Abstract
Although neurocognitive impairment is relatively common among patients with advanced lung disease, little is known regarding changes in neurocognition following lung transplantation. We therefore administered ten tests of neurocognitive functioning before and 6-months following lung transplantation and sought to identify predictors of change. Among the 49 study participants, native diseases included COPD (n = 22), cystic fibrosis (n = 12), non-fibrotic diseases (n = 11) and other (n = 4). Although composite measures of executive function and verbal memory scores were generally within normal limits both before and after lung transplantation, verbal memory performance was slightly better posttransplant compared to baseline (p < .0001). Executive function scores improved in younger patients but worsened in older patients (p = .03). A minority subset of patients (29%) exhibited significant cognitive decline (i.e., > 1 standard deviations on at least 20% of tests) from baseline to post-transplant. Patients who declined were older (p < .004) and tended to be less educated (p = .07). Lung transplantation, like cardiac revascularization procedures, appears to be associated with cognitive decline in a subset of older patients, which could impact daily functioning post-transplant.
Keywords: Neurocognition, Neuropsychological Testing, Lung Transplantation, Outcomes
For patients with end stage lung diseases and limited life expectancy, lung transplantation is an established treatment option that offers the chance of increased life expectancy and improved quality of life1. In the 10-year period between 2000 and 2010, the number of lung transplants performed in the United States grew by nearly 85%, and among recipients age 65 years and older, it increased from 30 to 148 - a 400% increase (based on OPTN data as of July 19, 2011).
Neurocognitive impairment is one of the consequences of end-stage lung disease 2. It may be attributable to factors such as decreased lung function, hypoxemia, hypercapnia, and/or inflammation 3. Deficits in neurocognitive functioning raise concerns about medication non-adherence 4, and independently have been associated with increased mortality 5. Cardiothoracic surgical interventions, such as coronary bypass surgery and heart transplantation, have been shown to be associated with increased neurocognitive impairment, particularly among older patients 6. However, to our knowledge, neurocognitive sequelae of lung transplantation have not been studied systematically. Therefore, the present study sought to determine whether there are objective changes in neurocognitive function following lung transplantation through prospective neurocognitive assessment and to examine predictors of neurocognitive change.
METHODS
Participants
Participants from the present study represent a subset of patients who participated in a randomized clinical trial known as the Investigational Study of Psychological Intervention in Recipients of Lung Transplant (INSPIRE) 7 study and subsequently underwent lung transplantation.
Procedures
Details of the patient population and assessment procedures have been published 2, 7. Briefly, participants were consenting adults who met the International Society for Heart and Lung Transplantation criteria for lung transplantation 8 and were on the lung transplant wait list. INSPIRE was a dual-center (Duke University and Washington University) RCT in which patients awaiting lung transplantation were randomized to either a coping skills training (CST) program or to an education control condition. The treatment program was delivered over the telephone. The primary results showed that the CST program improved quality of life, but it did not improve survival 7. Testing occurred between January 2001 and February 2006, and 48 of 49 transplants occurred prior to the implementation of the Lung Allocation Score system.
A neurocognitive substudy of INSPIRE was conducted only at the Duke site. Participants completed a battery of established neurocognitive tests assessing two domains of functioning: executive function and verbal memory, before and 6 months following lung transplantation. The tests were selected for their use in prior studies, availability of normative data, and measurement of different cognitive domains, especially verbal memory and executive function. All tests were individually administered by a trained psychometrician.
Neuropsychological function declines as part of the normal aging process, and it is important to avoid mischaracterizing the effects of normal aging as “cognitive impairment.” In order to address this issue demographically-adjusted neuropsychological test scores were used for all analyses of cognitive impairment. Specifically, raw test scores were standardized into t-scores (M = 50, SD = 10) using published normative data stratified by age, gender, and education. In addition, the t-scores were averaged together within cognitive domains to form composite measures of executive function and verbal memory. The grouping of subtests into neurocognitive domains were selected a priori and based upon prior research 2.
The executive function tests included:
The Stroop Color-Word Test 9 consists of three 100-item, timed trials. Participants first read from a list of color words as quickly as possible, and then identify colors from a list of colored stimuli as quickly as possible. The final trial uses an interference list consisting of color words, but the words themselves are colored in a different color ink than the color to which they refer. Participants are required to name the color of the word, but not the word itself. The Interference score is derived by comparing the predicted time to complete the final trial (based upon performance of the first two trials) to the actual time to complete the final trial. This is considered to be a measure of response inhibition and executive functioning.
