Abstract
Objective
Arterial stiffness decreases with weight loss in overweight/obese young adults. We aimed to determine the mechanisms by which this occurs.
Methods
We evaluated carotid-femoral pulse wave velocity (cfPWV) and brachial-ankle pulse wave velocity (baPWV) in 344 young adults (23% male, BMI 25–40 kg/m2) at baseline, 6, and 12 months in a behavioral weight loss intervention. Linear mixed models were used to evaluate associations between weight loss and arterial stiffness and to examine whether improvements in obesity-related factors explained these associations.
Results
At 6 months (7% mean weight loss), there was a significant median decrease of 47.5 cm/s in cfPWV (p<0.0001) and a mean decrease of 11.7 cm/s in baPWV (p=0.049). At 12 months (6% mean weight loss), only cfPWV remained reduced. In models adjusting for changes in mean arterial pressure and obesity-related factors, changes in BMI (p=0.01) and common carotid artery diameter (p=0.003) were positively associated with change in cfPWV. Reductions in heart rate (p<0.0001) and C-reactive protein (p=0.02) were associated with reduced baPWV and accounted for the association between weight loss and reduced baPWV.
Conclusions
Weight loss is associated with reduced cfPWV independently of changes in established hemodynamic and cardiometabolic risk factors, but its association with reduced baPWV is explained by concurrent reductions in heart rate and inflammation.
Keywords: arterial stiffness, obesity, lifestyle modification
INTRODUCTION
Obesity leads to poor vascular health and an increased risk of cardiovascular disease (CVD)[1, 2]. The metabolic requirements of excess weight necessitate increases in total blood volume and cardiac output, and these hemodynamic changes elevate arterial wall stress, smooth muscle cell proliferation, vessel wall thickness and diameter, and eventually arterial stiffness[1, 3]. These hemodynamic alterations, alongside other features of obesity, including chronic inflammation and endothelial dysfunction, impair vascular structure and function in obese individuals[4]. Weight loss lowers CVD risk[5, 6] and reverses many adverse vascular changes, including arterial stiffness[6–10], an established predictor of vascular events[11, 12]. Because weight loss improves many risk factors that correlate with arterial stiffness, such as blood pressure (BP) [13], inflammation[14], insulin resistance[13, 14], and renin-angiotensin-aldosterone system hyperactivity[13], improvements in some of these risk factors likely explain the effect of weight loss on arterial stiffness.
Reductions in arterial stiffness with weight loss in overweight/obese adults[8–10, 14, 15] may be independent of concurrent reductions in BP [10], though not all studies agree[16]. Among hemodynamic factors, elevated blood volume and cardiac output in obese individuals may be more important drivers of arterial stiffening than elevated BP[10], particularly in young individuals. Few studies have evaluated mechanisms by which weight loss may reduce arterial stiffness, and these have included small numbers of either middle-aged and older overweight/obese adults[8, 9] or severely obese adults[10]. The aim of this study was to determine potential mechanisms by which weight loss reduces arterial stiffness, as measured by carotid-femoral pulse wave velocity (cfPWV), a measure of aortic stiffness, and brachial-ankle pulse wave velocity (baPWV), a mixed measure of central and peripheral arterial stiffness, in young overweight/obese normotensive adults.
METHODS
CfPWV and baPWV were measured at baseline and 6 and 12 month visits in overweight/obese adults participating in the Slow Adverse Vascular Effects of excess weight study (SAVE), a randomized-controlled trial (NCT00366990) evaluating the effects of weight loss, increased physical activity, and reduced dietary sodium intake on vascular health.
Study Population
Eligible participants were men and women 20–45 years of age who were overweight/obese (body mass index (BMI) 25–39.9 kg/m2) and physically inactive (<8 months of physical activity (PA) during the past 12 months). Exclusions included 1) diabetes, 2) hypertension or average screening BP ≥140/90 mmHg, 3) cholesterol lowering, anti-psychotic, or vasoactive medication use and 4) current pregnancy or lactation. The study was approved by the University of Pittsburgh IRB and all participants provided written informed consent to participate in the study.
Intervention
All eligible participants received a one year lifestyle intervention consisting of diet and physical activity (PA). Participants were randomized to either 1) diet and PA alone (Control Na/lifestyle) or to 2) diet and PA plus reduced sodium intake (Low Na/lifestyle). The lifestyle intervention was delivered in group sessions that occurred weekly for months 1–4, biweekly for months 5–8, and monthly for months 9–12. The goal of the intervention was a 10% reduction in body weight over 6 months and continued maintenance of weight loss thereafter. The additional goal of the sodium reduction intervention was to gradually reduce daily sodium intake by approximately 50%.
Clinic Visits
Participants were to complete clinic visits at screening, baseline, and 6, 12, and 24 months following randomization. The present study is a secondary analysis using data from baseline, 6 and 12 months.
