakfast and then took home her meals for the remainder of that day. After 4 days on the controlled metabolic diet, patients collected two more 24-h urines (days 5–6; post-diet urine collections) to evaluate the effect of dietary therapy. Previous studies suggest calcium and sodium balance is achieved within four days of a diet change 9.
Urine chemistries
Urinary concentrations (24 hr) of oxalate, calcium, citrate execration and determinants of supersaturation were measured in Mayo Clinic Renal Testing Laboratory. Urinary oxalate was measured by oxalate oxidase. supersaturation was calculated using the EQUIL2 program 10.
Statistical Analyses
Demographics and biochemical information were analyzed using the JMP 8.0 software package (SAS Institute, Cary, NC, USA). Differences among baseline, time zero and final urine values were evaluated by two-sided paired t-tests. All reported results are expressed as means ± standard deviation, and p-values < 0.05 was considered statistically significant.
RESULTS
Nine subjects (8 women, 1 man) were recruited from the Mayo Nephrology Clinic with a history of calcium oxalate (CaOx) nephrolithiasis after bariatric surgery. Procedures included Roux-en-Y gastric bypass (6), jejunoileal bypass (2), and biliopancreatic diversion- duodenal switch (1). Patient characteristics are listed in Table 1. A minority of patients (3/9) had a nephrolithiasis history before their bariatric procedure, with a mean of 1.4 ± 1.2 stone events. Subjects were 10.9 ± 12.6 years out from their surgery and had an average of 1.9 ± 1.7 stone events postoperatively (median 2.0).
Table 1.
Patient characteristics
| Patient Characteristic | Total |
|---|---|
| Number | 9 |
| Gender (male: female) | 1:8 |
| Age (mean ± s.d.) | 51 ± 9 years |
| Type of surgery | |
| JIB | 2 |
| BPD-DS | 1 |
| RYGB | 6 |
| Stone events (mean ± s.d.) | 1.89 ± 1.69 |
| Years since surgery | 10.9 ± 12.6 |
The controlled metabolic diet was well-tolerated with no complications. Table 2 lists the urinary composition prior to the study on a free choice diet (Pre) and after one week on the controlled metabolic diet (Post). On this controlled diet urinary potassium increased and urinary osmolality decreased significantly (Figure 1). Other urinary components also changed in a direction that would favor reduced urinary supersaturation, although not significantly, including increases in urinary citrate and volume. The net result was that even though urinary oxalate excretion did not fall, overall CaOx supersaturation did. When broken down by type of surgery, response to the diet did not appear to vary (not shown). Limited data from the 4 individuals that completed a food frequency questionnaire to capture diet habits prior to the study suggested that the free choice diet was similar in protein (both 101 g), calcium (1102 vs 1038 mg), carbohydrates (289 vs 321g), and vitamin C (95 vs 121 mg). The free choice diet trended slightly higher in fat (82 vs 64 g; p=0.35) and contained significantly more oxalate (180 vs 78 g; p< 0.05).
Table 2.
Summary of urinary values on free choice diet (Baseline) and a controlled metabolic diet.
| Urine parameter | Baseline | Controlled diet | P-value |
|---|---|---|---|
| Creatinine (mg) | 1097 (470) | 1233 (240) | 0.25 |
| Sodium (mEq) | 156 (63) | 134 (37) | 0.31 |
| Phosphorus (mg) | 740 (429) | 931 (220) | 0.19 |
| Potassium (mEq) | 55 (30) | 73 (24) | 0.016 |
| Uric Acid (mg) | 377 (155) | 467 (127) | 0.08 |
| Chloride (mEq) | 145 (64) | 123 (31) | 0.38 |
| Calcium (mg) | 112 (109) | 108 (80) | 0.86 |
| Magnesium (mg) | 141 (87) | 141 (51) | 0.99 |
| Oxalate (mmol/l) | 0.69 (0.29) | 0.66 (0.38) | 0.76 |
| Osmolality (mOsm/kg) | 481 (63) | 362 (92) | 0.0055 |
| pH | 5.8 (0.6) | 6.1 (0.6) | 0.17 |
| Citrate Excretion (mg) | 443 (447) | 511 (340) | 0.48 |
| Sulfate (mEq) | 16 (5) | 24 (8) | 0.0253 |
| Urine Volume (ml) | 2047 (1042) | 2431 (640) | 0.34 |
| CaOx SS (DG) | 1.97 (0.49) | 1.13 (0.75) | 0.0111 |
| BR SS (DG) | −1.37 (1.49) | −1.90 (1.18) | 0.1 |
| HA SS (DG) | 2.32 (2.63) | 1.92 (2.09) | 0.52 |
| UA SS (DG) | −0.35 (3.35) | −0.91 (2.80) | 0.51 |
| NaUr SS (DG) | 0.54 (0.91) | −0.06 (0.42) | 0.048 |
Values are given as mean (s.d.). Values in bold were significantly different on controlled diet (p<0.05). p-values are for a paired t-test comparing baseline to values on the controlled diet. BR: Brushite; CaOx: Calcium oxalate; DG: delta gibbs; HA: Hydroxyapatite; NaUr: Sodium Urate; SS: Supersaturation UA: Uric acid.