The Animal Naming Task 10. This test requires participants to generate the names of as many animals as possible in 60 seconds. The score is determined by the number of animals named. This test measures semantic verbal fluency, and is considered an index of executive functioning.
The Controlled Oral Word Association (COWA) test 10 requires participants to generate as many different words as possible that begin with a particular letter, excluding proper nouns and suffix variations. Three letters are used, and there is a time limit of 60 seconds per letter. The score is determined by the total number of correct words across all three trials. Similar to the Animal Naming Task, the COWA measures associative verbal fluency and is considered an index of executive functioning.
The Ruff 2 and 7 Selective Attention Test 11 requires participants to visually search for and identify the digits 2 and 7, which are randomly embedded within 20 alternating sets of letter and digit distracters. The letter and digit trials are scored separately to reflect the greater cognitive burden of the digit trials. This test requires both sustained attention and executive functioning.
The Trail Making Test 12 consists of two parts, Parts A and B. The Trails A test requires participants to connect circles identified by the numbers 1 through 25 in order. The Trails B test requires participants to connect 25 circles identified by either a number or a letter, in alternating sequence (i.e., 1-A-2-B-3-C, and so on). Parts A and B are scored separately. The number of seconds to complete each part is the score, and lower scores are indicative of better performance. The test administrator points out and corrects errors in real time, effectively penalizing the score by prolonging the test. Trails A is considered a measure of sustained attention, whereas Trails B is sensitive to cognitive flexibility.
The verbal memory tests included:
Wechsler Memory Scale - Revised, Logical Memory I subtest 13 requires participants to repeat two paragraph-length stories immediately after they are read aloud by the examiner. Scores are determined by summing the number of story details correctly recalled. This subtest measures participant ability to accurately produce newly learned verbal information.
For the Wechsler Memory Scale - Revised, Verbal Paired Associates subtest 13, participants are verbally presented with a set of eight word pairs, half of which are semantically related (e.g., baby-cries) and half of which are unrelated (e.g., pen-grocery). Participants are then cued with one word from each pair and asked to produce the other word. The score is the number of correctly recalled word associations across the three trials.
Wechsler Adult Intelligence Scale – Revised, Digit Span subtest 14 requires participants to repeat progressively longer series of numbers immediately after they are read aloud by the examiner. In the Forward substest, participants repeat the numbers exactly as they are read. In the Backward subtest, participants repeat numbers in reverse sequence. Digit Span measures attention and working memory.
In addition, as part of the larger INSPIRE study, all participants in the neurocognitive substudy completed the Beck Depression Inventory 15 and the State-Trait Anxiety Inventory 16, both of which are well-established self-report measures of symptoms severity. Also, measures of pulmonary gas exchange (PCO2) and exercise tolerance (6-minute walk test) were recorded as part of the INSPIRE study and examined in the neurocognitive substudy. Baseline data were collected at the time of study enrollment. The average time between study enrollment and transplant was 39 weeks, with an interquartile range of 10 to 60 weeks.
Data analysis
Cross-sectional rates of neurocognitive impairment were examined by using demographically corrected t-scores to categorize participant performance on each test and at each time point as “not impaired” (i.e., for test scores > −1.00 standard deviations below normative data), “mildly impaired” (i.e., for test scores between −1.00 and −2.00 standard deviations below normative data), or “moderately-to-severely impaired” (i.e., for test scores below −2.00 standard deviations below normative data).
Changes in composite neurocognitive domain scores were examined with paired t-tests. The General Linear Model was used to examine the effects of age, education, and baseline PCO2 on post-transplant scores, controlling for pre-transplant scores. Exploratory analyses examined the contribution of diagnosis, 6-Minute walk test, depression, and anxiety.
Finally, post-transplant neurocognitive decline was examined by using an established standard by which participants are categorized as “impaired” if their performance on at least two neurocognitive tests declined between baseline and post-transplant testing by at least one standard deviation 17. For this final analysis, we examined raw test scores rather than t-scores.