Demographic and Physical Measures
Age, race, and smoking status were self-reported. Weight was measured in kilograms using a balance scale. Height was measured in centimeters using a stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured against the participant’s skin at the narrowest part of the torso between the ribs and the iliac crest. BP was measured with a mercury sphygmomanometer after participants sat quietly for 5 minutes with feet flat on the floor.
Laboratory Methods
Blood analytes were measured at the Heinz Laboratory at the University of Pittsburgh’s Graduate School of Public Health as previously described[17]. Valid 24-hour urine collections had volume 500–4000 mL, duration 22–26 hours, and total creatinine within the expected range[18]. Sodium and creatinine excretion were measured as previously described[17].
Pulse Wave Velocity
As earlier described[19], following ten minutes of rest, cfPWV and baPWV were simultaneously determined using the VP2000 system (Omron Health Care Co., Kyoto, Japan), a noninvasive automated waveform analyzer. PWV was calculated as the path length between arterial sites of interest divided by the time delay between the foot of the respective waveforms. Intraclass correlation coefficients (ICC) for within technologist replicate measures were 0.76 (cfPWV) and 0.97 (baPWV) and for between technologists replicates were 0.60 (cfPWV) and 0.87 (baPWV).
Carotid Ultrasound
Common carotid artery (CCA) intima-media thickness (IMT) and adventitial diameter (AD) measurements and readings were performed using an Acuson Sonoline Antares high resolution duplex scanner. IMT measures were determined as previously described[17]. CCA AD measures were calculated directly as the distance from the adventitial-medial interface on the near wall to the medial-adventitial interface on the far wall at end-diastole. The reading software used was the AMS system developed by Dr. Thomas Gustavsson[20]. For these analyses, the mean of the average IMT and AD values was used. Reproducibility of IMT and AD measures was excellent with between sonographer and within reader ICCs of >0.83.
Statistical Analysis
Descriptive statistics were calculated to summarize study variables by visit and presented as median/inter-quartile range (IQR) or mean (SD) for continuous variables and frequency and percentages for categorical variables. Whether changes were significant at follow-up visits was determined by testing time, as a nominal variable, in linear mixed models. Non-normally distributed variables were transformed as necessary. Intervention arm was included in every model for consistency with trial design. Interaction between intervention arm and time since baseline was included if significant at p<0.10. A mixed model was created for each PWV measure, adjusting for age, sex, race, smoking status, and years since baseline whenever significant at p<0.10. All possible second order interactions were evaluated.
Next, baseline BMI and change in BMI (or weight or waist circumference) were added to the mixed models to simultaneously determine cross-sectional and longitudinal associations between measures of body size and PWV. The measure of body size change showing the most statistically significant association with each PWV measure was kept for additional analyses. Baseline mean arterial pressure (MAP) and change in MAP were then added to the models. Next, other cardiovascular and metabolic risk factors that could potentially explain the relationship between weight loss and reduced arterial stiffness were added. Linear mixed models were used to determine whether these factors’ changes were associated with changes in BMI or waist circumference after adjustment for age, sex, race, and intervention arm. Only factors showing longitudinal association with at least one measure of body size were further examined.
Because participants who were not as successful with weight loss during the intervention may have been less likely to complete follow-up visits, non-ignorable mechanisms for the missing data were considered. Linear mixed effects pattern-mixture models were used to evaluate the influence of the missing data[21]. In the first model, a dichotomous variable for completion of the 12 month study visit was included as a covariate and this variable’s interactions with within-subject factors in the final mixed models were examined. In additional pattern-mixture models, missing data was multiply imputed under two different assumptions, one of which presumed that dropouts lost less weight than completers (see Supplementary Material for details). Values of p<0.05 were considered statistically significant. All analyses were performed using SAS (Statistical Analysis Software release 9.2, Cary, NC, USA).
RESULTS
The study population consisted of 344 participants in the SAVE trial who had baseline cfPWV and/or baPWV data. Five trial participants had missing or invalid data on both cfPWV and baPWV at baseline. Measurements were more commonly missing or invalid for cfPWV than baPWV (n=23 vs. 10 at baseline, 11 vs. 2 at 6 months, 14 vs. 6 at 12 months) among participants who attended the respective visit, mainly due to the technical difficulties of tonometry in obese individuals. The sample had a mean age of 37.9 years (SD 6.1) at baseline and consisted of 23% males and 16% African-Americans. Nine percent were current smokers at baseline. Mean values of key study measures during the intervention are shown in Table 1.
Table 1.