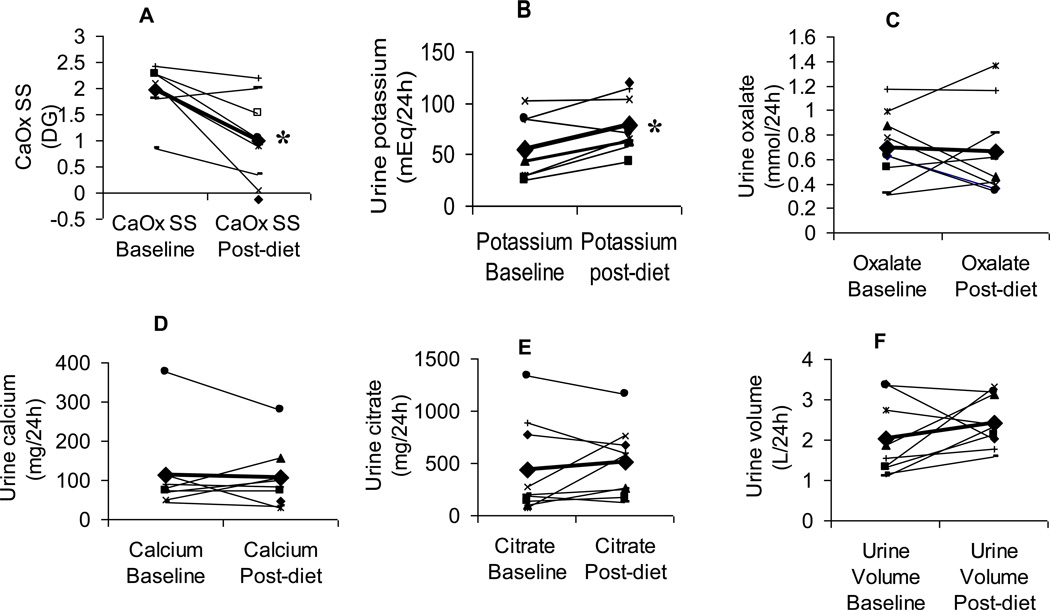
Figure 1. Changes in urinary chemistries on free-choice diet compared with a controlled metabolic low-oxalate diet.
CaOx SS (A) fell and urine potassium rose on the controlled metabolic diet for the majority of patients. Other key determinants of SS, including urinary oxalate (C), calcium (D), citrate excretion (E), and urine volume (F) did not change significantly. * denotes significance, p<0.05.
DISCUSSION
In this study, we observed that a diet reduced in fat and oxalate, normal in calcium, and moderate in sodium reduced overall urinary CaOx supersaturation in patients with a history of enteric hyperoxaluria and CaOx kidney stones after bariatric surgery. Urinary oxalate excretion did not fall significantly. Nevertheless it seems likely that this diet, or one similar to it, could reduce risk of recurrent stones, since in general the risk of CaOx nephrolithiasis parallels urinary supersaturation 11, although this hypothesis would need to be tested in a long-term study with kidney stone formation as an outcome.