RESULTS
Of 131 participants who completed neurocognitive assessments, 77 (59%) underwent lung transplantation, and 49 (64%) completed post-transplant neuropsychological testing. The 77 lung transplant recipients were similar to the 54 study participants who did not undergo lung transplantation during the course of this study in age, education, baseline medical variables (PCO2, 6-minute walk test), mood, anxiety, and diagnostic category, with all p’s > .10. Transplant recipients were more likely to be male (53% vs. 37%, p = .06), compared to participants who remained on the waitlist. Among the 77 transplant recipients, reasons for not completing posttransplant testing included death (n=7), recurrent hospitalization or medical illness (n=7), dropout/refusal (n = 5), and logistical barriers (e.g., patients who lived too far to return for the neurocognitive assessments; n = 8). The 49 participants who completed posttransplant testing were similar to the 28 participants who did not complete the testing in age, education, gender, baseline medical variables (PCO2, 6-minute walk test), mood, anxiety, and diagnostic category (all p’s > .10; see Table 1).
Table 1.
Baseline Data for INSPIRE Transplant Recipients: Comparing Posttransplant Testing Completers to Noncompleters
| Variable | Completers (n = 49) | Non-Completers (n = 28) | p |
|---|---|---|---|
| Age | 49.6 (12.9; 20–65) | 46.3 (11.9; 22–64) | .28 |
| Education in Years | 13.6 (2.7; 6–18) | 13.7 (2.5; 9–20) | .86 |
| Gender = Male, n (%) | 29 (59.2%) | 12 (42.3%) | .17 |
| PCO2 | 43.2 (6.5; 31–69) | 40.8 (5.7; 30–52) | .11 |
| 6-minute walk test (feet) | 1247.0 (372.9; 596–2100) | 1269.9 (470.6; 210–1985) | .82 |
| Beck Depression Inventory | 8.5 (6.5; 0–26) | 11.0 (6.9; 0–31) | .11 |
| State Trait Anxiety Inventory | 32.7 (8.9; 20–61) | 35.3 (10.5;21–56) | .25 |
| Lung Disease | |||
| COPD, n (%) | 22 (44.9%) | 8 (32.0%) | |
| CF, n (%) | 12 (24.5%) | 7 (28.0%) | |
| Other Fibrotic, n (%) | 11 (22.5%) | 6 (24.0%) | |
| Other, n (%) | 4 (8.2%) | 4 (16.0%) | .63 |
Notes: Data are provided as Mean (SD; Minimum-Maximum) or number (percentage). Among completers, the interquartile range for baseline PCO2 was 39–46.
Demographic data for the 49 participants are summarized in Table 1. Participants tended to be male (n = 29, 59%), middle-aged (age M = 49.6 yrs, SD = 12.9), Caucasian (n = 44, 90%), and college educated (M = 13.6 yrs, SD = 2.7). Native diseases included COPD (n = 22), cystic fibrosis (n = 12), non-cystic fibrotic diseases (n = 11) and other (n = 4). At the time of baseline testing, average time on the lung transplant waitlist was 51 weeks (SD = 76). Baseline testing occurred an average of 39 weeks before transplantation (SD = 42 weeks). The average time between baseline and 6-month posttransplant testing was 68 weeks (or 1.3 years; SD = 45 weeks). All participants received double lung transplants. One participant underwent CABG and required cardiopulmonary bypass. Of the 49 participants, 12 (25%) experienced rejection at 6 months posttransplant.
Clinical impairment ratings revealed that 82% of patients were classified as at least mildly impaired (i.e., score between 1 and 2 standard deviations below published normative values) on one or more of the neurocognitive measures at baseline, 39% of patients were classified as moderately-to-severely impaired (i.e., score > 2 standard deviations below published normative values) on at least one measure, and 16% were classified as moderately-to-severely impaired on at least two measures (Table 2). At six months posttransplant, 86% of patients were classified as at least mildly impaired on one or more measures, 22% of patients were classified as moderately-to-severely impaired on at least one measure, and 6% of patients evidenced moderate-to-severe impairment on 2 or more measures. Rates of impairment varied by test between 6% and 49% at baseline and between 8% and 37% at follow-up (see Table 2). COWAT, Animal Naming, and the Ruff 2 and 7 test were particularly sensitive to impairment in this setting, whereas Verbal Paired Associates was not.
Table 2.