Body size, cardiometabolic factors, and carotid artery geometry across the intervention
| Characteristic | Baseline (N=344) |
6 Months (N=284) |
12 Months (N=255) |
|---|---|---|---|
| Weight (kg) | 92.2 (14.9) | 85.7 (15.0)* | 85.5 (15.1)* |
| BMI (kg/m2) | 32.9 (3.8) | 30.4 (4.2)* | 30.4 (4.5)* |
| Waist Circumference (cm) | 100.4 (11.2) | 95.4 (11.5)* | 95.4 (12.2)* |
| SBP (mmHg) | 113.5 (10.5) | 110.2 (9.6)* | 110.1 (9.9)* |
| DBP (mmHg) | 72.9 (8.7) | 71.1 (8.4)* | 72.0 (8.2) |
| Glucose (mmol/L) | 5.4 (0.4) | 5.4 (0.5) | 5.4 (0.5) |
| Insulin (pmol/L)** | 86.8 (66.7, 120.8) | 80.6 (62.5, 108.3)* | 81.3 (65.3, 105.6)* |
| LDL-C (mmol/L) | 3.19 (0.86) | 3.15 (0.79) | 3.22 (0.80) |
| HDL-C (mmol/L) | 1.36 (0.35) | 1.38 (0.34) | 1.45 (0.37)* |
| Triglycerides (mmol/L)** | 1.31 (0.88, 1.92) | 1.05 (0.77, 1.56)* | 0.99 (0.78, 1.55)* |
| CRP (nmol/L)** | 24.8 (12.4, 55.2) | 19.0 (9.5, 41.9)* | 19.0 (8.6, 39.0)* |
| Leptin (nmol/L) | 1.62 (0.83) | 1.16 (0.74)* | 1.29 (0.83)* |
| Adiponectin (µg/mL) | 11.8 (5.9) | 11.9 (5.3) | 12.0 (5.4) |
| Ghrelin (pmol/L)** | 199 (162, 259) | 221 (180, 299) | 246 (189, 326) |
| Heart Rate (beats/min) | 64.1 (9.1) | 62.3 (8.3)* | 63.9 (9.0) |
| Mean IMT (mm) | 0.60 (0.08) | 0.61 (0.08) | 0.61 (0.09)* |
| Mean AD (mm) | 6.91 (0.53) | 6.86 (0.52)* | 6.83 (0.56)* |
Mean(SD) or median(IQR) are shown.
P<0.05 versus baseline in a linear mixed model with adjustment for intervention arm.
Log transformed for modeling.
CRP=C-reactive protein. IMT = common carotid artery intima-media thickness. AD = common carotid artery adventitial diameter.
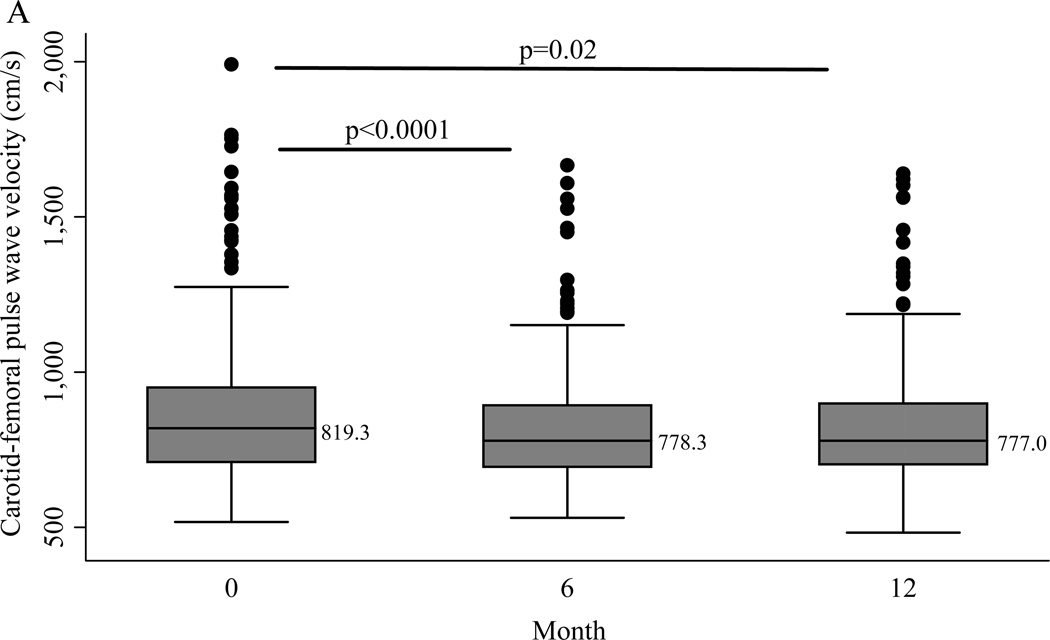
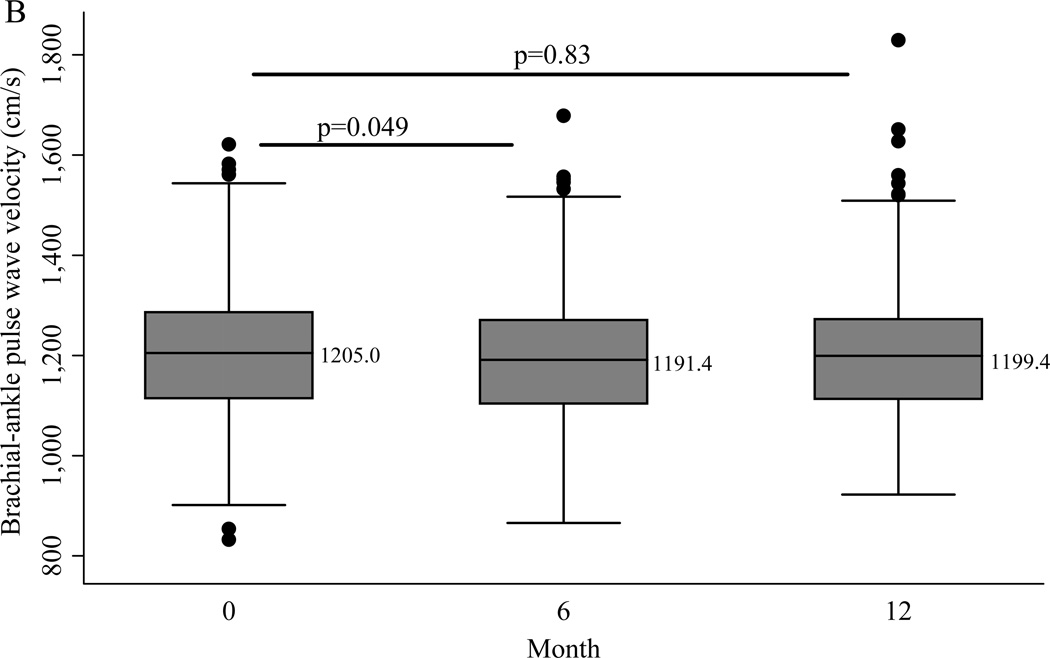
After 6 months an average weight loss of 7.1% was achieved, which was accompanied by a significant change in cfPWV (Mean −58.1 cm/s, SD 233; Median −47.5 cm/s, IQR −148, 44.5) (p<0.0001). Carotid-femoral PWV was log transformed to normalize its skewed distribution for further analyses. Six months into the intervention there was a mean change of −11.7 cm/s (SD 91.4) in baPWV (p=0.049). At the conclusion of the intervention, average weight loss was 6.4% and the change from baseline was statistically significant for cfPWV (Median −32.5 cm/s, IQR −140.5, 