Several recent reports describe an increasing number of persons with CaOx kidney stones after bariatric surgery 6, 12, 13. Risk factors include hyperoxaluria, low urinary volume, and low urinary citrate. Among currently performed procedures, hyperoxaluria has been documented after malabsorptive procedures like Roux-en-Y gastric bypass, but not after restrictive procedures such as gastric banding 14. Similar but perhaps more severe degrees of hyperoxaluria were seen in patients after jejunoileal bypass surgery for obesity, a more malabsorptive bariatric procedure no longer performed7. Factors that influenced the degree of urinary oxalate excretion in patients after jejunoileal bypass included the amount of fat, oxalate, and calcium in the diet 15. In addition oral calcium supplements, used as an oxalate binder, effectively reduced urinary oxalate levels among patients with fat malabsorption and hyperoxaluria, although typically doses in the range of 2000–3000 mg calcium were required 16.
In this study we demonstrate that a diet low in oxalate and fat, moderate in protein and normal in calcium does reduce overall urinary CaOx supersaturation in patients that have previously undergone modern bariatric surgical procedures. Urinary oxalate levels, however, did not fall. This is in contrast to similar studies we recently completed among idiopathic CaOx stone formers that confirmed a diet low in oxalate and normal in calcium and protein can reduce both urinary oxalate excretion and CaOx supersaturation 17. The decrease in supersaturation in the current study that included only post bariatric patients was due to favorable changes in multiple urinary components, including a slight increase of volume and citrate excretion. We presume that key overall differences in the composition of the controlled diet caused the positive urinary changes that included increased citrate, potassium and volume excretion. An increase in fruit and vegetable intake could cause these changes. However, the limited number of subjects that completed the pre-study FFQ did not permit this detailed analysis. The controlled diet contained on average 5 half-cup servings of fruit and 1 half-cup serving vegetables daily, which is higher than the typical US diet. In 1999–2000, only 40% of Americans ate an average of five or more ½-cup servings of fruits and vegetables per day 18.
It is notable that urinary oxalate levels remained high in the post bariatric surgery subjects on this diet which was fairly low in overall oxalate content (70–80 mg). Presumably, the percent absorption of oxalate was very high in these patients with fat malabsorption 19. We do not believe that noncompliance with the prescribed diet was a major issue, since subjects were given prepared meals, and other urinary components matched what would be predicted on this diet (e.g., urinary sodium). Furthermore, this diet was well below estimated baseline levels of oxalate (~180 mg), and reducing dietary oxalate even lower (less than 70–80 mg per day) is not likely to be practical over the long-term. Therefore, if hyperoxaluria itself, absent an increase in overall CaOx supersaturation, is a risk factor for kidney stones or renal damage, then other measures are required, since dietary restriction alone does not appear to suffice in this patient group. For example use of oral agents that bind or metabolize oxalate could be considered. It is also interesting that urine sulfate increased 50% on the controlled diet. Due to the high oxalate content of non-animal protein sources (nuts, soy, legumes), the controlled diet contained a high animal protein content. Therefore it was higher in animal protein than would normally be recommended. Total protein content was controlled to provide 15 – 20% of the calories, based on subjects personal preferences. This is not a high, but rather typical protein content for a typical American diet. On average, protein provided 17% of the calories for these subjects with a range of 15 – 19% protein calories. Overall 76% of total protein was from animal sources, including dairy products (range: 70 – 85%) with an average of 56 g meat and fish protein per day (range: 31 – 69 g protein), which is equivalent to 8 ounces. It is possible that protein intake was suboptimal in this post bariatric surgery on their free choice diet, and also that increased amounts of hydroxyproline were available on the experimental diet for conversion to oxalate 20. These unanswered questions deserve further study.
An important limitation of this study includes the lack of a parallel control group and a small sample size. With 9 subjects only changes equivalent to one standard deviation or more can be detected with 80% power.