Clinical Impairment Ratings for Individual Neuropsychological Tests (n = 49)
| Measure | Time Point | Not Impaired | Mildly Impaired | Moderately to Severely Impaired | Pre-to- Post Decline |
|---|---|---|---|---|---|
| Verbal Memory Tests | |||||
| Logical Memory | Pre-Txp | 73% | 27% | 8% | |
| Post-Txp | 82% | 18% | 0% | 4% | |
| Digit Span | Pre-Txp | 65% | 33% | 2% | |
| Post-Txp | 65% | 33% | 2% | 10% | |
| Verbal Paired Associates | Pre-Txp | 94% | 6% | 0% | |
| Post-Txp | 92% | 8% | 0% | 2% | |
|
| |||||
| Executive Function Tests | |||||
| Trail Making Test, Part A | Pre-Txp | 80% | 20% | 4% | |
| Post-Txp | 80% | 20% | 2% | 16% | |
| Trail Making Test, Part B | Pre-Txp | 80% | 20% | 6% | |
| Post-Txp | 88% | 12% | 2% | 8% | |
| Controlled Oral Word Association | Pre-Txp | 51% | 49% | 6% | |
| Post-Txp | 63% | 37% | 2% | 16% | |
| Animal Naming | Pre-Txp | 71% | 29% | 12% | |
| Post-Txp | 69% | 31% | 12% | 14% | |
| Ruff 2 and 7 Test, Letters | Pre-Txp | 71% | 29% | 12% | |
| Post-Txp | 69% | 31% | 0% | 4% | |
| Ruff 2 and 7 Test, Digits | Pre-Txp | 97% | 33% | 12% | |
| Post-Txp | 63% | 37% | 4% | 4% | |
| Stroop Interference | Pre-Txp | 94% | 6% | 0% | |
| Post-Txp | 78% | 22% | 6% | 20% | |
Mildly impaired corresponds to a score of −1 to −2 SD below age/education/gender corrected normative data, and moderately-to-severely impaired corresponds to greater than −2 SD below normative data. Pre-to-post decline refers to a posttransplant score of at least 1 SD below baseline.
The average composite test scores for verbal memory were considered to be within normal limits at baseline (t-score M = 48.6, SD = 6.2). Furthermore, composite verbal memory scores where higher at posttransplant compared to baseline (change in t-score M = 2.4, 95% CI = 1.2, 3.6, p < .0001). Further analysis at the level of individual tests revealed higher posttransplant scores for Logical Memory (change in t-score M = 7.2, 95% CI = 4.5, 9.5, p < .0001), but not the other verbal memory tests (Table 3). Age, education, and baseline PCO2 did not predict verbal memory test scores post-transplant (Table 4). In subsequent exploratory analyses, gender, diagnostic category, baseline performance for 6-Minute Walk Test, baseline BDI, baseline anxiety, and rejection at 6 months also did not contribute to posttransplant verbal memory scores.
Table 3.
Neurocognitive Test Scores in t-score units.
| Test | Pre-Txp M (SD; Min-Max) |
PostTxp M (SD; Min-Max) |
t | p |
|---|---|---|---|---|
| Verbal Memory Tests | 48.4 (6.2; 33–61) | 50.8 (4.8; 41–60) | 4.2 | <.0001 |
| Logical Memory | 48.1 (9.7; 27–67) | 55.3 (9.3; 33–70) | 5.4 | <.0001 |
| Digit Span | 47.1 (10.1; 30–73) | 46.2 (7.6; 30–60) | −0.9 | .39 |
| Verbal Paired Associates | 50.1 (5.0; 33–57) | 51.0 (4.2; 40–57) | 1.4 | .16 |
|
| ||||
| Executive Function Tests | 46.1 (6.1; 30–60) | 46.3 (5.4; 30–59) | 0.5 | .61 |
| Trail Making Test, Part A | 49.6 (11.8; 20–78) | 49.1 (9.7; 26–67) | ||
| Trail Making Test, Part B | 48.6 (11.4; 18–76) | 51.4 (10.4; 22–75) | ||
| Controlled Oral Word Association | 42.7 (8.5; 29–62) | 42.4 (7.4; 25–66) | ||
| Animal Naming | 45.0 (13.1; 13–74) | 44.7 (12.1; 13–74) | ||
| Ruff 2 and 7 Test, Letters | 45.4 (10.7; 21–66) | 46.3 (8.8; 31–67) | ||
| Ruff 2 and 7 Test, Digits | 43.8 (9.6; 22–72) | 44.7 (9.8; 25–77) | ||
| Stroop Interference | 47.4 (5.1; 36–58) | 45.7 (7.8; 26–61) | ||
Table 4.