84.5; p=0.02) but not baPWV (Mean −2.6 cm/s (SD 97.4)) (Figure 1). The only changes that differed even marginally by intervention arm were 24-hour urinary sodium and serum aldosterone. Mean urinary sodium was 186.1 mmol/24hr at baseline in the total sample and was reduced significantly more in the Low Na/lifestyle arm (48.9 mmol/day (SD 80.4)) than in the Control Na/lifestyle arm (8.6 mmol/day (SD 78.2)) at 6 months (p<0.001) but not at 12 months (p=0.20). The change in serum aldosterone was marginally greater in the Low Na/lifestyle arm than the Control Na/lifestyle arm at 6 months only (p=0.06).
Figure 1.
Boxplots of (A) carotid-femoral pulse wave velocity and (B) brachial-ankle pulse wave velocity across the intervention. P values are for follow-up versus baseline from linear mixed models with adjustment for intervention arm. Median values are shown at each time point.
As expected, changes in BMI and waist circumference were associated with changes in most cardiometabolic, hemodynamic, and vascular parameters (Table 2). In linear mixed models for log cfPWV that included baseline age, sex, race, and time since baseline, reductions in weight (p<0.0001), waist circumference (p=0.002), and BMI (p<0.0001) were all associated with decreases in log cfPWV during the lifestyle intervention. To determine which factors’ improvements might explain the association between weight loss and reduced cfPWV, risk factors were added sequentially to the model for log cfPWV. Because change in BMI had the most statistically significant parameter estimate of the three body size measures, it was kept in additional models. When baseline MAP (p<0.0001) and change in MAP (p=0.02) were added to the model, there was little change to the association between BMI reduction and cfPWV reduction (p=0.0003). Subsequently, when additional factors were added, only changes in heart rate (p=0.08) and carotid adventitial diameter (p=0.003) were associated at least marginally with change in log cfPWV. However, neither of these factors removed the statistical significance of the association between change in BMI and change in log cfPWV (Table 3). Results were very similar when waist circumference was evaluated in the models in place of BMI.
Table 2.