CONCLUSIONS
In conclusion, this study suggests that a diet, normal in calcium and moderate in protein can improve urinary CaOx supersaturation in patients after bariatric surgery. However, such a balanced low oxalate diet did normalize urinary oxalate excretion by itself. Therefore, in this patient group with known fat malabsorption additional measures to address hyperoxaluria may be necessary, such as the use of oxalate binders.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health including AT R21AT2534, the Mayo Clinic O’Brien Urology Research Center P50 DK083007, the Rare Kidney Stone Consortium U54 DK082908, and the Mayo Clinic Center for Translational Science Activities. We are also thankful to Ellen Olson, Beth Bjornson, and Ruth Kraft for coordinator support during the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Demaria EJ, Jamal MK. Surgical options for obesity. Gastroenterol Clin North Am. 2005;34:127–142. doi: 10.1016/j.gtc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe BM, Morton JM. Weighing in on bariatric surgery: procedure use, readmission rates, and mortality. Jama. 2005;294:1960–1963. doi: 10.1001/jama.294.15.1960. [DOI] [PubMed] [Google Scholar]
- 3.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 4.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 5.Puzziferri N, Blankenship J, Wolfe BM. Surgical treatment of obesity. Endocrine. 2006;29:11–19. doi: 10.1385/ENDO:29:1:11. [DOI] [PubMed] [Google Scholar]
- 6.Sinha MK, Collazo-Clavell ML, Rule A, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100–107. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 7.Requarth JA, Burchard KW, Colacchio TA, et al. Long-term morbidity following jejunoileal bypass. The continuing potential need for surgical reversal. Arch Surg. 1995;130:318–325. doi: 10.1001/archsurg.1995.01430030088018. [DOI] [PubMed] [Google Scholar]
- 8.Patterson RE, Kristal AR, Tinker LF, et al. : Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann.Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 9.Brinkley L, Pak CY. Pak CY, Adams PM. Techniques of patient-oriented research. New York: Raven Press; 1994. The metabolic balance regimen and nutritional aspects of clinical research; pp. 143–148. [Google Scholar]
- 10.Werness PJ, Brown CM, Smith LH, et al. EQUIL2: a BASIC computer program for the calculation of urinary saturation. J.Urol. 1985;134:1242–1244. doi: 10.1016/s0022-5347(17)47703-2. [DOI] [PubMed] [Google Scholar]
- 11.Parks JH, Coward M, Coe FL. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int. 1997;51:894–900. doi: 10.1038/ki.1997.126. [DOI] [PubMed] [Google Scholar]
- 12.Duffey BG, Pedro RN, Makhlouf A, et al. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J Am Coll Surg. 2008;206:1145–1153. doi: 10.1016/j.jamcollsurg.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Duffey BG, Alanee S, Pedro RN, et al. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg. 2010;211:8–15. doi: 10.1016/j.jamcollsurg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Penniston KL, Kaplon DM, Gould JC, et al. Gastric band placement for obesity is not associated with increased urinayr risk for urolithiasis compared to bypass. J.Urol. 2009;182:2340–2346. doi: 10.1016/j.juro.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Stauffer JQ. Hyperoxaluria and intestinal disease. The role of steatorrhea and dietary calcium in regulating intestinal oxalate absorption. Am J Dig Dis. 1977;22:921–928. doi: 10.1007/BF01076170. [DOI] [PubMed] [Google Scholar]
- 16.Hylander E, Jarnum S, Nielsen K. Calcium treatment of enteric hyperoxaluria after jejunoileal bypass for morbid obesity. Scand J Gastroent. 1980;15:349–352. doi: 10.3109/00365528009181482. [DOI] [PubMed] [Google Scholar]
- 17.Lieske JC, Tremaine WJ, De Simone C, et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 2010;78:1178–1185. doi: 10.1038/ki.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guenther PM, Dodd KW, Reedy J, et al. Most Americans eat much less than recommended amounts of fruits and vegetables. J Am Diet Assoc. 2006;106:1371–1379. doi: 10.1016/j.jada.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Earnest DL, Johnson G, Williams HE, et al. Hyperoxaluria in patients with ileal resection: an abnormality in dietary oxalate absorption. Gastroenterology. 1974;66:1114–1122. [PubMed] [Google Scholar]
- 20.Jiang J, Johnson LC, Knight J, et al. Metabolism of 13c5-Hydroxyproline in Vitro and in Vivo: Implications for Primary Hyperoxaluria. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00331.2011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]