Baseline predictors of posttransplant neurocognitive function
| Predicting Posttransplant Executive Function Scores | Predicting Posttransplant Verbal Memory Scores | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baseline Variable | b | SEb | p | Baseline Variable | b | SEb | p |
| Executive Function | .70 | .12 | <.0001 | Verbal Memory | .57 | .08 | <.0001 |
| Age | −.13 | .60 | .03 | Age | −.03 | .04 | .45 |
| Education | −.17 | .24 | .49 | Education | .21 | .18 | .24 |
| PCO2 | −.02 | .10 | .80 | PCO2 | .06 | .07 | .37 |
b = standardized beta coefficient from the regression equation predicting posttransplant scores.
SEb = standard error of the beta coefficient.
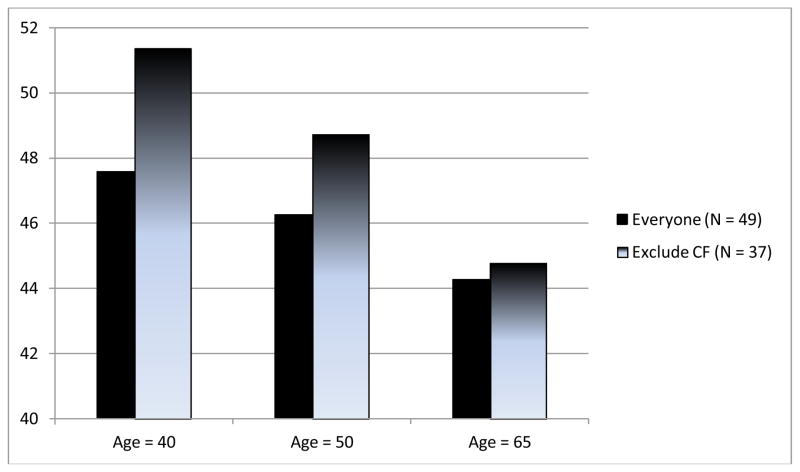
Average composite test scores for executive function were considered to be within normal limits at baseline (t-score M = 46.0, SD = 6.1); composite executive function scores at posttransplant were similar to baseline scores (p = .61; Table 3). However, older age predicted lower posttransplant composite executive function scores after controlling for pretransplant scores (b for Age = −0.13, 95% CI = −0.23, −0.03, p = .03), whereas education and baseline PCO2 did not (Table 4). Based upon this model, the predicted change in composite executive function t-score for a typical participant was +1.6 higher at age 40, +0.3 at age 50, and −1.7 lower at age 65. Exploratory analyses revealed that gender, diagnostic category, baseline 6-minute walk test, baseline BDI, baseline anxiety, and rejection at 6 months did not predict posttransplant executive function scores. When the analyses were repeated with a sample limited to non-CF patients (n = 37), the effect of age on posttransplant executive function scores was more pronounced (b = −.26, 95% CI = −0.11, −0.42, p < .0001), producing predicted change in composite executive function t-scores of +3.7 at age 40, +1.1 at age 50, and −2.9 at age 65 suggesting that older age was associated with worse performance (see Figure 1).
Figure 1.
Predicted posttransplant composite executive function at three different ages, for both the sample as a whole and for a subsample excluding patients diagnosed with CF.
In addition to examination of mean changes in neurocognitive performance, we also examined the proportion of participants who exhibited declines of at least 1 standard deviation on each neuropsychological test between baseline and post-transplant testing are provided in Table 2. Stroop Interference, Trail Making Test Part A, Controlled oral Word Association Test, and Animal Naming appeared to be particularly sensitive to neurocognitive decline. Fourteen (29%) of participants met the a priori criteria for significant neurocognitive decline (i.e., post-transplant performance of at least 1 SD lower on at least 2 tests, compared to pre-transplant performance). The rate of neurocognitive decline was similar among participants with a diagnosis of rejection at 6-months (2 of 14, 17%), compared to participants without a diagnosis of rejection at 6 months (12 of 37, 32%; p = .29). Participants who were classified as exhibiting neurocognitive decline were older (age in years M = 57.1, SD = 12.7, t = −2.8, p = .004) and tended to report fewer years of formal education (education in years M = 12.7, SD = 1.9, p = .07) compared to participants who were not (age in years M = 46.5, SD = 14.0; education in years M = 14.0; SD = 2.6).