Longitudinal associations between changes in body size and changes in cardiometabolic factors and common carotid artery geometry
| Change in BMI (kg/m2) | Change in Waist Circumference (cm) | |||||
|---|---|---|---|---|---|---|
| Dependent Variable | Estimate | Standard Error |
P | Estimate | Standard Error |
P |
| MAP (mm Hg) | 0.66 | 0.12 | <0.0001 | 0.21 | 0.04 | <0.0001 |
| LDL-C (mmol/L) | 0.055 | 0.011 | <0.0001 | 0.019 | 0.004 | <0.0001 |
| HDL-C (mmol/L) | −0.010 | 0.004 | 0.01 | −0.003 | 0.001 | 0.02 |
| Triglycerides (mmol/L)* | 0.001 | 0.0002 | <0.0001 | 0.0005 | 0.00005 | <0.0001 |
| Insulin (pmol/L)* | 0.42 | 0.042 | <0.0001 | 0.14 | 0.014 | <0.0001 |
| Aldosterone (pmol/L)* | −0.017 | 0.025 | 0.53 | −0.003 | 0.008 | 0.68 |
| Adiponectin (µg/mL) | −0.41 | 0.06 | <0.0001 | −0.13 | 0.02 | <0.0001 |
| Leptin (nmol/L) | 0.15 | 0.009 | <0.0001 | 0.044 | 0.003 | <0.0001 |
| Ghrelin (pmol/L)* | −0.003 | 0.002 | 0.18 | −0.001 | 0.0006 | 0.09 |
| CRP (nmol/L)* | 1.05 | 0.095 | <0.0001 | 0.29 | 0.048 | <0.0001 |
| Sodium Excretion (mmol/24hr)** | 5.84 | 1.34 | <0.0001 | 1.37 | 0.46 | 0.003 |
| IMT (mm) | 0.001 | 0.0009 | 0.20 | 5.9×10−6 | 0.0003 | 0.98 |
| AD (mm) | 0.03 | 0.005 | <0.0001 | 0.006 | 0.002 | 0.0001 |
Each dependent variable is the outcome in a linear mixed effects model. Random intercept and years since baseline effects were included in each model. Intervention arm, baseline age, race, sex, and baseline BMI or waist circumference were included as fixed effects.
Log transformed for modeling.
MAP = mean arterial pressure. IMT = common carotid artery intima-media thickness. AD = common carotid artery adventitial diameter. Number of subjects = 344. Number of observations = 882.
Number of subjects = 324. Number of observations = 674.
Table 3.
Fully- adjusted multivariable linear mixed effects model for log carotid-femoral pulse wave velocity
| Variable | Estimate | Standard Error | P |
|---|---|---|---|
| Change in MAP (mm Hg) | 0.002 | 0.001 | 0.07 |
| Change in Heart Rate (beats/min) | 0.002 | 0.001 | 0.08 |
| Change in BMI (kg/m2) | 0.01 | 0.004 | 0.01 |
| Change in AD (mm) | 0.11 | 0.03 | 0.001 |
| Time since Baseline (years) | −0.001 | 0.02 | 0.93 |
Random intercept and years since baseline effects were included in this model. In addition to those variables listed, intervention arm and baseline age, race, sex, MAP, heart rate, BMI, and AD were included as fixed effects. Carotid-femoral pulse wave velocity was log transformed. MAP = mean arterial pressure. AD = common carotid artery adventitial diameter. Number of subjects = 326. Number of observations = 804.
In linear mixed models for baPWV that included baseline age, sex, race, and time since baseline, reductions in weight (p=0.002), waist circumference (p=0.0009), and BMI (p=0.005) were associated with decreases in baPWV during the intervention. To determine which factors’ improvements might explain the association between weight loss and reduced baPWV, risk factors were added sequentially to the model for baPWV. Because change in waist circumference had the most statistically significant parameter estimate of the three body size measures, it was kept in additional models. When baseline MAP (p<0.0001) and change in MAP (p<0.0001) were added to the model, reduction in waist circumference (p=0.02) remained a statistically significant determinant of decrease in baPWV. When other factors were added, only changes in heart rate (p<0.0001) and CRP (p=0.005) were individually associated with change in baPWV. Both of these factors, separately or together, removed the statistical significance of the association between change in waist circumference and change in baPWV (Table 4). In models that examined BMI in place of waist circumference, change in BMI was similarly not significant (p=0.99) in the fully-adjusted model for baPWV, and the results for all other factors were similar to those from the models that included waist circumference. No statistically significant interactions were detected in any model for either PWV measure.
Table 4.
Fully-adjusted multivariable linear mixed effects model for brachial-ankle pulse wave velocity
| Variable | Estimate | Standard Error | P |
|---|---|---|---|
| Change in MAP (mm Hg) | 2.59 | 0.55 | <0.0001 |
| Change in Heart Rate (beats/min) | 2.42 | 0.50 | <0.0001 |
| Change in Waist Circumference (cm) | 0.38 | 0.60 | 0.53 |
| Change in CRP (nmol/L)* | 1.10 | 0.49 | 0.03 |
| Time since Baseline (years) | 6.28 | 5.47 | 0.25 |
A random intercept was included in this model. In addition to those variables listed, intervention arm and baseline age, race, sex, MAP, heart rate, waist circumference, and CRP were included as fixed effects. MAP = mean arterial pressure. CRP = C-reactive protein.
Log transformed. Number of subjects = 335. Number of observations = 832.