CONCLUSIONS
To our knowledge, this is the first prospective analysis of changes in neurocognitive functioning in patients undergoing lung transplantation. Our results suggest that patients with advanced lung disease exhibit compromised neurocognitive functioning, with more than 80% of our sample exhibiting at least mildly-impaired neurocognition on one or more tests prior to and following surgery. Despite these deficits, we found that most patients remained stable, and neurocognitive functioning improved in some cases following surgery. For example, verbal memory scores improved following lung transplantation, particularly for contextual verbal memory, where average participant performance improved by more than two-thirds of a standard deviation. We adopted a strategy for describing meaningful changes in neurocognition that had been used successfully in patients who underwent coronary artery bypass surgery 17. We found that 16% of our sample evidenced moderate-to-severe impairment on two or more neurocognitive measures at the pretransplant assessment, compared to only 6% at the six-month posttransplant assessment.
For most patients, executive functioning remained relative stable following surgery, although younger lung transplant recipients appeared to show improvement in executive functioning, whereas older recipients exhibited a decline. An exploratory analysis in which the CF patients, who are younger, were removed from the sample suggested that greater declines in neurocognition may be evident among older patients with COPD. Indeed, we would predict that an otherwise-average 65 year-old participant would lose approximately 0.30 standard deviations in composite executive functioning. Although this effect size is modest, the impact of these changes on everyday functioning is not known, and should be the focus of future studies.
Our sample evidenced no improvement, on average, in the basic connect-the-dots trial, and only slight performance in the complex set-shifting trial. These results contrast with prior studied, in whence moderate to large improvements in verbal memory, as well as moderate improvement in Trail Making Test performance, were observed among recipients of kidney transplants (N = 20) 18 and heart transplants (N = 27) 19 one year following transplantation.
While comparison of mean changes in test scores was unremarkable, interestingly 29% of the sample exhibited significant neurocognitive declines between their baseline and post-transplant assessments. These findings also have been reported in longitudinal studies of patients who undergo coronary artery bypass grafting 6, 17. For example, while mean neurocognitive scores improved from baseline to 6 months, 24% of patients exhibited a decline of at least 1 standard deviation on at least 1 of 4 composite measures of neurocognition 6 in patients who underwent CABG. In our sample, patients who exhibited significant neurocognitive decline were an average of 10 years older, and tended to have less formal education, compared to patients who did not. Similar findings have been reported among CABG patients 6.
Baseline measures of medical and psychosocial function did not predict neurocognitive outcomes in this sample. For example, patients’ level of depression or anxiety was unrelated to changes in neurocognitive functioning. However, most patients were neither clinically anxious nor depressed, and the small sample limited the power to detect an effect had one been present. Diagnosis of rejection at 6 months also did not predict neurocognitive outcomes, although because the sample was small, we may not have had sufficient statistical power to detect group differences.
The small sample size of this study raises concern about the representativeness of this sample. However, the demographic and clinical characteristics of the 49 transplant recipients included in the final sample were similar to those of the 28 transplant recipients who did not complete post-transplant testing. Furthermore, all 77 transplant recipients were similar to the 54 subjects who did not undergo lung transplantation in baseline and demographic data with the exception of gender, and change in neurocognition was not moderated by gender. While we acknowledge the small sample, we conclude that our sample of transplant recipients is representative of the larger population of patients who were listed for lung transplantation at the time.
At the time when these data were collected, lung transplantation was limited to patients aged 65 years-old or younger. Some transplant centers have raised or eliminated their maximum age for lung transplantation, and in the last decade there has been tremendous growth in the rates of lung transplantation among patients age 65 years and older. Thus, the potentially adverse effects of lung transplantation on neurocognition in the current transplant population may be more pronounced in older transplant recipients.
The current findings encourage further study of the effects of lung transplantation on neurocognition, particularly among older patients. Future studies could benefit from the inclusion of a demographically-matched, non-transplant control condition, which may help to clarify the amount of impairment specifically attributable to transplantation. Also, the dominant surgery mechanisms thought to be responsible for neurocognitive decline in CABG patients (e.g., longer time on cardiopulmonary bypass pump; particulate and gas emboli secondary to clamping) are unlikely to be relevant to this population because most lung transplants at this center are conducted without cardiopulmonary bypass. Future studies should examine possible mechanisms responsible for neurocognitive decline among a subset of patients undergoing lung transplantation, such as hypoxia, hypercarbia, hypotension, cerebral edema from impaired outflow during superior vena cava retraction, and medications (e.g. side effects of immunosuppressant medication 20). Finally, future research should examine mechanisms which may place older adults at greater risk for post-transplant neurocognitive decline, such as the presence of established pre-operative cerebrovascular risk factors, greater sensitivity to medication side effects, and greater medical comorbidities.
Acknowledgments
This study was supported by NHLBI grants HL 65503-01 and HL 065503-06A1. Also, this work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Orens JB, Garrity ER., Jr General overview of lung transplantation and review of organ allocation. Proc Am Thorac Soc. 2009 Jan 15;6(1):13–19. doi: 10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- 2.Parekh PI, Blumenthal JA, Babyak MA, et al. Gas exchange and exercise capacity affect neurocognitive performance in patients with lung disease. Psychosom Med. 2005;67(3):425–432. doi: 10.1097/01.psy.0000160479.99765.18. [DOI] [PubMed] [Google Scholar]
- 3.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35(4):913–922. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 4.Arciniegas DB, Filley CM. Implication of impaired cognition for organ transplant candidacy. Curr Opin Org Transpl. 1999;4:168–180. [Google Scholar]
- 5.Antonelli-Incalzi R, Corsonello A, Pedone C, et al. Drawing impairment predicts mortality in severe COPD. Chest. 2006;130(6):1687–1694. doi: 10.1378/chest.130.6.1687. [DOI] [PubMed] [Google Scholar]
- 6.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344(6):395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal JA, Babyak MA, Keefe FJ, et al. Telephone-based coping skills training for patients awaiting lung transplantation. J Consult Clin Psychol. 2006;74(3):535–544. doi: 10.1037/0022-006X.74.3.535. [DOI] [PubMed] [Google Scholar]
- 8.Maurer JR, Frost AE, Estenne M, Higenbottam T, Glanville AR. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. Heart Lung. 1998;27(4):223–229. doi: 10.1016/s0147-9563(98)90033-4. [DOI] [PubMed] [Google Scholar]
- 9.Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, Illinois: Skoelting; 1978. [Google Scholar]
- 10.Lezak MD. Neuropsychological assessment. 3. Oxford; New York: Oxford University Press; 1995. [Google Scholar]
- 11.Ruff RM, Allen CC. Ruff 2 & 7 Selective Attention Test. Odessa, FL: Psychological Assessment Resources, Inc; 1996. [Google Scholar]
- 12.Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Tuscon: Reitan Neuropsychological Laboratories, Inc; 1979. [Google Scholar]
- 13.Wechsler D. Wechsler Memory Scale-Revised. New York: The Psychological Corporation; 1987. [Google Scholar]
- 14.Wechsler D. Wechsler Adult Inteilligence Scale (WAIS-R) Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 15.Beck AT, Steer RA, Brown GK. Beck Depression Inventory. 2. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 16.Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists’ Press; 1970. [Google Scholar]
- 17.Mahanna EP, Blumenthal JA, White WD, et al. Defining neuropsychological dysfunction after coronary artery bypass grafting. Ann Thorac Surg. 1996;61(5):1342–1347. doi: 10.1016/0003-4975(95)01095-5. [DOI] [PubMed] [Google Scholar]
- 18.Harciarek M, Biedunkiewicz B, Lichodziejewska-Niemierko M, Debska-Slizien A, Rutkowski B. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int. 2011;79(12):1353–1360. doi: 10.1038/ki.2011.40. [DOI] [PubMed] [Google Scholar]
- 19.Deshields TL, McDonough EM, Mannen RK, Miller LW. Psychological and cognitive status before and after heart transplantation. Gen Hosp Psychiat. 1996;18(6 Suppl):62S–69S. doi: 10.1016/s0163-8343(96)00078-3. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q, Marescauz C, Wolff V, et al. Tacrolimus-associated posterior reverisble encephalpathy syndrome after solid organ transplnatation. Eur Neurol. 2010;64(3):169–177. doi: 10.1159/000319032. [DOI] [PubMed] [Google Scholar]