In this analysis, participants with missing PWV data at 6 months were more likely than those with non-missing 6 month data to be male and in the low sodium intervention arm (p<0.05 for both). Study subjects missing 12 month PWV data were more likely to be male, to have higher baseline BMI, and to have achieved lesser weight and BP reductions at 6 months than those with 12 month data (p<0.05 for all). In addition to these differences, it is likely that completers and non-completers differed with regard to unobserved factors. However, in sensitivity analyses using pattern-mixture modeling and multiple imputation, marginal parameter estimates differed little from those in the original fully-adjusted mixed models (See supplemental Tables 1–6).
DISCUSSION
Among normotensive overweight/obese young adults, weight loss is associated with a reduction in aortic stiffness as measured by cfPWV. This association is independent of concurrent improvements in established obesity-related cardiometabolic and hemodynamic risk factors. Diameter reduction of the common carotid artery is also strongly associated with aortic stiffness reduction, and this association is statistically stronger than those between changes in aortic stiffness and either BP or heart rate. In contrast, weight loss is not independently associated with changes in baPWV, a more peripheral measure of arterial stiffness; this latter association appears to be explained by concurrent changes in heart rate and CRP. These findings are important because firstly, this is the first large study of young overweight/obese otherwise healthy adults to demonstrate improvements in PWV during a lifestyle intervention. Secondly, we investigated concurrent changes in a wide variety of obesity-related factors that might explain the association between weight loss and reduced arterial stiffness, and thirdly, sensitivity analyses that realistically assumed less successful weight loss among non-completers of the study yielded largely unchanged results.
These data suggest that BP is not the primary mechanism linking weight loss to improvements in either central or peripheral arterial stiffness in healthy normotensive young adults. Though weight loss, through either lifestyle modification or bariatric surgery, at least partially reverses obesity-related vascular alterations[6–10, 14, 15], the precise mechanisms by which this occurs are poorly understood. Only a few studies have evaluated mechanisms other than BP reduction by which weight loss may reduce arterial stiffness, and these studies have not focused on young adults[8–10]. In a sample of middle-aged obese adults who achieved dietary or surgical weight loss, Rider et al. found that only BMI reduction, not concurrent hormonal or metabolic factor changes, independently correlated with aortic PWV reduction[9]. In a study of middle-aged and older overweight/obese adults, only BMI reduction, total weight loss, or total fat loss independently predicted cfPWV reduction when other adiposity-related factors were included in a multivariable model[8]. Similarly, Ikonomidis et al. found that BMI reduction was the strongest independent determinant of reduced thoracic aortic stiffness index in severely obese young and middle-aged adults who underwent bariatric surgery; concurrent changes in BP, lipids, glucose, or indices reflecting elevated circulating blood volume were not as strong determinants[10]. This study, similar to the present investigation, found an association between reductions in BMI and diastolic aortic diameter, suggesting that lowered circulating blood volume may partly drive the improvement in arterial stiffness with weight loss[10]. Altogether, the evidence suggests that weight loss impacts aortic stiffness independently of concurrent changes in BP and other established cardiometabolic and hemodynamic risk factors.
In addition to the factors investigated, several others may explain the reductions in arterial stiffness that occur with weight loss. Micro-structural properties of the aortic wall, for example the extent of cross-linking of extracellular matrix proteins and the balance between matrix protein synthesis and degradation, may be altered by weight loss and physical activity[22]. Furthermore, improvements in sympathovagal balance during weight loss may influence arterial stiffness[23]. Changes in nitric oxide bioavailability and local renin-angiotensin-aldosterone system activity may also contribute to improvements in arterial stiffness with weight loss[23]. Finally, the effects of obesity differ across aortic segments[9]. Some studies have shown that weight loss has a greater effect on the stiffness of the abdominal aorta than the more proximal segments[9].
In addition to the possible segment-specific effects of obesity on aortic stiffness, excess weight appears to have heterogeneous effects on peripheral and central arterial stiffness. In the present study, a larger and more sustained reduction was seen in cfPWV than baPWV, and only the reduction in cfPWV with weight loss was independent of concurrent risk factor changes. This finding is consistent with that of another study in which a small group of healthy middle-aged males participated in an aerobic exercise intervention that promoted weight loss; in that study cfPWV decreased significantly (mean = 58 cm/s) whereas baPWV did not[24]. Cross-sectionally, greater BMI and body fat are associated with higher stiffness of both muscular and elastic arteries, and for elastic arteries the association may be strongest in young adults[1].Thus, though obesity affects arterial stiffness throughout the arterial tree, in young overweight/obese adults weight loss may be particularly beneficial in reducing central arterial stiffness. This is a promising for CVD prevention, as cfPWV is an established predictor of incident vascular events[11].
Brachial-ankle PWV is highly correlated with cfPWV, exhibits similar associations with cardiovascular risk factors in some studies[24, 25], and has been found to predict cardiovascular and total mortality in Japanese older adults[12]. Larger studies with longer follow-up times, however, are needed to substantiate these findings. There have been few longitudinal studies using baPWV, and to our knowledge none of these have attempted to determine the mechanisms by which weight change influences baPWV[24, 26]. A recent Mendelian randomization study reported that functional polymorphisms in the gene encoding CRP are not associated with aortic PWV in the general population, suggesting that CRP does not cause aortic stiffening[27]. However, it remains possible that chronic inflammation in obese individuals affects arterial stiffness, and this may be more evident in peripheral arteries given the vasoconstrictive phenotype promoted by CRP[28]. Similar to the present findings, it was recently found that higher baseline heart rate and an increase in heart rate were associated with greater increases in baPWV over 5–6 years[29]. The relationship between reductions in heart rate and baPWV may result from decreases in sympathetic activation, improvements in physical fitness, reductions in cyclic stretching of the arteries, or simply the frequency dependence of the viscoelasticity of the artery walls[30]. The greater strength of the longitudinal association between heart rate and baPWV than between CRP and baPWV, and the finding that both baPWV and heart rate were unchanged from baseline at the end of the intervention suggest that heart rate rather than inflammation may explain the influence of weight loss on baPWV. However, future studies are needed to determine whether these factors play causal roles in arterial stiffening in overweight/obese individuals.
This study had several strengths. First, many factors were measured in order to explore numerous potential mechanisms by which weight loss improves arterial stiffness. A second strength was the stability of the results under various hypothesized distributions for the missing data. Another strength was the absence of antihypertensive or vasoactive medication use, thus minimizing treatment related confounding. One limitation was the small number of males and non-white participants, which limited power to detect subgroup effects, though no significant interactions were detected with these factors.
Conclusions
Weight loss reduces aortic stiffness independently of concurrent changes in commonly measured cardiometabolic and hemodynamic risk factors and common carotid artery geometry in young normotensive overweight/obese adults. In contrast, reductions in heart rate and CRP are determinants of reductions in baPWV, a more peripheral measure of arterial stiffness, and these factors may drive the effect of weight loss on baPWV. Though PWV reductions in this study were not as large as in some intervention studies[8, 9], likely due to the low risk study sample, the predictive power of cfPWV and baPWV in the general population suggests that even these small reductions made early in adulthood may reduce vascular events in the long-term.
Supplementary Material
Highlights.
How does weight loss reduce arterial stiffness in overweight/obese young adults?
We study 344 young adult participants in a one year lifestyle intervention.
Weight loss reduces cfPWV independently of other vascular risk factors.
Influence of weight loss on baPWV is explained by reductions in heart rate and CRP.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grant R01 HL077525-01 and Jennifer Cooper is supported by F31 HL106986, both from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
Contributor Information
Jennifer N. Cooper, Email: jnn9@pitt.edu.
Jeanine M. Buchanich, Email: jeanine@pitt.edu.
Ada Youk, Email: ayouk@pitt.edu.
Maria Mori Brooks, Email: brooks@edc.pitt.edu.
Emma Barinas-Mitchell, Email: barinas@edc.pitt.edu.
Molly B. Conroy, Email: conroymb@upmc.edu.
Kim Sutton-Tyrrell, Email: tyrrell@edc.pitt.edu.
REFERENCES
- 1.Zebekakis PE, Nawrot T, Thijs L, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J. Hypertens. 2005;23:1839–1846. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Balady GJ, Criqui MH, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA Task Force on Risk Reduction. American Heart Association. Circulation. 1998;97:1876–1887. doi: 10.1161/01.cir.97.18.1876. [DOI] [PubMed] [Google Scholar]
- 3.De Michele M, Panico S, Iannuzzi A, et al. Association of obesity and central fat distribution with carotid artery wall thickening in middle-aged women. Stroke. 2002;33:2923–2928. doi: 10.1161/01.str.0000038989.90931.be. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton PA, James ME, Goodwill AG, et al. Obesity and vascular dysfunction. Pathophysiology. 2008;15:79–89. doi: 10.1016/j.pathophys.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skilton MR, Sieveking DP, Harmer JA, et al. The effects of obesity and non-pharmacological weight loss on vascular and ventricular function and structure. Diabetes Obes Metab. 2008;10:874–884. doi: 10.1111/j.1463-1326.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- 6.Karason K, Wikstrand J, Sjostrom L, et al. Weight loss and progression of early atherosclerosis in the carotid artery: a four-year controlled study of obese subjects. Int. J. Obes. Relat. Metab. Disord. 1999;23:948–956. doi: 10.1038/sj.ijo.0801024. [DOI] [PubMed] [Google Scholar]
- 7.Mavri A, Stegnar M, Sentocnik JT, et al. Impact of weight reduction on early carotid atherosclerosis in obese premenopausal women. Obes. Res. 2001;9:511–516. doi: 10.1038/oby.2001.67. [DOI] [PubMed] [Google Scholar]
- 8.Dengo AL, Dennis EA, Orr JS, et al. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension. 2010;55:855–861. doi: 10.1161/HYPERTENSIONAHA.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rider OJ, Tayal U, Francis JM, et al. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity (Silver Spring) 2010;18:2311–2316. doi: 10.1038/oby.2010.64. [DOI] [PubMed] [Google Scholar]
- 10.Ikonomidis I, Mazarakis A, Papadopoulos C, et al. Weight loss after bariatric surgery improves aortic elastic properties and left ventricular function in individuals with morbid obesity: a 3-year follow-up study. J. Hypertens. 2007;25:439–447. doi: 10.1097/HJH.0b013e3280115bfb. [DOI] [PubMed] [Google Scholar]
- 11.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 12.Miyano I, Nishinaga M, Takata J, et al. Association between brachial-ankle pulse wave velocity and 3-year mortality in community-dwelling older adults. Hypertens. Res. 2010;33:678–682. doi: 10.1038/hr.2010.56. [DOI] [PubMed] [Google Scholar]
- 13.Fogari R, Zoppi A, Corradi L, et al. Effect of body weight loss and normalization on blood pressure in overweight non-obese patients with stage 1 hypertension. Hypertens. Res. 2010;33:236–242. doi: 10.1038/hr.2009.220. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg Y, Boaz M, Matas Z, et al. Weight loss induced by nutritional and exercise intervention decreases arterial stiffness in obese subjects. Clin. Nutr. 2009;28:21–25. doi: 10.1016/j.clnu.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal JA, Babyak MA, Hinderliter A, et al. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch. Intern. Med. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balkestein EJ, van Aggel-Leijssen DP, van Baak MA, et al. The effect of weight loss with or without exercise training on large artery compliance in healthy obese men. J. Hypertens. 1999;17:1831–1835. doi: 10.1097/00004872-199917121-00008. [DOI] [PubMed] [Google Scholar]
- 17.Njoroge JN, El Khoudary SR, Fried LF, et al. High urinary sodium is associated with increased carotid intima-media thickness in normotensive overweight and obese adults. Am. J. Hypertens. 2011;24:70–76. doi: 10.1038/ajh.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis D, Lloyd C, Becker DJ, et al. The changing course of diabetic nephropathy: Low-density lipoprotein cholesterol and blood pressure correlate with regression of proteinuria. Am. J. Kidney Dis. 1996;27:809–818. doi: 10.1016/s0272-6386(96)90518-1. [DOI] [PubMed] [Google Scholar]
- 19.Cooper JN, Tepper P, Barinas-Mitchell E, et al. Serum Aldosterone Is Associated with Inflammation and Aortic Stiffness in Normotensive Overweight and Obese Young Adults. Clin. Exp. Hypertens. 2011 doi: 10.3109/10641963.2011.618200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendelhag I, Gustavsson T, Suurkula M, et al. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin. Physiol. 1991;11:565–577. doi: 10.1111/j.1475-097x.1991.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 21.Hedeker D, Gibbons RD. Application of Random-Effects Pattern-Mixture Models for Missing Data in Longitudinal Studies. Psychological Methods. 1997;2:64–78. [Google Scholar]
- 22.Greenwald SE. Ageing of the conduit arteries. J. Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 23.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriosclerosis Thrombosis and Vascular Biology. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara J, Hayashi K, Yokoi T, et al. Brachial-ankle pulse wave velocity: an index of central arterial stiffness? J. Hum. Hypertens. 2005;19:401–406. doi: 10.1038/sj.jhh.1001838. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J. Hypertens. 2009;27:2022–2027. doi: 10.1097/HJH.0b013e32832e94e7. [DOI] [PubMed] [Google Scholar]
- 26.Yamada J, Tomiyama H, Matsumoto C, et al. Overweight body mass index classification modifies arterial stiffening associated with weight gain in healthy middle-aged Japanese men. Hypertens. Res. 2008;31:1087–1092. doi: 10.1291/hypres.31.1087. [DOI] [PubMed] [Google Scholar]
- 27.Schumacher W, Cockcroft J, Timpson NJ, et al. Association between C-reactive protein genotype, circulating levels, and aortic pulse wave velocity. Hypertension. 2009;53:150–157. doi: 10.1161/HYPERTENSIONAHA.108.117622. [DOI] [PubMed] [Google Scholar]
- 28.Ferri C, Croce G, Cofini V, et al. C-reactive protein: interaction with the vascular endothelium and possible role in human atherosclerosis. Curr. Pharm. Des. 2007;13:1631–1645. doi: 10.2174/138161207780831301. [DOI] [PubMed] [Google Scholar]
- 29.Tomiyama H, Hashimoto H, Tanaka H, et al. Synergistic relationship between changes in the pulse wave velocity and changes in the heart rate in middle-aged Japanese adults: a prospective study. J. Hypertens. 2010;28:687–694. doi: 10.1097/HJH.0b013e3283369fe8. [DOI] [PubMed] [Google Scholar]
- 30.Sa Cunha R, Pannier B, Benetos A, et al. Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. J. Hypertens. 1997;15:1423–1430. doi: 10.1097/00004872-199715120-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.